Sustainable and Cost-Effective Gel Documentation
Abstract
1. Introduction
2. Materials and Equipment
- Free standing UV transilluminating box (for documenting gels with ethidium bromide).
- Costs for a transilluminator vary from $900–$2500 USD. The cost is lower if purchased from a supplier of used scientific equipment, with some suppliers offering warranties. Gel boxes are long-lasting pieces of equipment and a good investment. Our lab’s UV light box, a Cole-Parmer 97500 series, was inherited from a dismantled lab, and is 30+ years old, requiring only a change of bulbs and fluorescent bulb starters. For safety and performance reasons, the UV light box should be obtained from a reputable manufacturer or scientific supplier (see Discussion). Repurposing a light box with UV bulbs is not recommended due to cost and safety concerns.
- Consider all DNA detection dyes that will be used by the lab and ensure that the excitation wavelengths for the dyes are able to pass through the transillumination surface plate. The more expensive, all-in-one Gel Documentation Systems provide flexibility regarding dye usage, as multiple excitation wavelengths are possible and multiple bandpass filter sets are provided with the instrument. Our lab uses dilute ethidium bromide to stain gels in routine genotyping or quality and quantity checking, and SYBR gold when downstream applications require extraction of stained DNA [9,10]. Ethidium bromide’s fluorescence excitation peak, 312 nm [19], is a standard excitation wavelength for UV transilluminating boxes. SYBR gold has a blue light excitation peak at 495 nm, and a second, weaker excitation band at ~300 nm, energies emitted from a UV transilluminator [20]. While LED diodes with 312 nm emission are available, there are currently no off-the-shelf replacements for mercury fluorescent bulbs in boxes optimized for 312 nm excitation, and many bulbs marketed to the public as UV or germicidal do not actually emit UV below 400 nm.
- If both UV and blue light illumination (for SYBR) are desired, and if UV transilluminator wavelengths are not sufficient for SYBR (due to blockage of blue wavelengths), blue light converter screens have been developed (Syngene). These are placed on top of the UV transilluminator and convert UV to blue light. Alternatively, an additional box can be configured for SYBR dyes. A separate dual blue/white light box can also be useful for detection of Coomassie stained protein gels.
- For UV transilluminators that pass wavelengths under 495 nm, imaging SYBR gels without incurring UV-induced damage to the DNA is possible by placing an acrylic sheet underneath the gel. Acrylic (PMMA poly(methyl methacrylate) or Plexiglas® Perfexion™ UF-5 blocks 95% of UV (Plexiglas U825-UVA: [21]). Small sheets of UV-resistant acrylic are sold as glass substitutes for picture frames; larger sizes can be purchased from a plastics manufacturer (EMCO industrial plastics).
- UV protection goggles. The photography hood covers the entire illumination plate, protecting users from UV during photography, but when excising DNA from gels, the plastic cover and UV goggles should be used. Opaque paper towels should cover exposed areas of the UV light box and users should also wear UV protection goggles.
- White light illumination box for photography of protein gels
- These are relatively inexpensive, when new, and can be obtained from photography or art suppliers. Newer equipment is outfitted with LED lamps.
- Blue LED bulbs (495 mn) are suitable for SYBR gold excitation. Newer gel boxes are outfitted with both white and blue lamps for viewing Coomassie stained protein gels or SYBR-stained DNA gels.
- Care should be taken to avoid placing wet gels on the surface of the light box. They are not water-tight, and Coomassie stain can build up on the surface.
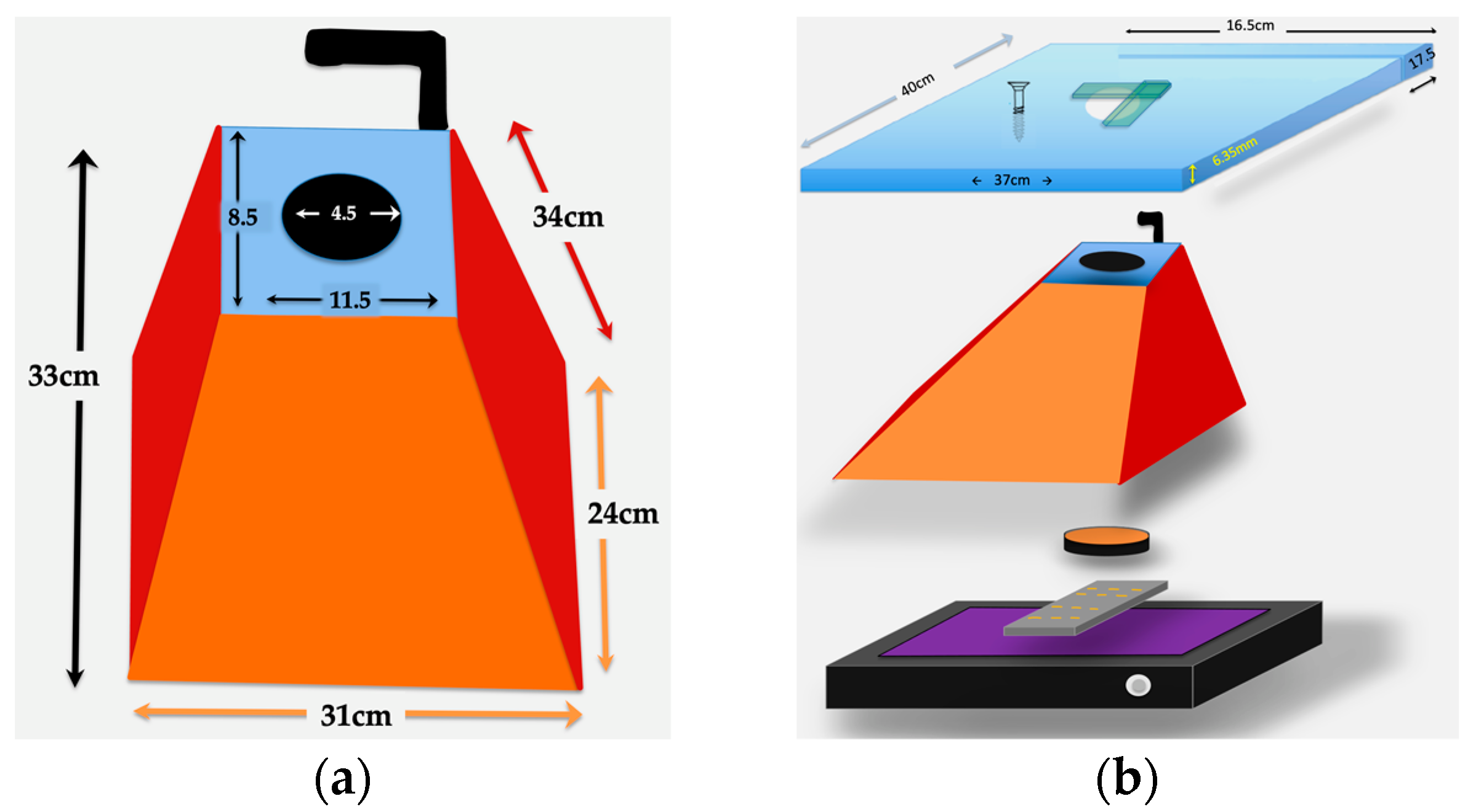
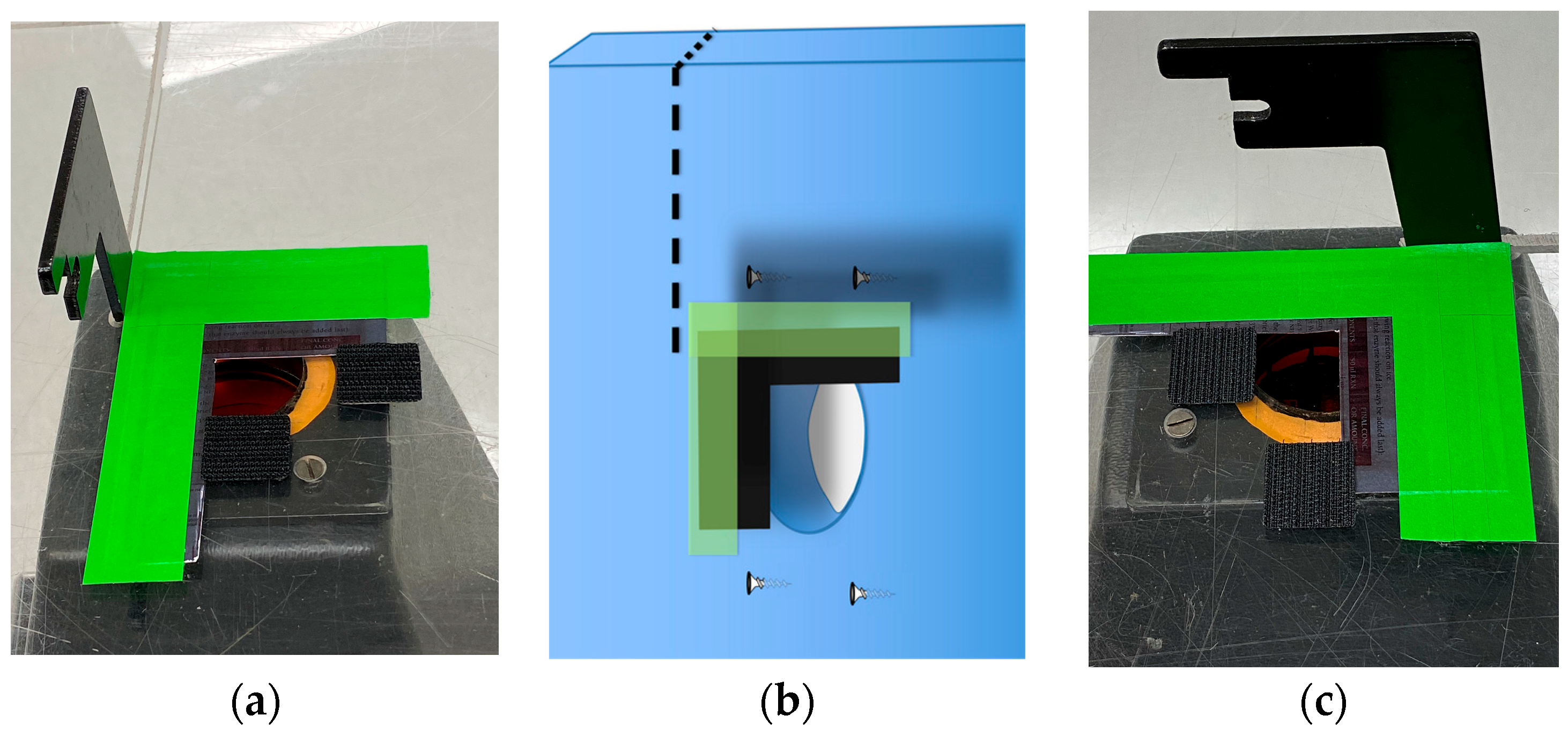
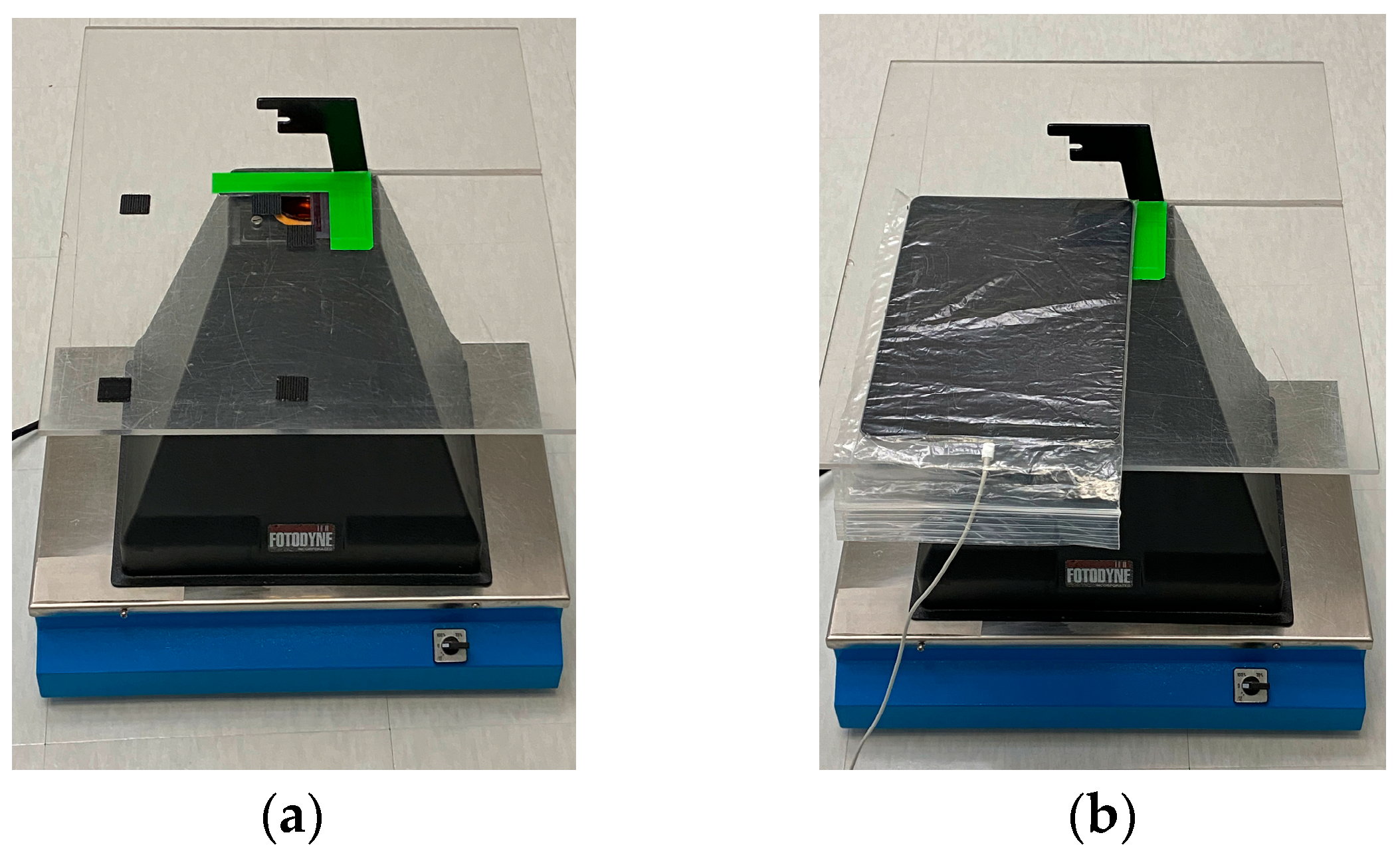
- Light shielding photography hood. The simple Fotodyne photography hood we describe here is difficult to find as new equipment. The hoods can be purchased from suppliers of used equipment for $10–$80 USD. With the dimensions provided in Figure 1A, a similar hood could easily be constructed using lightweight wood or plywood. The purpose of the hood is to shield the experiment from light, protect the user from UV, and provide a stable platform for the camera. Other shapes of hoods will likely work as well. An appropriately-sized Styrofoam shipping box might serve well—it would be waterproof, and a sturdy strip of plastic, such as a ruler, could be glued to its back wall to serve as a handle. A coating of matte black paint or spray paint on the Styrofoam would improve image quality.
- Bandpass filters: 590 nm for ethidium bromide; 530/40 nm for SYBR gold.
- Obsolete electronics with camera. The device should be wiped clean of data and apps and removed from online visibility. Power and data transfer cords are needed. Images here used a 1st gen iPad Air with 5 MP camera and 9.7 in display.
- Transparent, sealable plastic bag to protect the electronics.
- Data storage. A secure method should be devised to easily transfer images to working laboratory computers or cloud storage.
- Squares of hook-and-loop style fasteners (Velcro® brand) with adhesive backing.
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, B.J. SDS polyacrylamide gel electrophoresis of proteins. Methods Mol. Biol. 1994, 32, 23–34. [Google Scholar] [CrossRef]
- Maniatis, T.; Fritsch, E.F.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: New York, NY, USA, 1982; p. 545. [Google Scholar]
- Green, M.R.; Sambrook, J. Analysis of DNA by Agarose Gel Electrophoresis. Cold Spring Harb. Protoc. 2019. [Google Scholar] [CrossRef]
- Koontz, L. Agarose gel electrophoresis. Methods Enzym. 2013, 529, 35–45. [Google Scholar] [CrossRef]
- Sonagra, A.D.; Dholariya, S.J. Electrophoresis; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Williams, L.R. Staining nucleic acids and proteins in electrophoresis gels. Biotech. Histochem. 2001, 76, 127–132. [Google Scholar] [CrossRef]
- Smejkal, G.B. The Coomassie chronicles: Past, present and future perspectives in polyacrylamide gel staining. Expert Rev. Proteom. 2004, 1, 381–387. [Google Scholar] [CrossRef]
- Neidle, S. The molecular basis for the action of some DNA-binding drugs. Prog. Med. Chem. 1979, 16, 151–221. [Google Scholar] [CrossRef]
- Huang, Q.; Fu, W.-L. Comparative analysis of the DNA staining efficiencies of different fluorescent dyes in preparative agarose gel electrophoresis. Clin. Chem. Lab. Med. 2011, 43, 49–52. [Google Scholar] [CrossRef]
- Han, X.; Wang, W.; Cui, Y.; Lin, Y.; Chen, H.; An, R.; Liang, X.; Komiyama, M. The staining efficiency of cyanine dyes for single-stranded DNA is enormously dependent on nucleotide composition. Electrophoresis 2019, 40, 1708–1714. [Google Scholar] [CrossRef]
- Porch, T.G.; Erpelding, J.E. Low-cost conversion of the Polaroid MD-4 land camera to a digital gel documentation system. J. Biochem. Biophys. Methods 2006, 67, 1–5. [Google Scholar] [CrossRef]
- Scott, T.M.; Dace, G.L.; Altschuler, M. Low-cost agarose gel documentation system. Biotechniques 1996, 21, 68–70, 72. [Google Scholar] [CrossRef]
- Medberry, S.; Gallagher, S.; Moomaw, B. Overview of digital electrophoresis analysis. Curr. Protoc. Protein Sci. 2005, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Zyzik, A.; Loeschke, S.; Lindsay, W.; Vollmer, E. Cost-effective gel documentation using a web-cam. J. Biochem. Biophys. Methods 2001, 50, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Cunha, E.N.; de Souza, M.F.B.; Lanza, D.C.F.; Lima, J. A low-cost smart system for electrophoresis-based nucleic acids detection at the visible spectrum. PLoS ONE 2020, 15, e0240536. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Gonzalez-Garcia, M.; Perez-Ballestero, R. Low-cost modular systems for agarose gel documentation. Biotechniques 2022, 73, 227–232. [Google Scholar] [CrossRef]
- Nilsen, E. Biden administration moves to phase out compact fluorescent light bulbs and push market toward LEDs. In CNN Politics; Warner Brothers Disocvery Company: Atlanta, Georgia, 2022. [Google Scholar]
- Bennich, P.; Scholand, M. Evidence of the Availability of Mercury-Free Alternative Products to Certain Fluorescent Lamps. 2019. Available online: https://www.mercuryconvention.org/sites/default/files/documents/submission_from_government/Norway1_Lamp.pdf (accessed on 20 December 2022).
- Pohl, F.M.; Jovin, T.M.; Baehr, W.; Holbrook, J.J. Ethidium bromide as a cooperative effector of a DNA structure. Proc. Natl. Acad. Sci. USA 1972, 69, 3805–3809. [Google Scholar] [CrossRef]
- Tuma, R.S.; Beaudet, M.P.; Jin, X.; Jones, L.J.; Cheung, C.Y.; Yue, S.; Singer, V.L. Characterization of SYBR Gold nucleic acid gel stain: A dye optimized for use with 300-nm ultraviolet transilluminators. Anal. Biochem. 1999, 268, 278–288. [Google Scholar] [CrossRef]
- GSPlasticOpticsWebsite. Transmission Curves for Plastic Optics. Available online: https://www.gsoptics.com/transmission-curves/ (accessed on 20 December 2022).
- Tiselius, A. Electrophoresis of serum globulin: Electrophoretic analysis of normal and immune sera. Biochem. J. 1937, 31, 1464–1477. [Google Scholar] [CrossRef]
- Tiselius, A. Reflections from both sides of the counter. Annu. Rev. Biochem. 1968, 37, 1–24. [Google Scholar] [CrossRef]
- Kunkel, H.G.; Slater, R.J. Zone electrophoresis in a starch supporting medium. Proc. Soc. Exp. Biol. Med. 1952, 80, 42–44. [Google Scholar] [CrossRef]
- Smithies, O. Zone electrophoresis in starch gels: Group variations in the serum proteins of normal human adults. Biochem. J. 1955, 61, 629–641. [Google Scholar] [CrossRef]
- Hjerten, S. A new method for preparation of agaraose for gel electrophoresis. Biochim. Biophys. Acta 1962, 62, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Bonner, P.L.R.; Hargreaves, A. Basic Bioscience Laboratory Techniques: A Pocket Guide; John Wiley & Sons: Oxford, UK; Hoboken, NJ, USA, 2011; p. xix. 212p. [Google Scholar]
- GlobalNewswire. Global Electrophoresis Market Size To Grow USD 3.8 Billion By 2030; GlobalNewswire: Los Angeles, CA, USA, 2022. [Google Scholar]
- Shipley, R.A.; Stern, K.G.; White, A. Electrophoresis of Anterior Pituitary Proteins. J. Exp. Med. 1939, 69, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Toepler, A. Optischen Studien nach der Methode der Schlierenbeobachtung. Poggendorfs Ann. Phys. Chem. 1967, 131, 33–35. [Google Scholar]
- Alberty, R.A. An Introduction to Electrophoresis. J. Chem. Educ. 1948, 25, 426–433. [Google Scholar] [CrossRef]
- Takagi, T.; Kubota, H.; Oishi, S. Application of schlieren optics to real-time monitoring of protein electrophoresis in crosslinker-free linear polyacrylamide solution. Electrophoresis 1991, 12, 436–438. [Google Scholar] [CrossRef]
- Enviromental Health & Safety Working Group. Ethidium Bromide and Alternative DNA Stains: Precautions, Waste Treatment and Disposal. Available online: https://ehs.berkeley.edu/sites/default/files/publications/ethidium-bromide-fact-sheet.pdf (accessed on 20 December 2022).
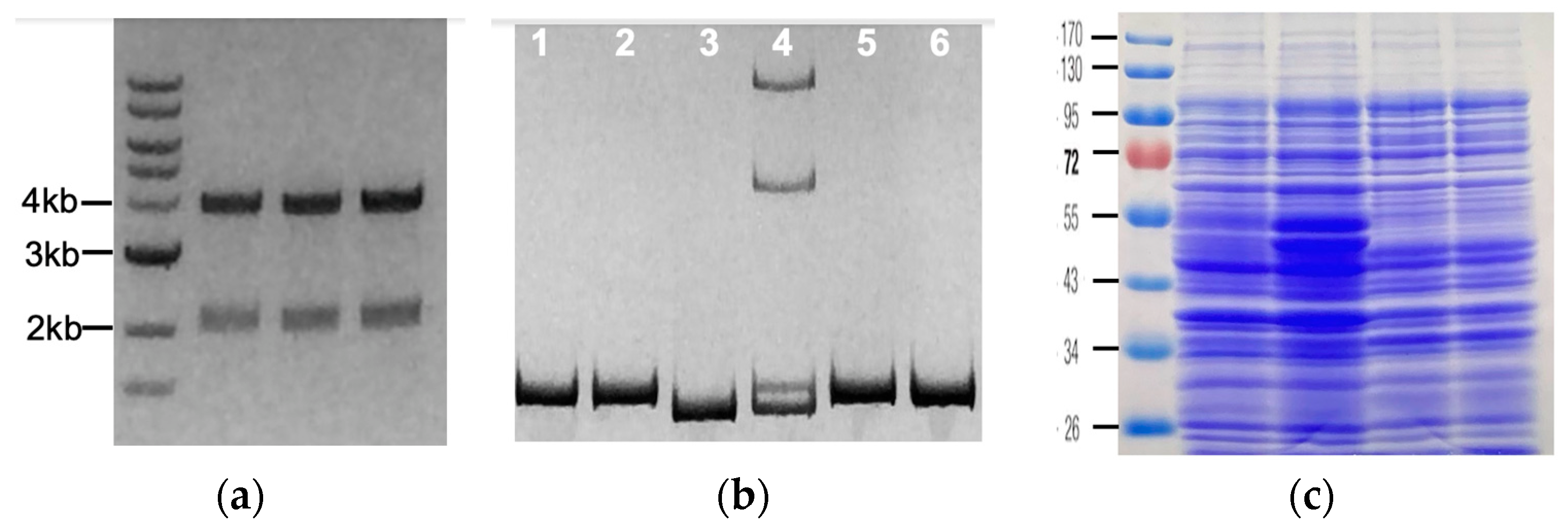
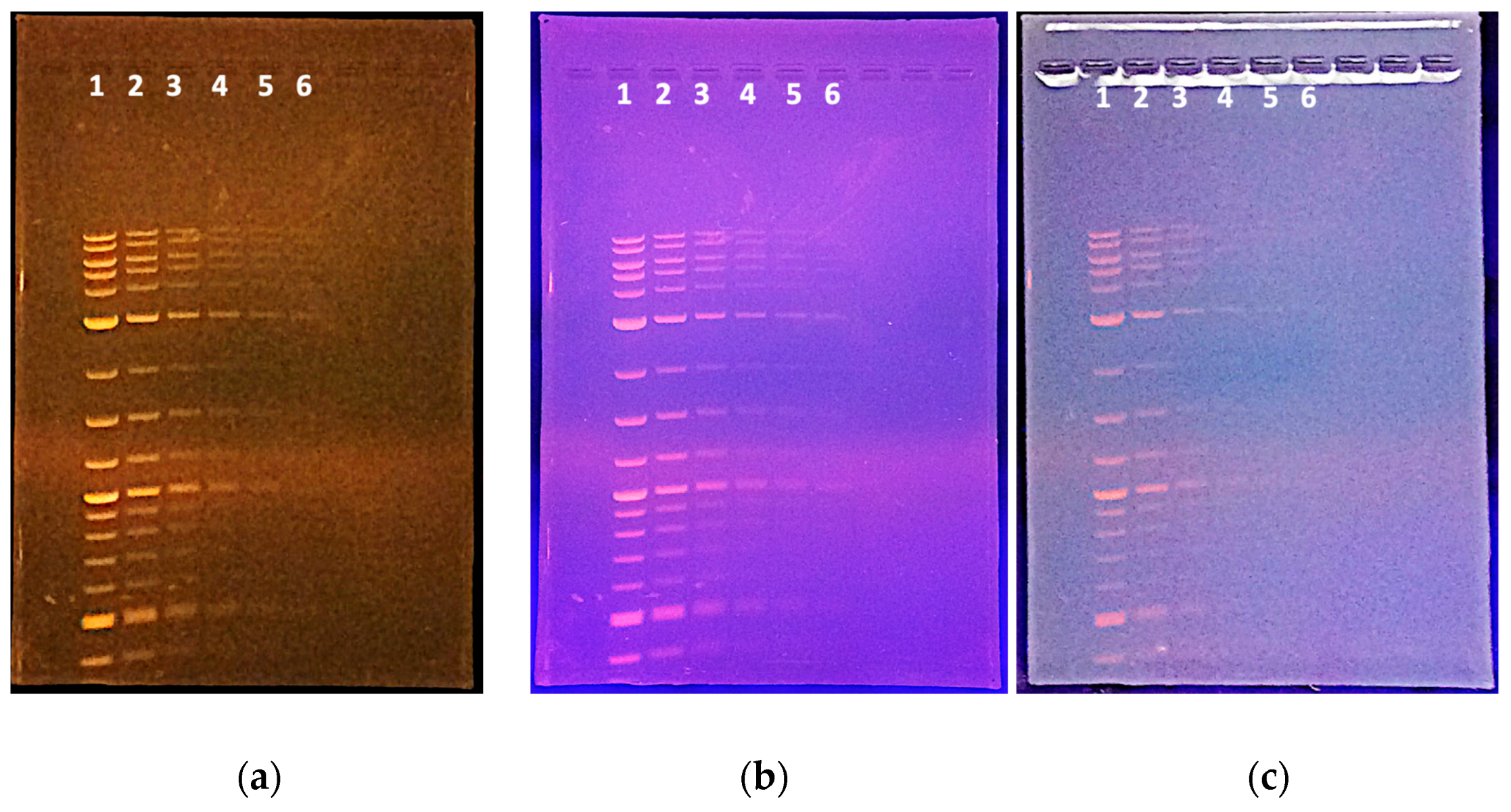
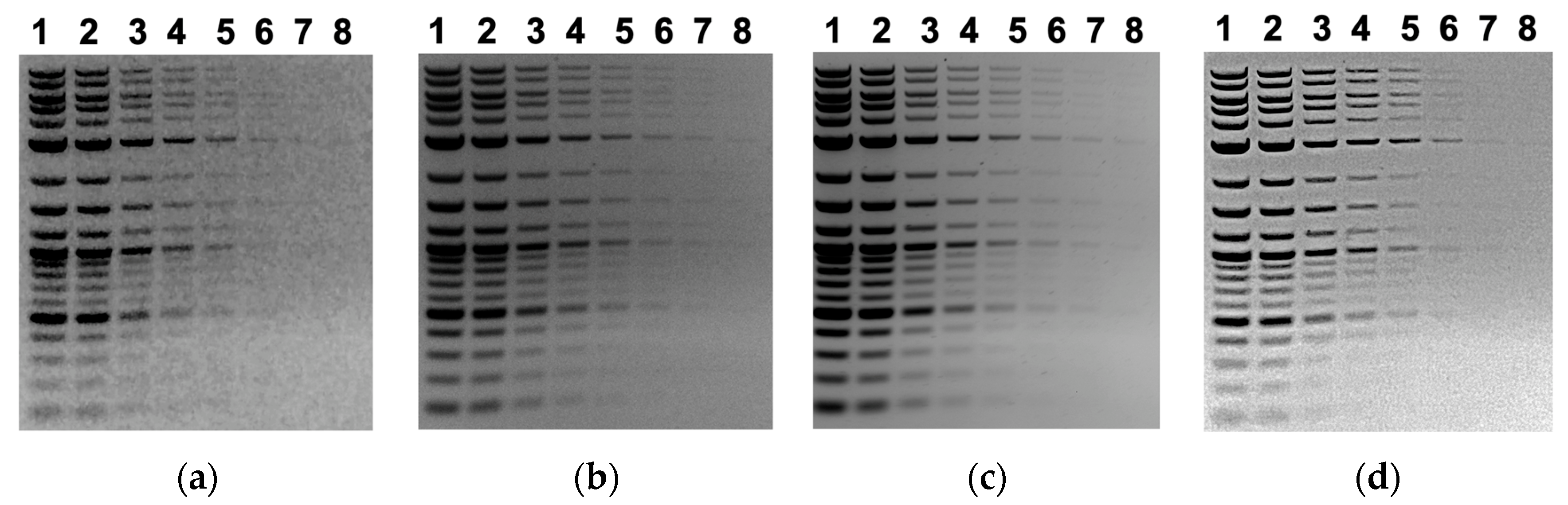
| Lane 1 | Lane 2 | Lane 3 | Lane 4 | Lane 5 | Lane 6 | |
|---|---|---|---|---|---|---|
| Length | Mass (ng) | Mass (ng) | Mass (ng) | Mass (ng) | Mass (ng) | Mass (ng) |
| 10 kb | 40 | 20.0 | 10.0 | 5.0 | 2.5 | 1.3 |
| 8 kb | 40 | 20.0 | 10.0 | 5.0 | 2.5 | 1.3 |
| 6 kb | 48 | 24.0 | 12.0 | 6.0 | 3.0 | 1.5 |
| 5 kb | 40 | 20.0 | 10.0 | 5.0 | 2.5 | 1.3 |
| 4 kb | 32 | 16.0 | 8.0 | 4.0 | 2.0 | 1.0 |
| 3 kb | 120 | 60.0 | 30.0 | 15.0 | 7.5 | 3.8 |
| 2 kb | 40 | 20.0 | 10.0 | 5.0 | 2.5 | 1.3 |
| 1.5 kb | 57 | 28.5 | 14.3 | 7.1 | 3.6 | 1.8 |
| 1.2 kb | 45 | 22.5 | 11.3 | 5.6 | 2.8 | 1.4 |
| 1 kb | 122 | 61.0 | 30.5 | 15.3 | 7.6 | 3.8 |
| 0.9 kb | 34 | 17.0 | 8.5 | 4.3 | 2.1 | 1.1 |
| 0.8 kb | 31 | 15.5 | 7.8 | 3.9 | 1.9 | 1.0 |
| 0.7 kb | 27 | 13.5 | 6.8 | 3.4 | 1.7 | 0.8 |
| 0.6 kb | 23 | 11.5 | 5.8 | 2.9 | 1.4 | 0.7 |
| 0.5 kb | 124 | 62.0 | 31.0 | 15.5 | 7.8 | 3.9 |
| 0.4 kb | 49 | 24.5 | 12.3 | 6.1 | 3.1 | 1.5 |
| 0.3 kb | 37 | 18.5 | 9.3 | 4.6 | 2.3 | 1.2 |
| 0.2 kb | 32 | 16.0 | 8.0 | 4.0 | 2.0 | 1.0 |
| 0.1 kb | 61 | 30.5 | 15.3 | 7.6 | 3.8 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asad, N.; Cregg, S.; Shakya, S.; Stegman, S.; Timmons, L. Sustainable and Cost-Effective Gel Documentation. Methods Protoc. 2023, 6, 21. https://doi.org/10.3390/mps6020021
Asad N, Cregg S, Shakya S, Stegman S, Timmons L. Sustainable and Cost-Effective Gel Documentation. Methods and Protocols. 2023; 6(2):21. https://doi.org/10.3390/mps6020021
Chicago/Turabian StyleAsad, Nadeem, Scott Cregg, Sudeep Shakya, Sutton Stegman, and Lisa Timmons. 2023. "Sustainable and Cost-Effective Gel Documentation" Methods and Protocols 6, no. 2: 21. https://doi.org/10.3390/mps6020021
APA StyleAsad, N., Cregg, S., Shakya, S., Stegman, S., & Timmons, L. (2023). Sustainable and Cost-Effective Gel Documentation. Methods and Protocols, 6(2), 21. https://doi.org/10.3390/mps6020021






