Process Intensification for the Synthesis of 6-Allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol from 1-Allylpyridinium Salt Using a Continuous UV-Light Photoflow Approach
Abstract
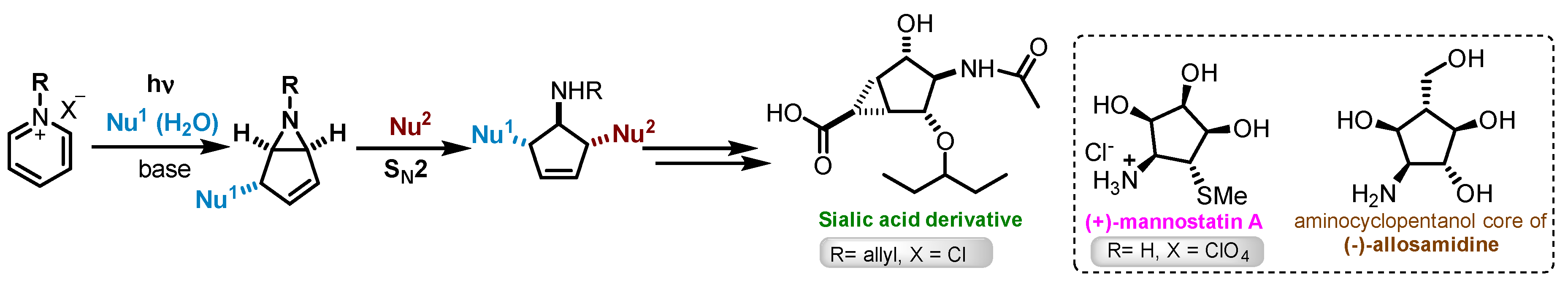
1. Introduction
2. Experimental Design
2.1. Materials
- Pyridine (Carlo Erba, CAS no. 110-86-1, Product Sku 469622)
- Allyl bromide (Sigma-Aldrich (97%), CAS no. 106-95-6)
- Potassium carbonate (Sigma-Aldrich (99%), CAS no. 584-08-7)
- Dichloromethane (Valentim Ribeiro 99–100%, Ref. VR0370)
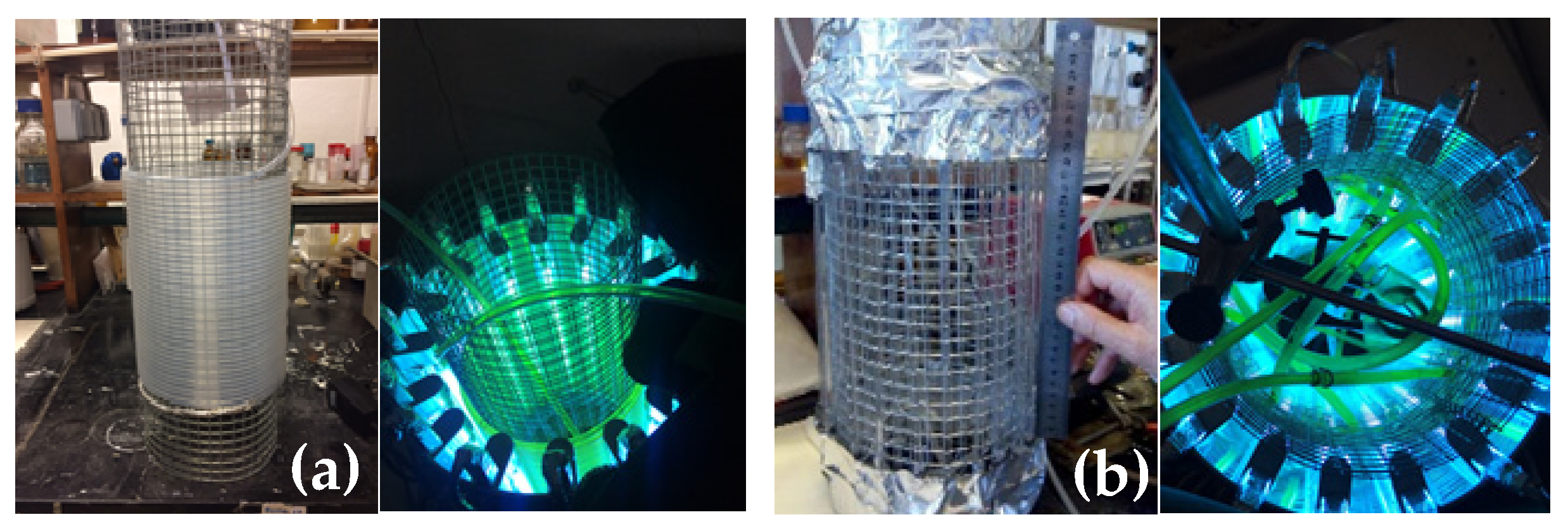
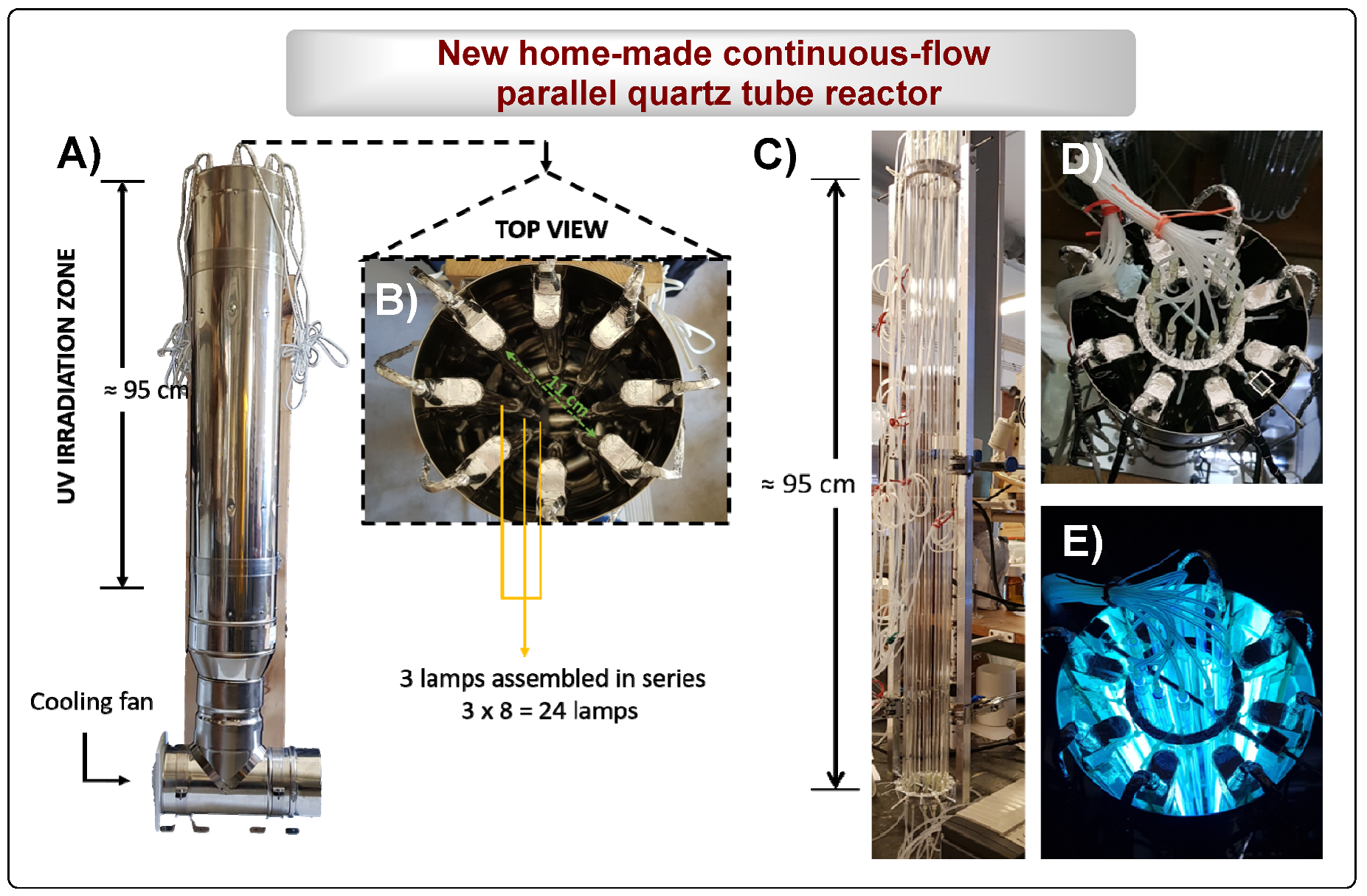
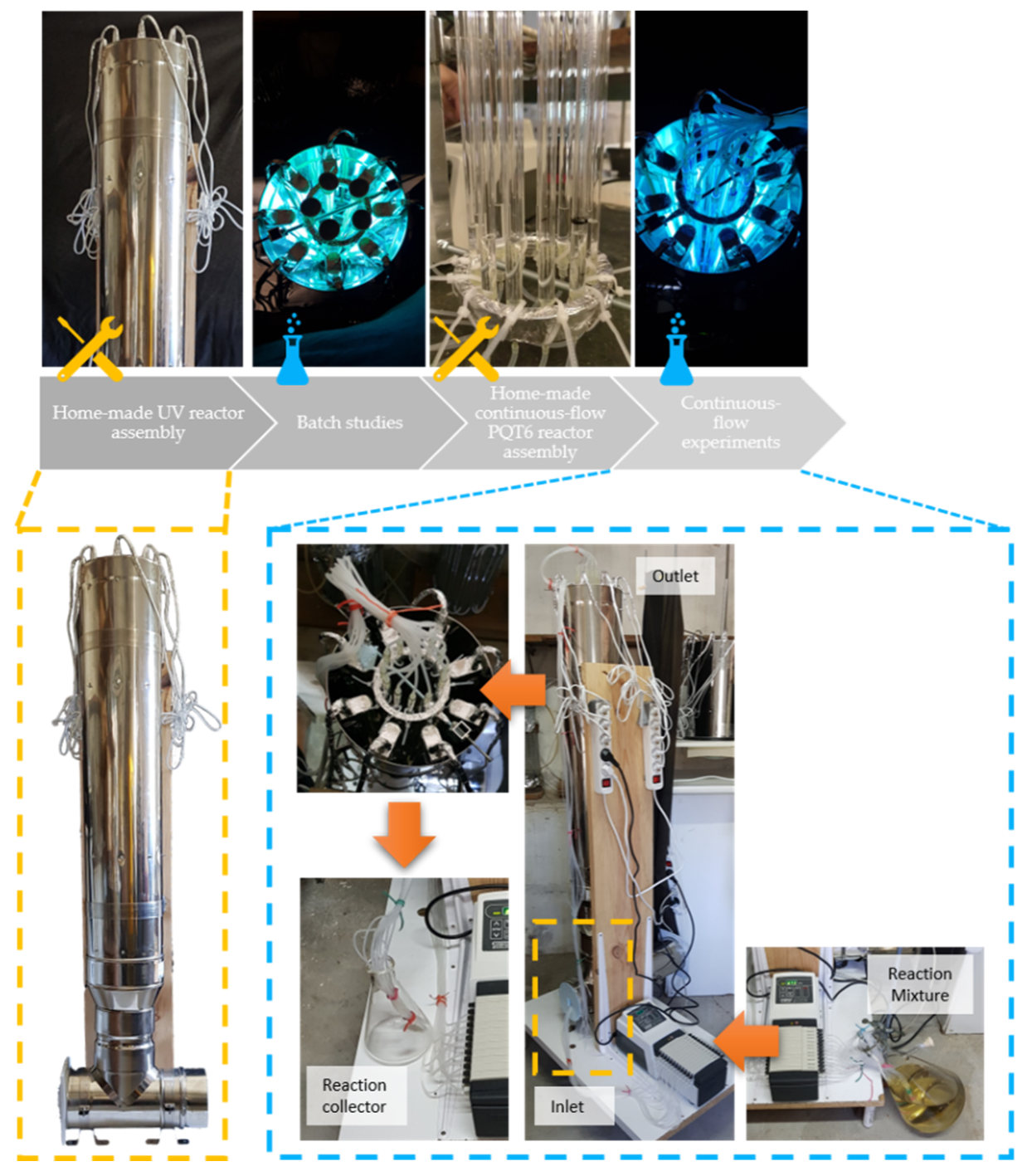
2.2. Equipment
- Home-made UV reactor containing 24 PURITEC HNS Germicidal Lamps Ref. HNS 8W G5 G5 (8W at 254 nm) with 144 cm length and 95 cm of irradiation zone (3 lamps assembled in line).
- Quartz tubes (QT): [total length (l), length under irradiation zone (l) and internal diameter (d)];
- Quartz tubes’ support for the home-made UV reactor.
- Home-made continuous-flow parallel quartz tube reactor (PQT6): [12 tubes: 95 cm under irradiation (l) × 0.6 cm (d)].
- Multichannel cassette pumps Watson Marlow 205S.
- 24 silicon tubes 1 × 3 mm, of ±2 m each, Ref. 350013.
3. Procedure
3.1. General Methods
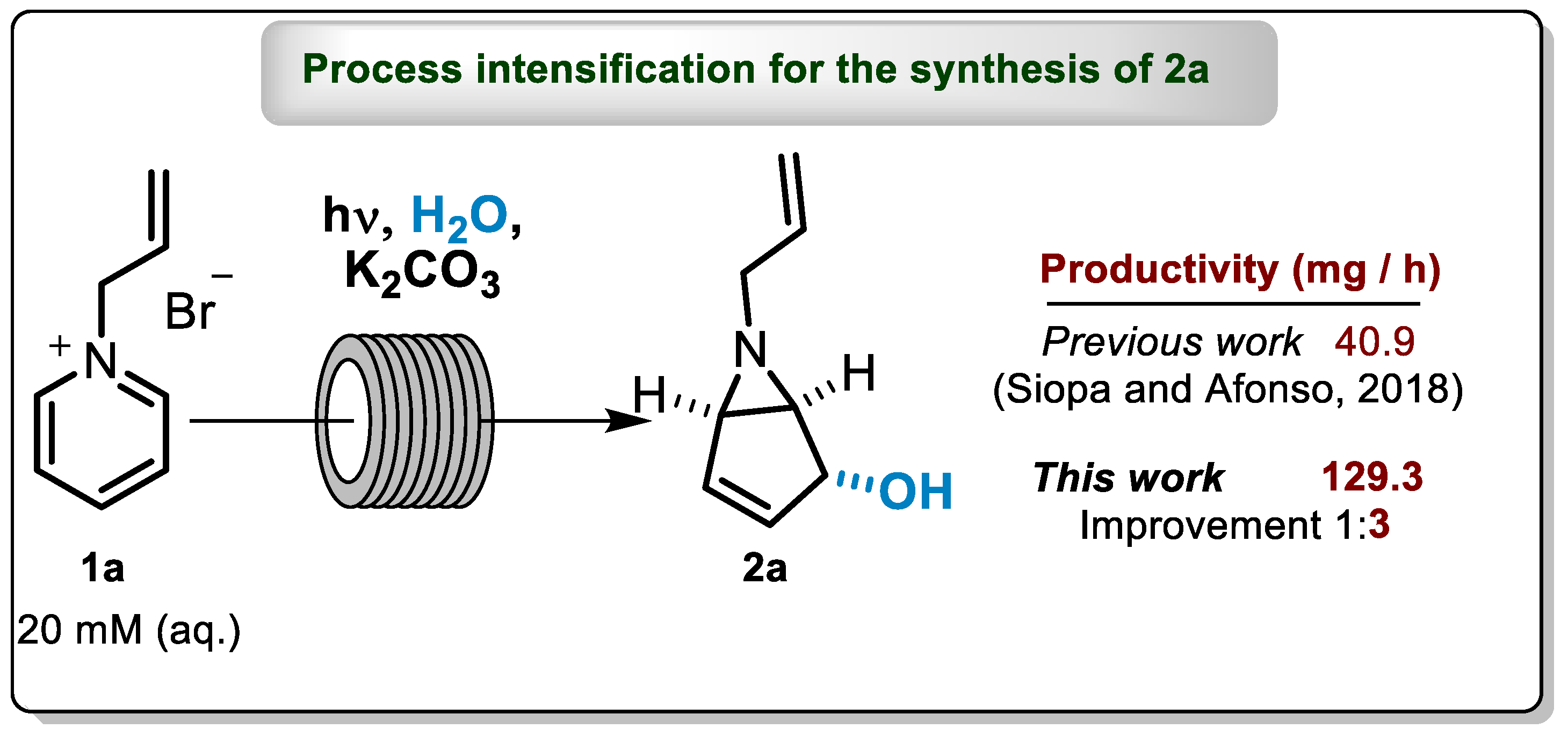
3.2. General Procedure for Batch Photochemical Transformation of 1a to 2a
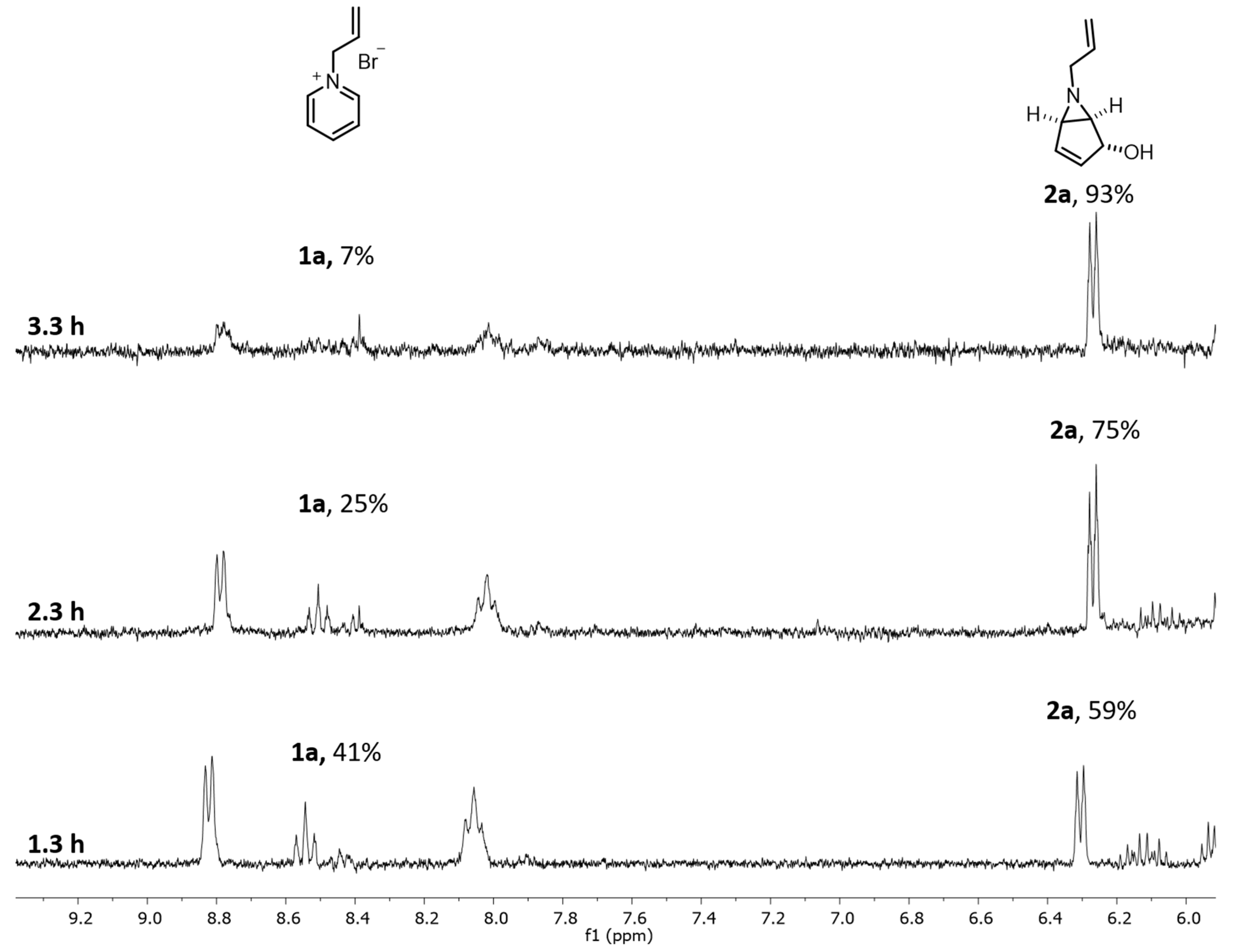
3.3. Optimization of the Reaction Conditions for Continuous-Flow Photochemical Transformation of 1a to 2a
3.3.1. Using PQT6 with 12 QTs under Continuous-Flow Conditions
- PAUSE STEP In order to save resources from the laboratory, the experiences described in Section 3.3.2 used 11 QTs under recirculating continuous-flow conditions, with the solution already irradiated in Section 3.3.1. These 11 tubes were not used for analysis of the reaction, they were only used to mimic the continuous operation conditions using 12 tubes.
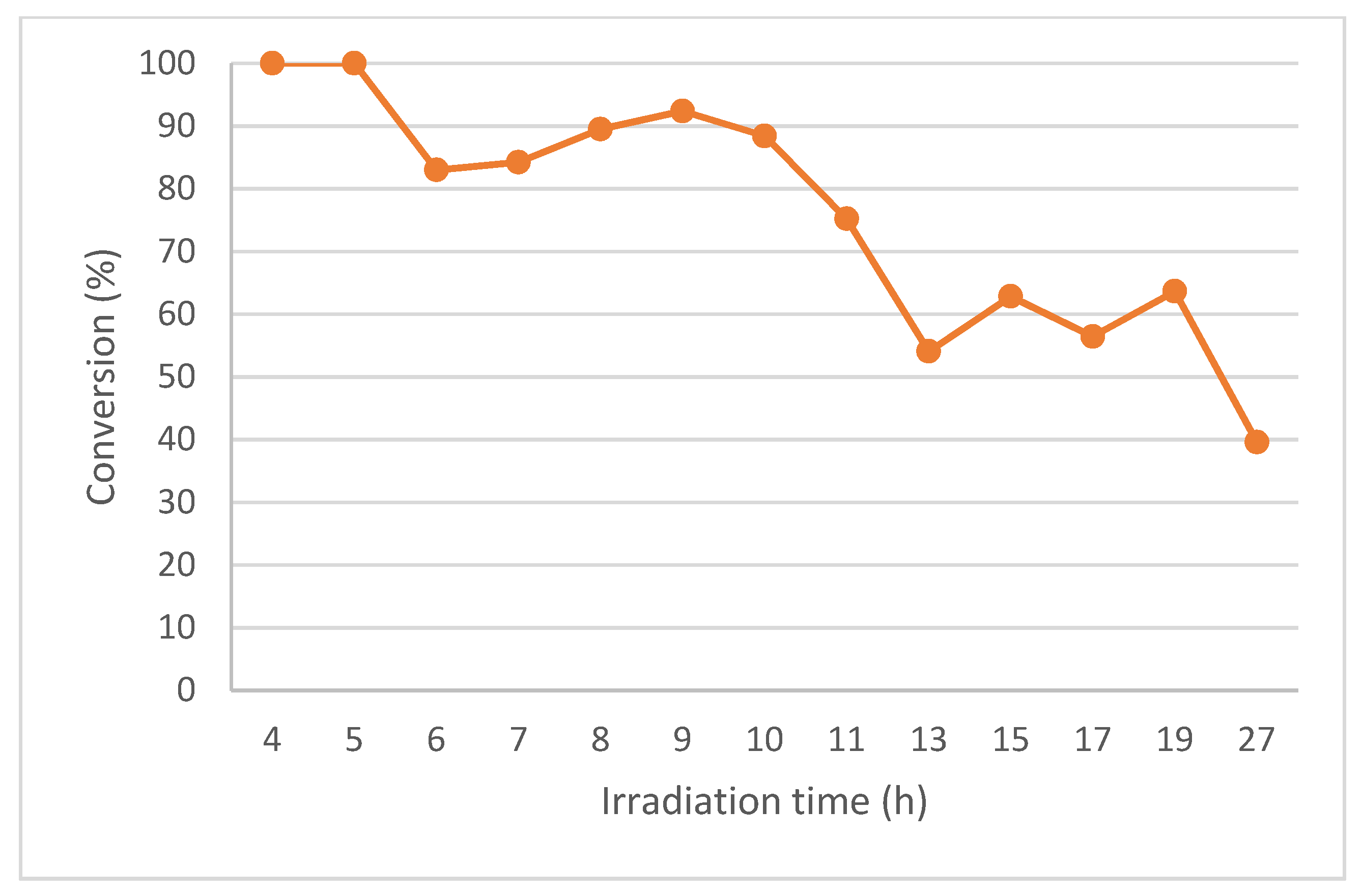
3.3.2. Using PQT6 with 1 QT under Continuous-Flow and 11 QTs under Recirculating Continuous-Flow
3.4. General Procedure for Continuous-Flow Photochemical Transformation of 1a to 2a
4. Results
4.1. Batch Results
4.2. Continuous-Flow Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Damiano, T.; Morton, D.; Nelson, A. Photochemical transformations of pyridinium salts: Mechanistic studies and applications in synthesis. Org. Biomol. Chem. 2007, 5, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Mariano, P.S. The synthetic potential of pyridinium salt photochemistry. Photochem. Photobiol. Sci. 2008, 7, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Mariano, P.S.; Lam, Y.F. A concise synthesis of the (-)-allosamizoline aminocyclopentitol based on pyridinium salt photochemistry. Tetrahedron Lett. 2001, 42, 4755–4757. [Google Scholar] [CrossRef]
- Ling, R.; Mariano, P.S. A Demonstration of the Synthetic Potential of Pyridinium Salt Photochemistry by Its Application to a Stereocontrolled Synthesis of (+)-Mannostatin A1. J. Org. Chem. 1998, 63, 6072–6076. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Pinto, B.M.; Bernardi, A.; Bennet, A.J. Synthesis and evaluation of influenza A viral neuraminidase candidate inhibitors based on a bicyclo[3.1.0]hexane scaffold. Org. Biomol. Chem. 2016, 14, 6539–6553. [Google Scholar] [CrossRef] [PubMed]
- Hook, B.D.A.; Dohle, W.; Hirst, P.R.; Pickworth, M.; Berry, M.B.; Booker-Milburn, K.I. A Practical Flow Reactor for Continuous Organic Photochemistry. J. Org. Chem. 2005, 70, 7558–7564. [Google Scholar] [CrossRef] [PubMed]
- Siopa, F.; António, J.P.M.; Afonso, C.A.M. Flow-Assisted Synthesis of Bicyclic Aziridines via Photochemical Transformation of Pyridinium Salts. Org. Process Res. Dev. 2018, 22, 551–556. [Google Scholar] [CrossRef]
- Snieckus, V.; Allais, C. Flow Photochemical Synthesis of Bicyclic Aziridines from Pyridinium Salts. Synfacts 2018, 14, 0903. [Google Scholar]
- Cambie, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noel, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Straathof, N.J.W.; Hessel, V.; Noel, T. Photochemical Transformations Accelerated in Continuous-Flow Reactors: Basic Concepts and Applications. Chem. Eur. J. 2014, 20, 10562–10589. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.P.; Elliott, L.D.; Booker-Milburn, K.I. Flow photochemistry: Old light through new windows. Beilstein J. Org. Chem. 2012, 8, 2025–2052. [Google Scholar] [CrossRef] [PubMed]
| Entry | Tube 2 | S/V Ratio (m2/m3) | 1a mM | Reaction Time (h) | Conv. (%) 3 | Productivity (mg h−1) |
|---|---|---|---|---|---|---|
| 1 | QT2 | 2000 | 60 | 2 | 100 | 12.3 |
| 2 | QT4 | 1000 | 60 | 4 | 100 | 24.6 |
| 3 | QT6 | 667 | 20 | 1 | 100 | 73.7 |
| 4 | QT6 | 667 | 40 | 6 | 100 | 24.6 |
| 5 | QT6 | 667 | 60 | 4 | 82 | 47.5 |
| 6 | QT6 | 667 | 80 | 8 | 77 | 28.4 |
| Entry | Flow Rate mL/min | rpm | Residence Time (h) | Conv. (%) 1 |
|---|---|---|---|---|
| 1 | 0.35 | 8.75 | 1.3 | 59 2 |
| 2 | 0.21 | 5 | 2.3 | 75 3 |
| 3 | 0.14 | 3.5 | 3.3 | 93 3 |
| Entry | Reaction Time (h) | Cycle | Conv. (%) 2 | Volume Out of the Reactor (mL) |
|---|---|---|---|---|
| 1 | 0–4 | 0 | 49 | 350 |
| 2 | 4–8 | 1 | 92 | 360 |
| 3 | 8–13 | 2 | 82 | 450 |
| 4 | 13–19 | 3 | 66 | 550 |
| 5 | 19–27 | 4 | 41 | 710 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, M.A.G.; Ly, C.-P.; Siopa, F.; Afonso, C.A.M. Process Intensification for the Synthesis of 6-Allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol from 1-Allylpyridinium Salt Using a Continuous UV-Light Photoflow Approach. Methods Protoc. 2019, 2, 67. https://doi.org/10.3390/mps2030067
Fortunato MAG, Ly C-P, Siopa F, Afonso CAM. Process Intensification for the Synthesis of 6-Allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol from 1-Allylpyridinium Salt Using a Continuous UV-Light Photoflow Approach. Methods and Protocols. 2019; 2(3):67. https://doi.org/10.3390/mps2030067
Chicago/Turabian StyleFortunato, Milene A. G., Chi-Phong Ly, Filipa Siopa, and Carlos A. M. Afonso. 2019. "Process Intensification for the Synthesis of 6-Allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol from 1-Allylpyridinium Salt Using a Continuous UV-Light Photoflow Approach" Methods and Protocols 2, no. 3: 67. https://doi.org/10.3390/mps2030067
APA StyleFortunato, M. A. G., Ly, C.-P., Siopa, F., & Afonso, C. A. M. (2019). Process Intensification for the Synthesis of 6-Allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol from 1-Allylpyridinium Salt Using a Continuous UV-Light Photoflow Approach. Methods and Protocols, 2(3), 67. https://doi.org/10.3390/mps2030067






