Synthesis Chalones and Their Isomerization into Flavanones and Azaflavanones
Abstract
:1. Introduction
2. Experimental Design
2.1. Materials
- Acetophenones (2’-aminoacetophenone and 2’-hydroxyacetophnone from Aldrich, Saint Louis, MO, USA).
- Benzaldehydes (e.g., benzaldehyde and p-methoxybenzaldehyde from Merck, Kenilworth, NJ, USA; and p-nitrobenzaldehyde from Riedel-Haën, Seelze, Germany).
- Sodium hydride from Aldrich (dry, 90%, or 60% dispersion in mineral oil).
- Montmorillonite K10 from Fulka AG.
- Proton sponge from Aldrich.
- Solvents (THF, methanol, ethanol, hexane, ethyl acetate, and dichloromethane p.a. grade from Riedel-Haën).
- Solid supports (silica gel 60 and celite from Merck).
2.2. Equipment
- Stirring plate and water circulating system.
- UV lamp (λ = 254 nm).
- Ethos MicroSynth Labstation microwave (Milestone Inc., Sorisole, Italy).
- NMR spectrometer (Bruker Avance 300).
3. Procedure
3.1. Synthesis of Chalcone Derivatives (Time for Completion: 45 min)
- Add 2’-hydroxyacetophenone 1a or 2’-aminoacetophenone 1b (8.2 mmol), dried THF (20.0 mL), and NaH (24.7 mmol) to a round-bottom reactor equipped with a stir bar.
CRITICAL STEP. The NaH should be added slowly (if necessary, a glass funnel can be used) and with gentle stirring. The round-bottom reactor can be put in an ice bath placed on a stirring plate. This will prevent the uncontrolled release of hydrogen.
- OPTIONAL STEP. The THF can be dried by the students following a described procedure [13] or molecular sieves can be added to the flask the day before the class.
PAUSE STEP. After the addition of the NaH, the mixture should be stirred at room temperature for 10 min.
- Add 1.5 molar equiv. of the desired benzaldehyde 2a–c and place the reactor in the microwave accordingly to the apparatus shown in Figure 1.
- Maintain the reaction mixture, stirring 15 min and with 400 W irradiation (for 2’-hydroxychalcones 3a–c) or 20 min and with 50 W irradiation (for 2’-aminochalcones 3d–f).
PAUSE STEP. After stopping, allow the reaction mixture to cool down to room temperature.
- Pour the reaction mixture into a vessel containing ice and add hydrochloric acid (1 mol dm−3 solution) to adjust pH to below 4 and simultaneously precipitate the desired chalcones.
- Filter the formed solid using a vacuum filtration apparatus.
- OPTIONAL STEPS. Recrystallize from hot ethanol (1 g of product in 5 mL of hot ethanol or dissolve with dichloromethane and purify by chromatography (using a mixture of hexane/ethyl acetate [7:3]).
3.2. Synthesis of Flavanone Derivatives (Time for Completion: 24 h)
- Add the appropriate 2’-hydroxychalcones 3a–c (0.2 mmol), methanol (3.0 mL), dichloromethane (3.0 mL), and proton sponge (0.5 mmol) to a 10 mL round-bottom flask equipped with a stir bar.
- Reflux during 24 h (Figure 2).
CRITICAL STEP. The 2’-hydroxychalcone isomerization is slow, so the reaction progress is controlled by TCL (using a mixture of hexane/dichloromethane [3:7]), and after 12 h, more or less half of the 2’-hydroxychalcone is converted. The reaction can be stopped if an additional step of purification is important for students.
- Pour the reaction mixture into a vessel containing ice and add hydrochloric acid to adjust pH (near 6).
- Extract with dichloromethane and evaporate to dryness.
- OPTIONAL STEP. Dissolve with dichloromethane and purify by chromatography (using a mixture of hexane/dichloromethane [3:7]).
- Recrystallize from hot ethanol (1 g of product in 5 mL of hot ethanol).
3.3. Synthesis of 2-aryl-2,3-dihydroquinolin-4(1H)-one Derivatives (Time for Completion: 45 min)
- Add 2’-aminochalcone 3d–f (0.1 g), and montmorillonite K10 (1.0 g) to a microwave tube.
CRITICAL STEP. The mixture of the reagents should be homogeneous, so it can be mixed using a mortar and pestle prior.
- Place the microwave tube accordingly into the apparatus shown in Figure 3 and irradiate at a constant power of 400 W for 20 min for the derivatives 4e,f and 900 W for 30min for the derivative 4d.
PAUSE STEP. After stopping, allow the reaction mixture to cool down to room temperature.
- Filter the reaction mixture through celite, using ethyl acetate to remove the desired compound.
- Evaporate the solvent.
- OPTIONAL STEP. Recrystallize from hot ethanol.
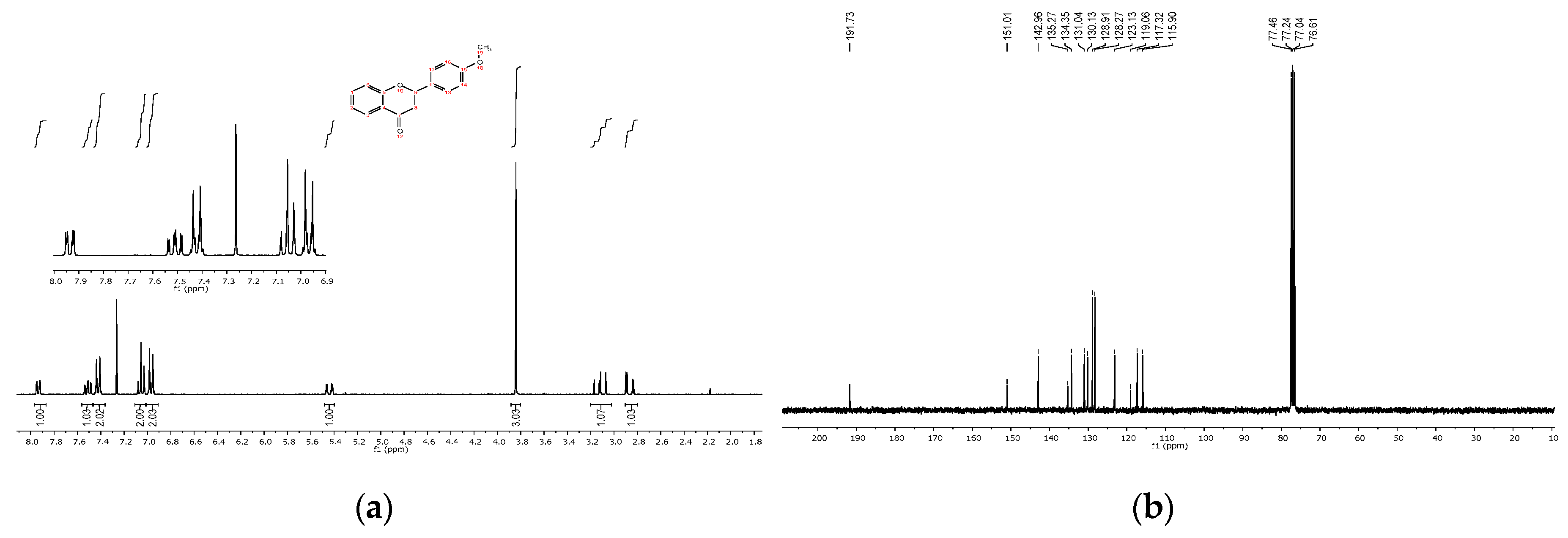
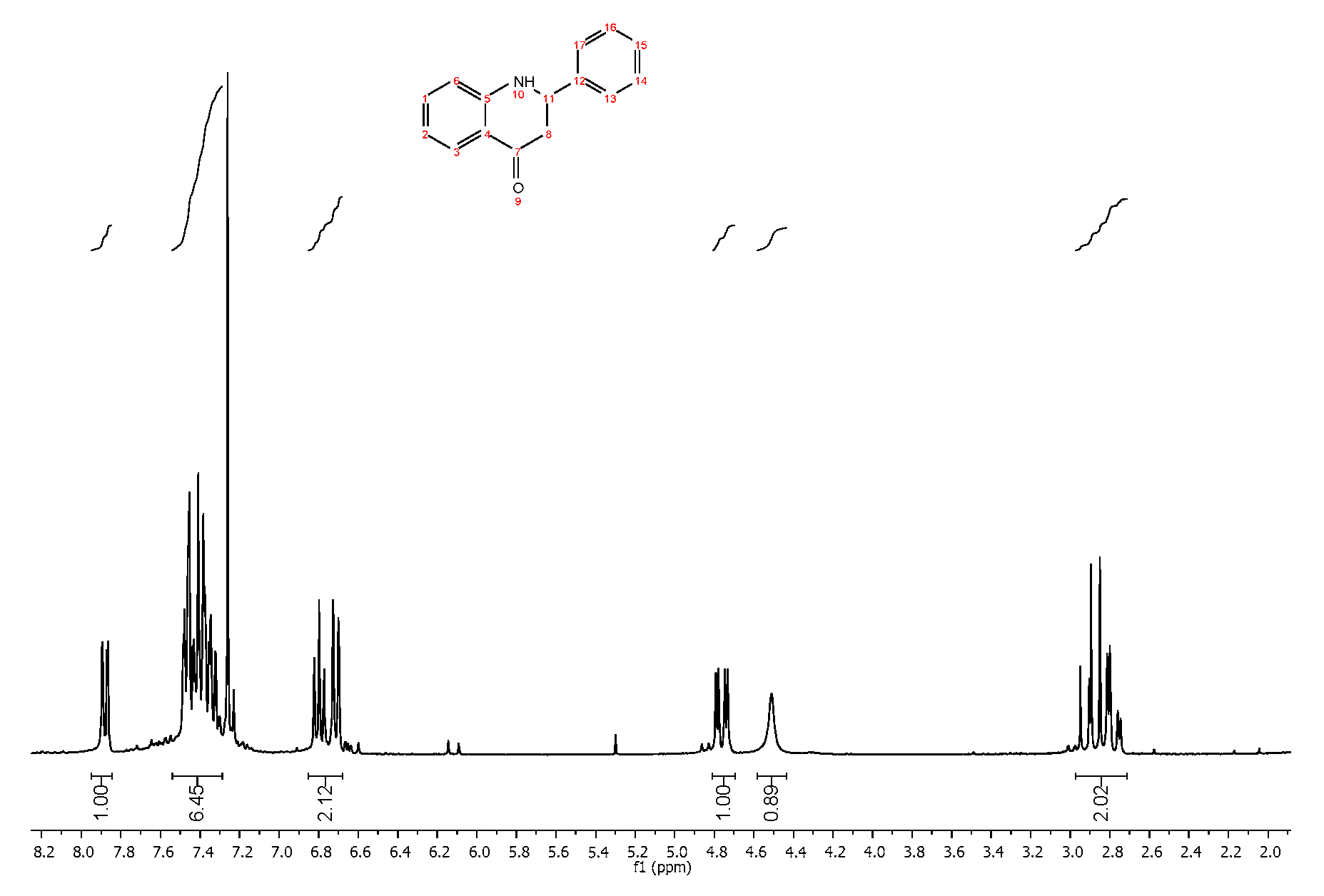
4. Expected Results
4.1. Reaction Conditions
4.2. Product Characterization
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosa, G.P.; Seca, A.M.L.; Barreto, M.C.; Silva, A.M.S.; Pinto, D.C.G.A. Chalcones and flavanones bearing hydroxyl and/or methoxyl groups: Synthesis and biological assessments. App. Sci. 2019, 9, 2846. [Google Scholar] [CrossRef]
- Tomás-Navarro, M.; Vallejo, F.; Tomás-Barberán, F.A. Bioavailability and metabolism of citrus fruit beverage flavanones in Humans. In Polyphenols in Human Health and Disease; Academic Press: Annapolis, MD, USA, 2014; Volume 1, pp. 537–551. [Google Scholar]
- Murkovic, M. Phenolic compounds. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Academic Press: Annapolis, MD, USA, 2003; pp. 4507–4514. [Google Scholar]
- European Medicines Agency. EMA/175398/2019. Available online: https://www.ema.europa.eu/en/documents/referral/quinolone-fluoroquinolone-article-31-referral-disabling-potentially-permanent-side-effects-lead_en.pdf (accessed on 15 July 2019).
- Shinde, B.; Kamble, S.B.; Pore, D.M.; Gosavi, P.; Gaikwad, A.; Jadhav, H.S.; Karale, B.K.; Burungale, A.S. pH-Transformed ZnO-NPs/NaPTS: The first room-temperature brisk synthesis of flavanones in aqueous medium. Chem. Select 2018, 3, 13197–13206. [Google Scholar] [CrossRef]
- Gupta, A.; Jamatia, R.; Patil, R.A.; Ma, Y.R.; Pal, A.K. Copper oxide/reduced graphene oxide nanocomposite-catalyzed synthesis of flavanones and flavanones with triazole hybrid molecules in one pot: A green and sustainable approach. ACS Omega 2018, 3, 7288–7299. [Google Scholar] [CrossRef]
- Chelghoum, M.; Bahnous, M.; Bouraiou, A.; Bouacida, S.; Belfaitah, A. An efficient and rapid intramolecular aza-Michael addition of 2’-aminochalcones using ionic liquids as recyclable reaction media. Tetrahedron Lett. 2012, 53, 4059–4061. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, L.; Yu, S. Enantioselective synthesis of azaflavanones using organocatalytic 6-endo aza-Michael addition. Adv. Synth. Catal. 2014, 356, 982–986. [Google Scholar] [CrossRef]
- Sakirolla, R.; Yaeghoobi, M.; Rahman, N.A. Synthesis of flavanones, azaflavanones, and thioflavanones catalyzed by PMA-SiO2 as a mild, efficient, and reusable catalyst. Monastsh. Chem. 2012, 143, 797–800. [Google Scholar] [CrossRef]
- McMurry, J. Organic Chemistry, 9th ed.; Cengage Learning: Boston, MA, USA, 2016. [Google Scholar]
- Silva, A.M.S.; Tavares, H.R.; Barros, A.I.N.R.A.; Cavaleiro, J.A.S. NMR and structural and conformational features of 2′-hydroxychalcones and flavones. Spectrosc. Lett. 1997, 30, 1655–1667. [Google Scholar] [CrossRef]
- Barros, A.I.N.R.A.; Silva, A.M.S. Efficient synthesis of nitroflavones by cyclodehydrogenation of 2’-hydroxychalcones and by Baker-Venkataraman method. Monatsh. Chem. 2006, 137, 1505–1528. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman Scientific & Technical: Harlow, UK, 1989. [Google Scholar]
- Silva, A.M.S. Synthesis and Structural Characterization of Flavonoids and Related Compounds. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 1993. [Google Scholar]
- Rocha, D.H.A. Synthesis and Transformation of Chromones and 4-Quinolones. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 2015. [Google Scholar]
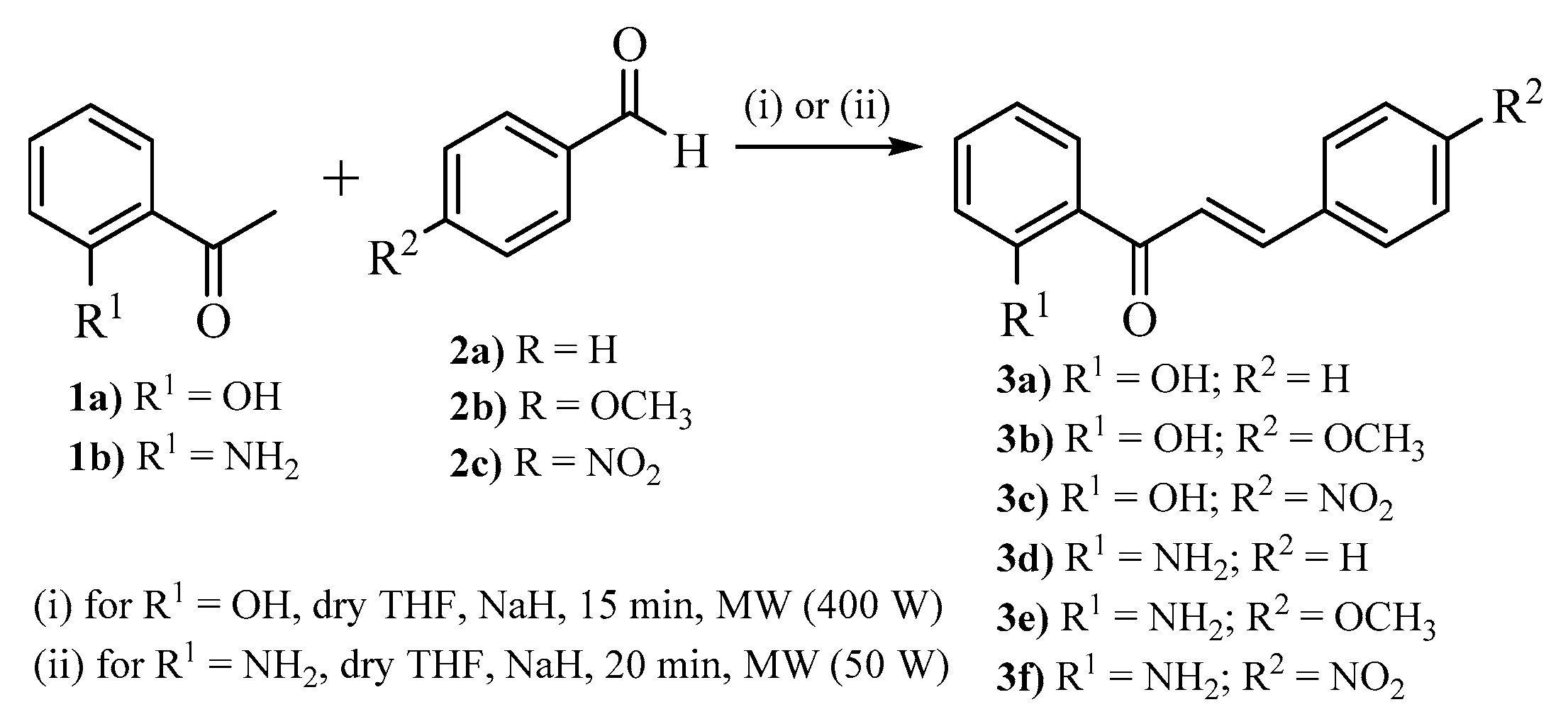




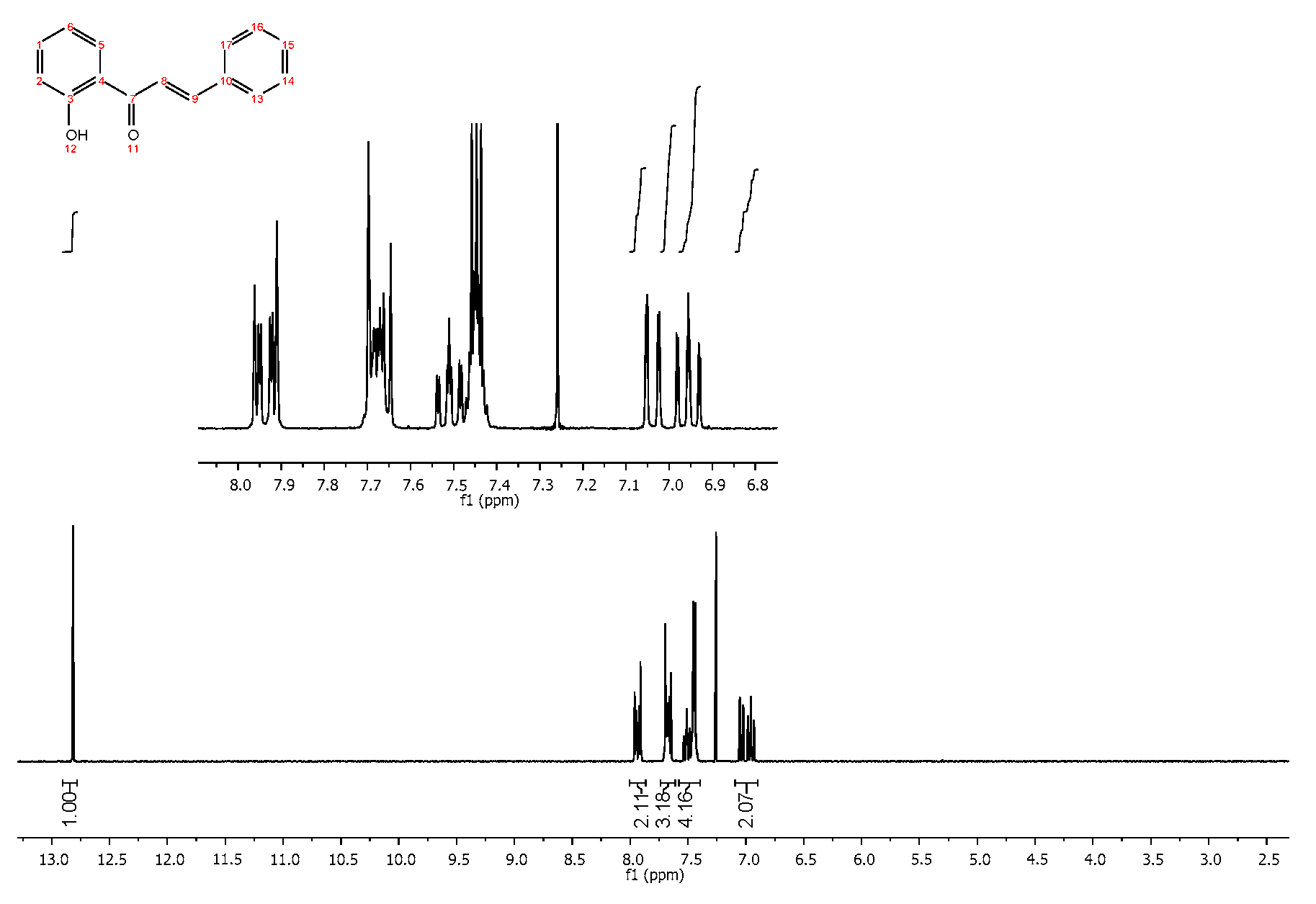
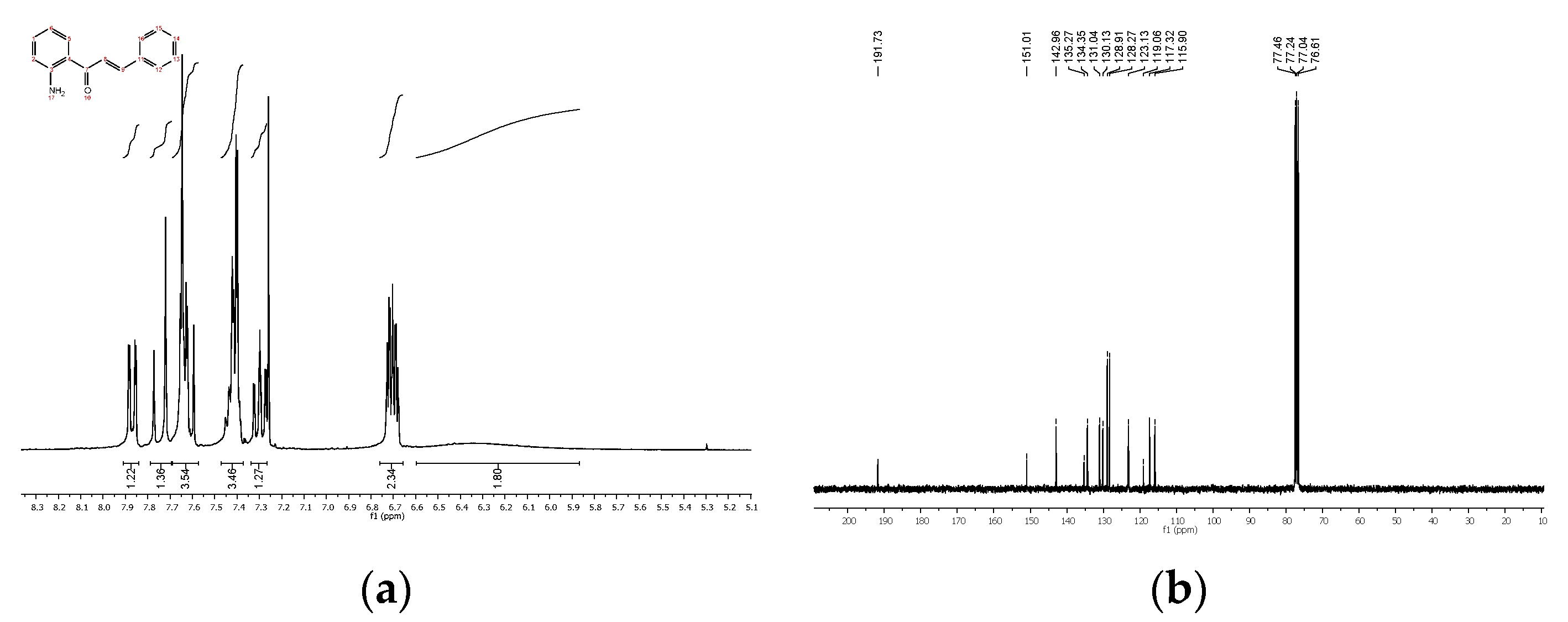
| Compound | R 1 | R 2 | Yield * (%) |
|---|---|---|---|
| 3a | OH | H | 84 |
| 3b | OH | OCH3 | 88 |
| 3c | OH | NO2 | 80 |
| 3d | NH2 | H | 87 |
| 3e | NH2 | OCH3 | 83 |
| 3f | NH2 | NO2 | 78 |
| 4a | OH | H | 70 |
| 4b | OH | OCH3 | 74 |
| 4c | OH | NO2 | 69 |
| 4d | NH | H | 80 |
| 4e | NH | OCH3 | 84 |
| 4f | NH | NO2 | 60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, D.H.A.; Vaz, P.A.A.M.; Pinto, D.C.G.A.; Silva, A.M.S. Synthesis Chalones and Their Isomerization into Flavanones and Azaflavanones. Methods Protoc. 2019, 2, 70. https://doi.org/10.3390/mps2030070
Rocha DHA, Vaz PAAM, Pinto DCGA, Silva AMS. Synthesis Chalones and Their Isomerization into Flavanones and Azaflavanones. Methods and Protocols. 2019; 2(3):70. https://doi.org/10.3390/mps2030070
Chicago/Turabian StyleRocha, Djenisa H. A., Patrícia A. A. M. Vaz, Diana C. G. A. Pinto, and Artur M. S. Silva. 2019. "Synthesis Chalones and Their Isomerization into Flavanones and Azaflavanones" Methods and Protocols 2, no. 3: 70. https://doi.org/10.3390/mps2030070
APA StyleRocha, D. H. A., Vaz, P. A. A. M., Pinto, D. C. G. A., & Silva, A. M. S. (2019). Synthesis Chalones and Their Isomerization into Flavanones and Azaflavanones. Methods and Protocols, 2(3), 70. https://doi.org/10.3390/mps2030070