On-Site Multisample Determination of Chlorogenic Acid in Green Coffee by Chemiluminiscent Imaging
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
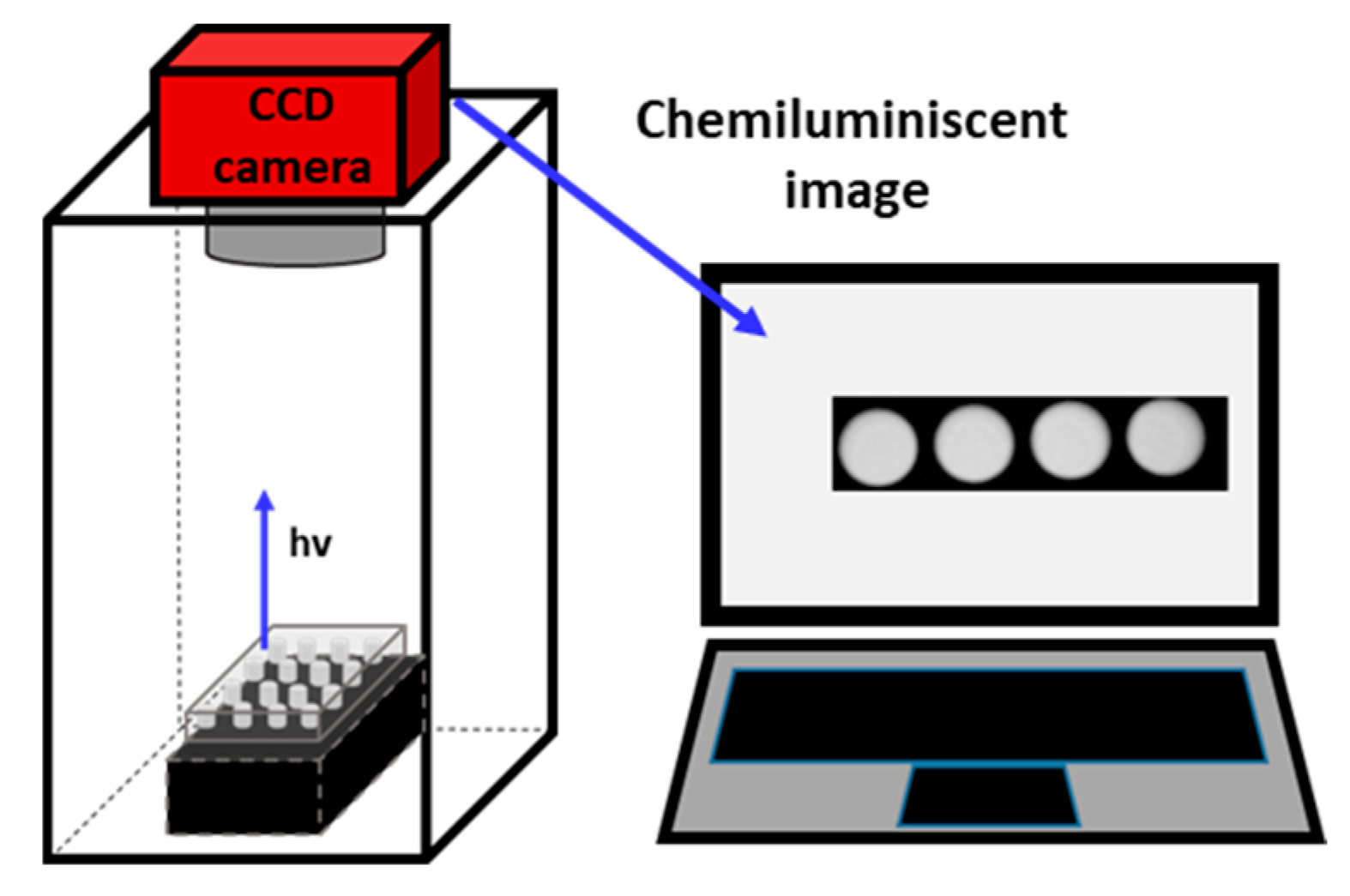
2.2. Equipment
2.3. Procedure
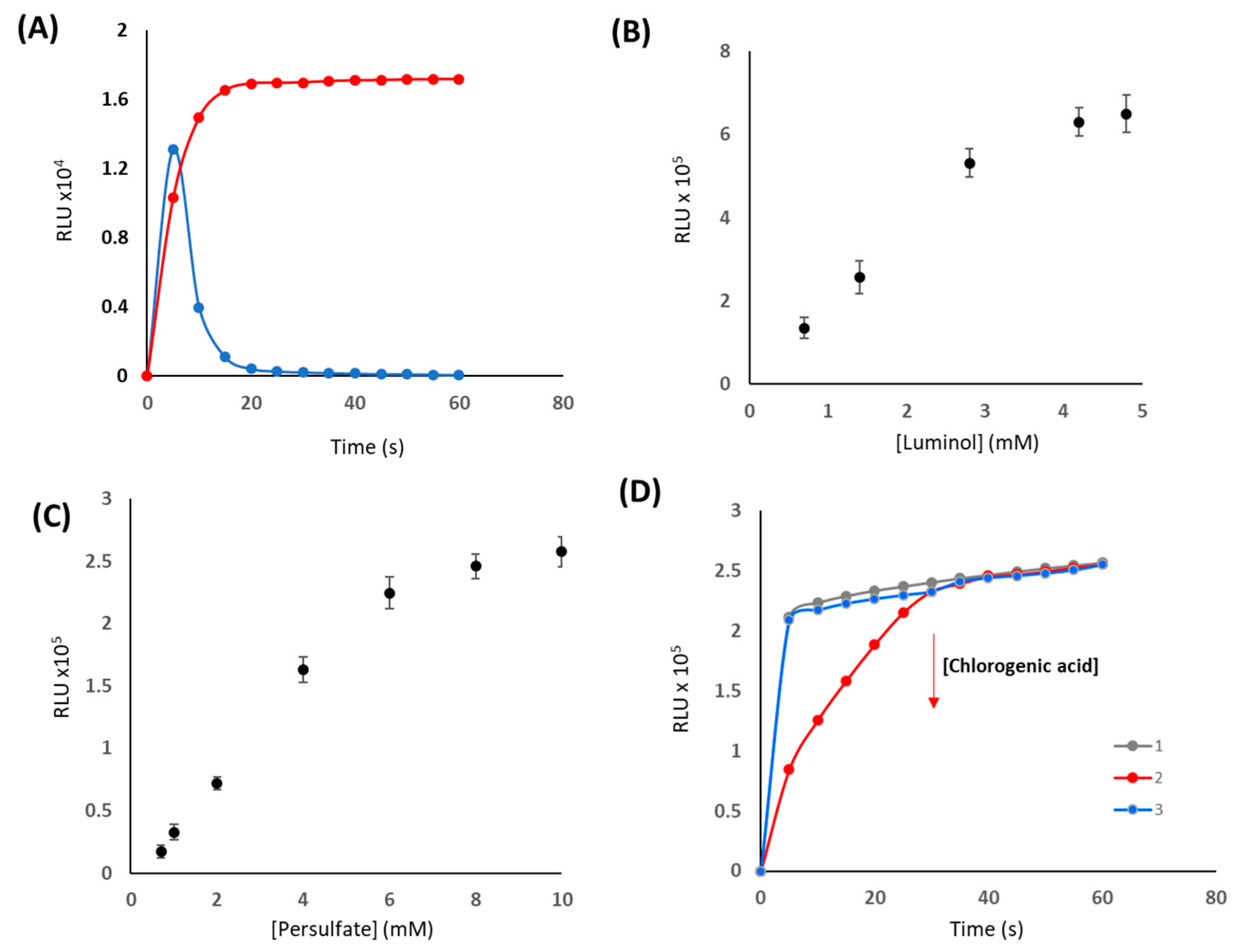
2.3.1. Optimization and Measurement of the CL Response
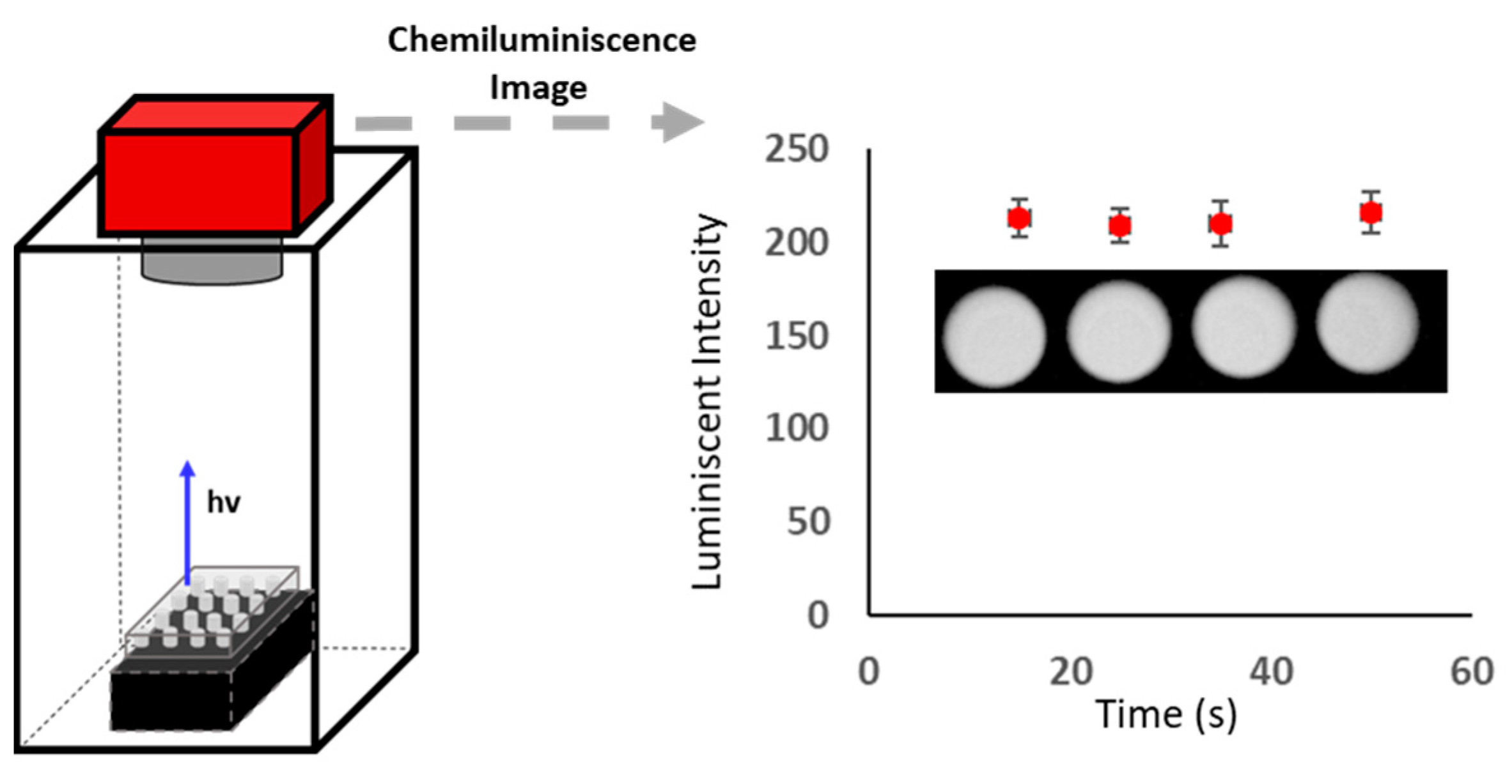
2.3.2. CCD Measurements
2.4. Analysis of Commercial Green Coffee Samples
3. Procedure
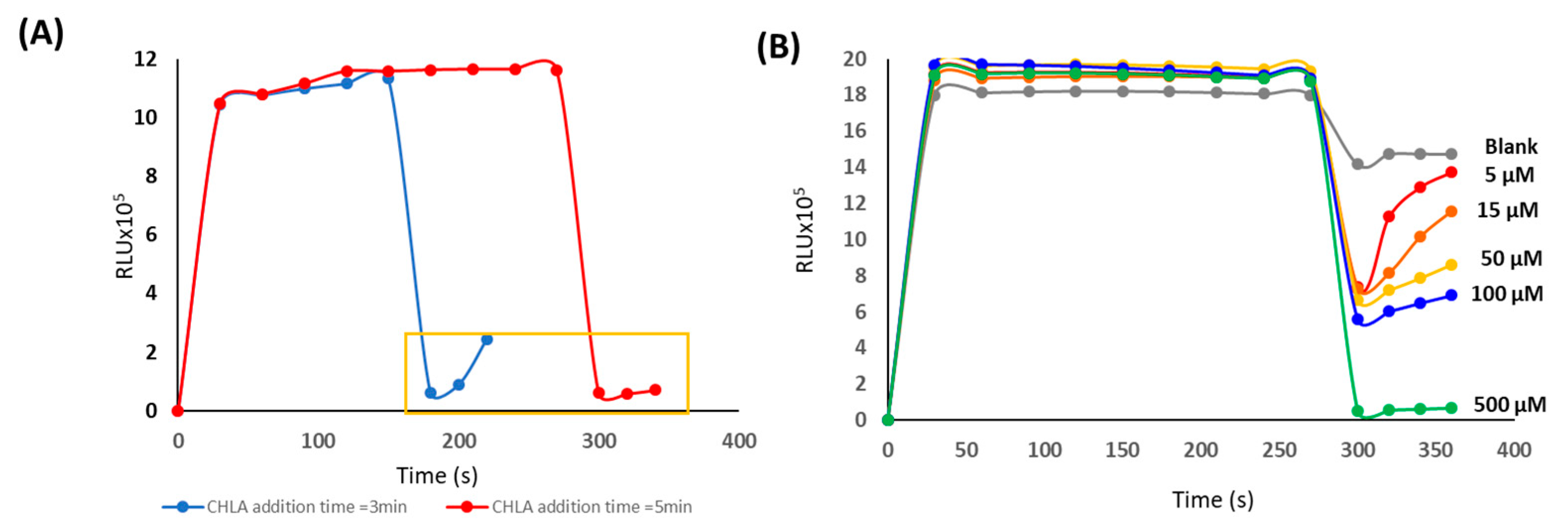
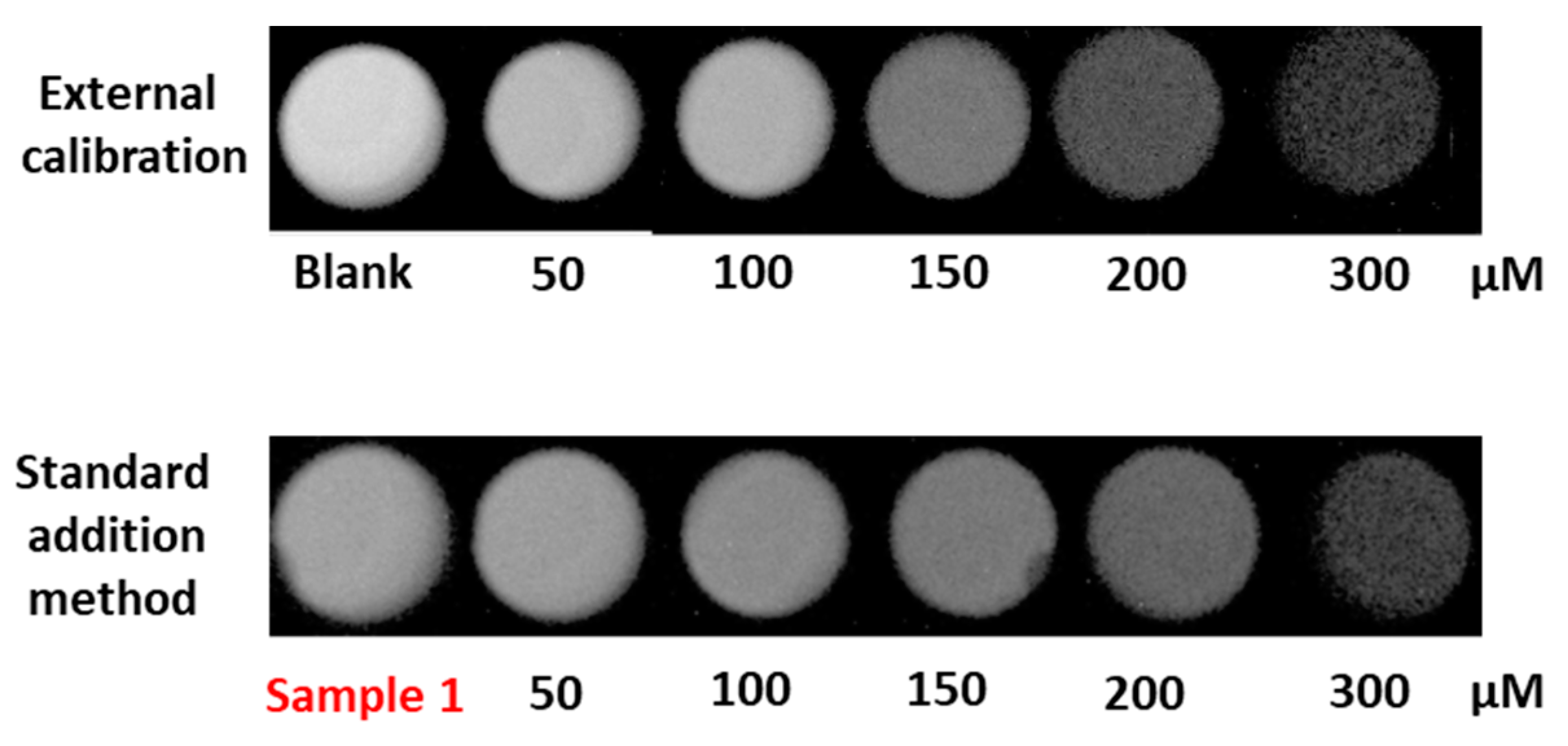
3.1. Study of Chemiluminiscent Imaging as an Analytical Response
3.2. Analytical Parameters of the Procedure
3.3. Procedure Validation: Application to the Analysis of Green Coffee Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogge, M.; Kumar, P.; Grundmann, O.; ACCP Public Policy Committee. Front-Line Health Care Professionals Lack Critical Knowledge in Dietary Supplement and Nutraceutical Products: A Call to Action for Comprehensive Educational Opportunities. J. Clin. Pharmacol. 2022, 62, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Binns, C.W.; Lee, M.K.; Lee, A.H. Problems and Prospects: Public health regulations of dietary supplements. Annu. Rev. Public Health 2018, 39, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Joko, S.; Watanabe, M.; Fuda, H.; Takeda, S.; Furukawa, T.; Hui, S.-P.; Shrestha, R.; Chiba, H. Comparison of chemical structures and cytoprotection abilities between direct and indirect antioxidants. J. Funct. Foods 2017, 35, 245. [Google Scholar] [CrossRef]
- Bandara, S.B.; Urban, A.; Liang, L.G.; Parker, J.; Fung, E.; Maier, A. Active pharmaceutical contaminants in dietary supplements: A tier-based risk assessment approach. Regul. Toxicol. Pharmacol. 2022, 123, 104955. [Google Scholar] [CrossRef]
- Coskun, S.H.; Wise, S.A.; Kuszak, A.J. The Importance of Reference Materials and Method Validation for Advancing Research on the Health Effects of Dietary Supplements and Other Natural Products. Front. Nutr. 2021, 8, 786261. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. A review on electrochemical sensors and biosensors used for chlorogenic acid electroanalysis. Int. J. Mol. Sci. 2021, 22, 13138. [Google Scholar] [CrossRef]
- Vidal-Casanella, O.; Núñez, O.; Granados, M.; Saurina, J.; Sentellas, S. Sentellas. Analytical methods for exploring nutraceuticals based on phenolic acids and polyphenols. Appl. Sci. 2021, 11, 8276. [Google Scholar] [CrossRef]
- Jia, W.; Shi, L.; Zhang, F.; Chang, J.; Chu, X.G. High-Throughput mass spectrometry scheme for screening and quantification of flavonoids in antioxidants nutraceuticals. J. Chromatogr. A. 2019, 1608, 460408. [Google Scholar] [CrossRef]
- Fantoukh, O.I.; Wang, Y.H.; Parveen, A.; Hawwal, M.E.; Ali, Z.; Al-Hamoud, G.A.; Chittiboyina, A.G.; Joubert, E.; Viljoen, A.; Khan, I.A. Chemical fingerprinting profile and targeted quantitative analysis of phenolic compounds from Rooibos tea and dietary supplements by using UHPLC-PDA-MS. Separations 2022, 9, 159. [Google Scholar] [CrossRef]
- Brzezicha, J.; Błażejewicz, D.; Brzezińska, J.; Grembecka, M. Green coffee vs dietary supplements: A comparative analysis of bioactive compounds and antioxidant activity. Food Chem. Toxicol. 2021, 155, 112377. [Google Scholar] [CrossRef]
- Saar-Reismaa, P.; Koel, M.; Tarto, R.; Vaher, M. Extraction of bioactive compounds from Dpsacus fullonum leaves using Deep eutectic solvents. J. Chromatogr. A 2022, 1677, 463330. [Google Scholar] [CrossRef] [PubMed]
- Krzyminski, K.K.; Roshal, A.D.; Rudnicki-Velasquez, P.B.; Zamojc, K. On the use of acridinium indicators for the chemiluminiscent determination of the total antioxidant capacity of dietary supplements. Luminiscence 2019, 34, 512. [Google Scholar] [CrossRef] [PubMed]
- Jornet-Martínez, N.; Moliner-Martínez, Y.; Molins-Legua, C.; Campíns-Falcó, P. Trends for the Development of In Situ Analysis Devices. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–23. [Google Scholar]
- Poppa, C.V.; Visilescu, A.; Litescu, S.C.; Albu, C.; Danet, A.F. Metal nano-oxide based colorimetric sensor array for the determination of polyphenols with antioxidant properties. Anal. Lett. 2020, 53, 627. [Google Scholar] [CrossRef]
- Puangbanlang, C.; Sirivibulkovit, K.; Nacapricah, D.; Sameenoi, Y. A paper-based device for simultaneous determination of antioxidant activity and total phenolic content in food samples. Talanta 2019, 198, 542. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Kumar, A.; Sachan, M.; Gupta, S.; Nara, S. Aptamer-gold nanozyme based competitive lateral flow assay for rapid detection of CA125 in human serum. Biosens. Bioelectr. 2020, 165, 112368. [Google Scholar] [CrossRef]
- Chládková, G.; Kunovská, K.; Chocholouš, P.; Polášek, M.; Sklenářová, H. Automatic Screening of Antioxidants Based on the Evaluation of Kinetics of Suppression of Chemiluminescence in a Luminol-Hydrogen Peroxide System Using a Sequential Injection Analysis Setup with a Flow-Batch Detection Cell. Anal. Meth. 2019, 11, 2531. [Google Scholar] [CrossRef]
- González-Fuenzalida, R.A.; Molins-Legua, C.; Calabria, D.; Mirasoli, M.; Guardigli, M.; Roda, A.; Campins-Falcó, P. Sustainable and green persulfate-based chemiluminescent method for on-site estimation of chemical oxigen demand in water. Anal. Chim. Acta 2022, 1223, 340196. [Google Scholar] [CrossRef]
- Velkiska-Markovska, L.; Jankulovska, M.S.; Petanovska-Ilievska, B.; Hristovski, K. Development and validation of RRLC-UV method for determination of chlorogenic acid in green coffee. Acta Chromatogr. 2020, 32, 34. [Google Scholar] [CrossRef]
- Chen, X.; Mao, J.; Wen, F.; Xu, X. Determination of phenolic acids in botanical pharmaceutical products by capillary electrophoresis with chemiluminiscence detection. Anal. Lett. 2021, 54, 8861765. [Google Scholar] [CrossRef]
- Xia, Z.; Sun, Y.; Cai, C.; He, Y.; Nie, P. Determination of chlorogenic acid, luteoloside and 3,5-O-dicaffeoylquinic acid in Chrysanthemum using near—Infrared spectroscopy. Sensors 2019, 19, 1981. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Simultaneous determination of caffeic acid and ferrulic acid using carbon nanofiber-based screen-printed sensor. Sensors 2022, 22, 4689. [Google Scholar] [CrossRef] [PubMed]

| Analytical Parameters | |
|---|---|
| Working interval (µM) | 50–400 |
| a ± sa | 200 ± 4 |
| b ± sb (µM−1) | 0.470 ± 0.017 |
| R2 | 0.999 |
| LOD (µM) | 20 |
| RSD intraday (%) | 2.0 |
| RSD interday (%) | 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallorca-Cebria, S.; Moliner-Martinez, Y.; Molins-Legua, C.; Campins-Falcó, P. On-Site Multisample Determination of Chlorogenic Acid in Green Coffee by Chemiluminiscent Imaging. Methods Protoc. 2023, 6, 20. https://doi.org/10.3390/mps6010020
Mallorca-Cebria S, Moliner-Martinez Y, Molins-Legua C, Campins-Falcó P. On-Site Multisample Determination of Chlorogenic Acid in Green Coffee by Chemiluminiscent Imaging. Methods and Protocols. 2023; 6(1):20. https://doi.org/10.3390/mps6010020
Chicago/Turabian StyleMallorca-Cebria, Sergi, Yolanda Moliner-Martinez, Carmen Molins-Legua, and Pilar Campins-Falcó. 2023. "On-Site Multisample Determination of Chlorogenic Acid in Green Coffee by Chemiluminiscent Imaging" Methods and Protocols 6, no. 1: 20. https://doi.org/10.3390/mps6010020
APA StyleMallorca-Cebria, S., Moliner-Martinez, Y., Molins-Legua, C., & Campins-Falcó, P. (2023). On-Site Multisample Determination of Chlorogenic Acid in Green Coffee by Chemiluminiscent Imaging. Methods and Protocols, 6(1), 20. https://doi.org/10.3390/mps6010020









