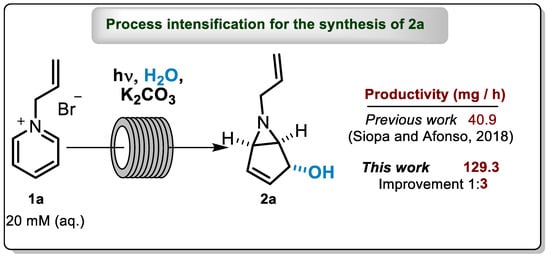
Abstract
A new home-made UV photochemical reactor (95 cm of irradiation zone) consisting of a 12 parallel quartz tubes flow reactor, PQT6 (95 cm under irradiation and an internal diameter of 0.6 cm) was assembled to perform photochemical transformations in continuous-flow. PQT6 was evaluated for the photoreaction of 1-allylpyridinium bromide (1a) to 6-allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol (2a), in a continuous process. This technology provides reduced reaction times, continuous production of 2a, and a productivity of 129 mg h−1, corresponding to 1.94 g of isolated 2a after 15 h of irradiation.
1. Introduction
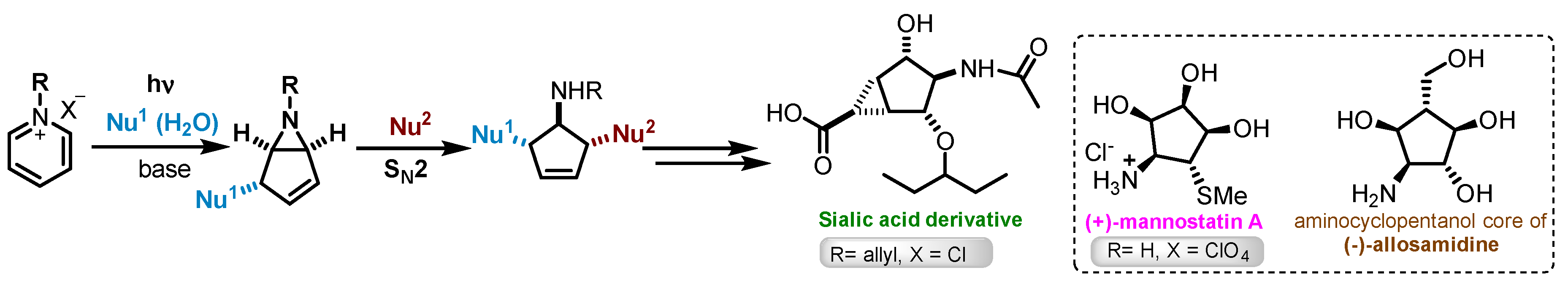
Bicyclic aziridines (α-hydroxycyclopenteno-aziridines) prepared by photochemical transformation of pyridinium salts are key intermediates used for the total synthesis of several aminocyclopentitols (Scheme 1) [1,2,3,4]. In 2016, Bennet and co-workers, described the synthesis of sialic acid derivatives using this synthetic methodology. Under batch conditions, 1-allylpyridinium chloride was irradiated during 16 h to afford 630 mg (60% yield) of 6-allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol (2a), with a productivity of 39.4 mg h−1 [5].
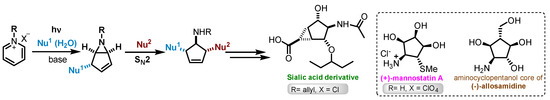
Scheme 1.
General synthetic pathway for aminocyclopentitols via photoreaction of pyridinium salts [1,2,3,4].
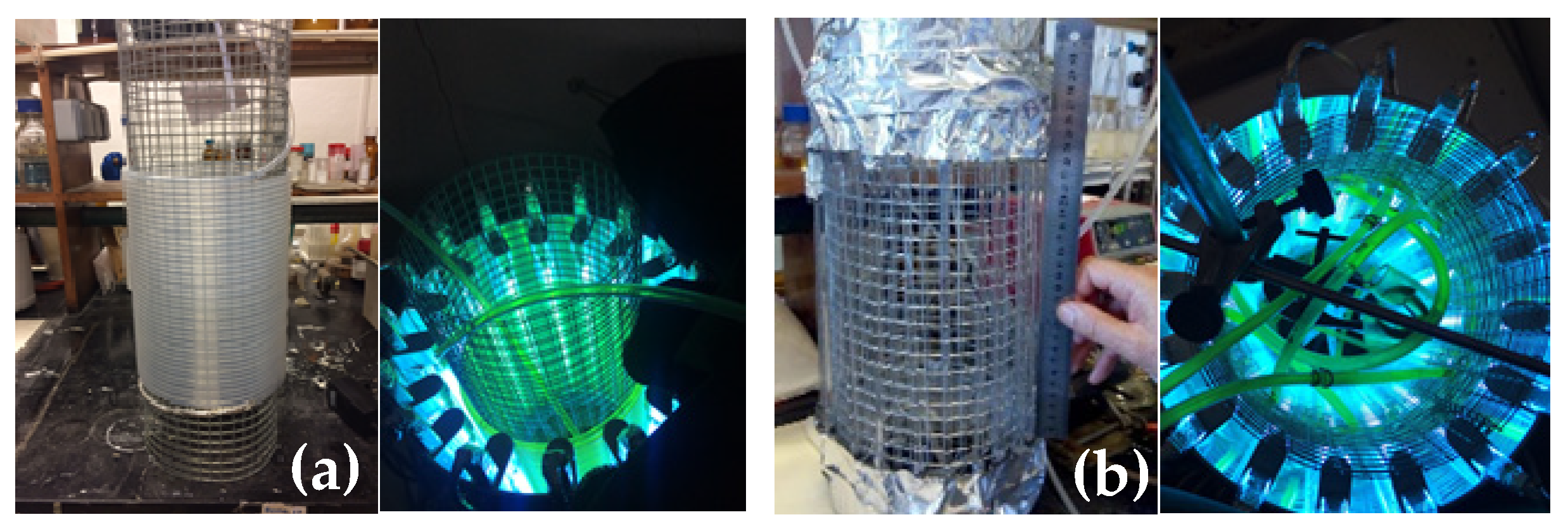
Recently, we developed different types of home-made continuous-flow reactors to overcome the reduced productivity problem of this photoreaction (Scheme 1). A fluorinated ethylene propylene (FEP4) tube reactor (internal diameter of 0.4 cm, Figure 1a) similar to that described by Booker-Milburn [6], and two parallel quartz tube (PQT) reactors (internal diameters 0.4 cm denoted as PQT4, Figure 1b; and 0.2 cm) were used [7].
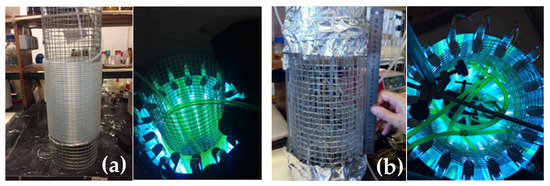
Figure 1.
Previously reported home-made continuous-flow reactors. (a) FEP4 reactor [(2296 cm (l) × 0.4 cm (d)] around metal grid (left), and top-view under irradiation (right). (b) Parallel quartz tube reactor (PQT4), [24 tubes in vertical orientation and parallel to each other: 22.5 cm (l) × 0.4 cm (d)] (left) and top-view under irradiation (right) [7].
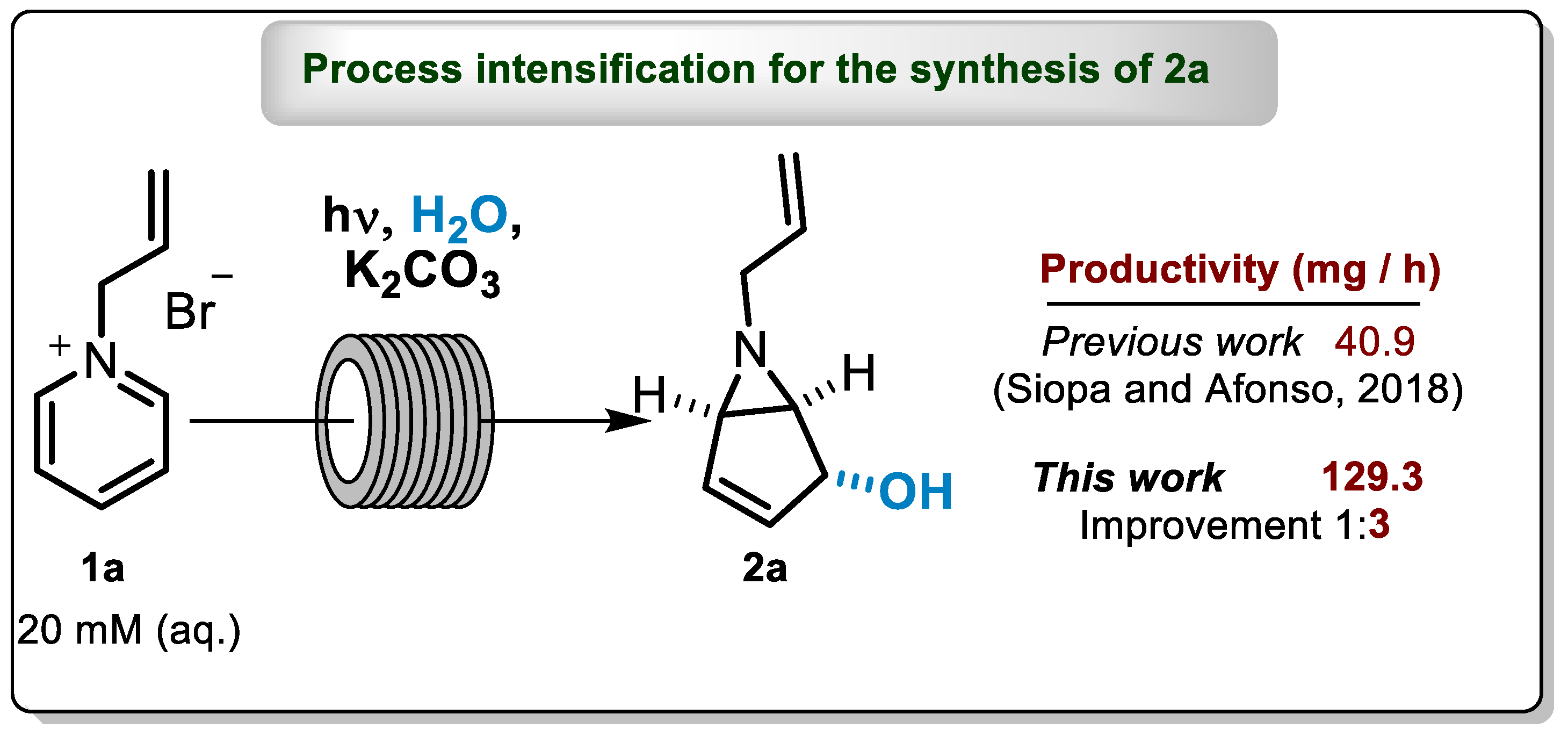
The reactors were assessed for the photoflow transformation of 1-allyl and 1-n-butylpyridinium salts to the correspondent bicyclic aziridines, under recirculating (FEP4, PTQ4 and PTQ2) and continuous-flow conditions (FEP4) [7]. Under recirculating conditions, PQT4 gave the best productivity results for both bicyclic aziridines. Furthermore, regarding the isolated 6-allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol, (2a) (Scheme 2) and 6-butyl-6-azabicyclo[3.1.0]hex-3-en-2-ol (2b), this reactor was capable of producing 40.9 mg h−1 and 87.0 mg h−1, respectively [7], as highlighted by Snieckus, V. and Allais, C. in Synfact in 2018 [8]. Despite these outstanding productivity results, the PQT4 reactor presents some drawbacks as a consequence of its design, such as a large dead-volume, resulting from the connections between the different quartz tubes (Figure 1b). Such limitation has so far prevented the use of PQT4 reactor in a continuous process.
Scheme 2.
Synthetic methodology for the synthesis of 2a.
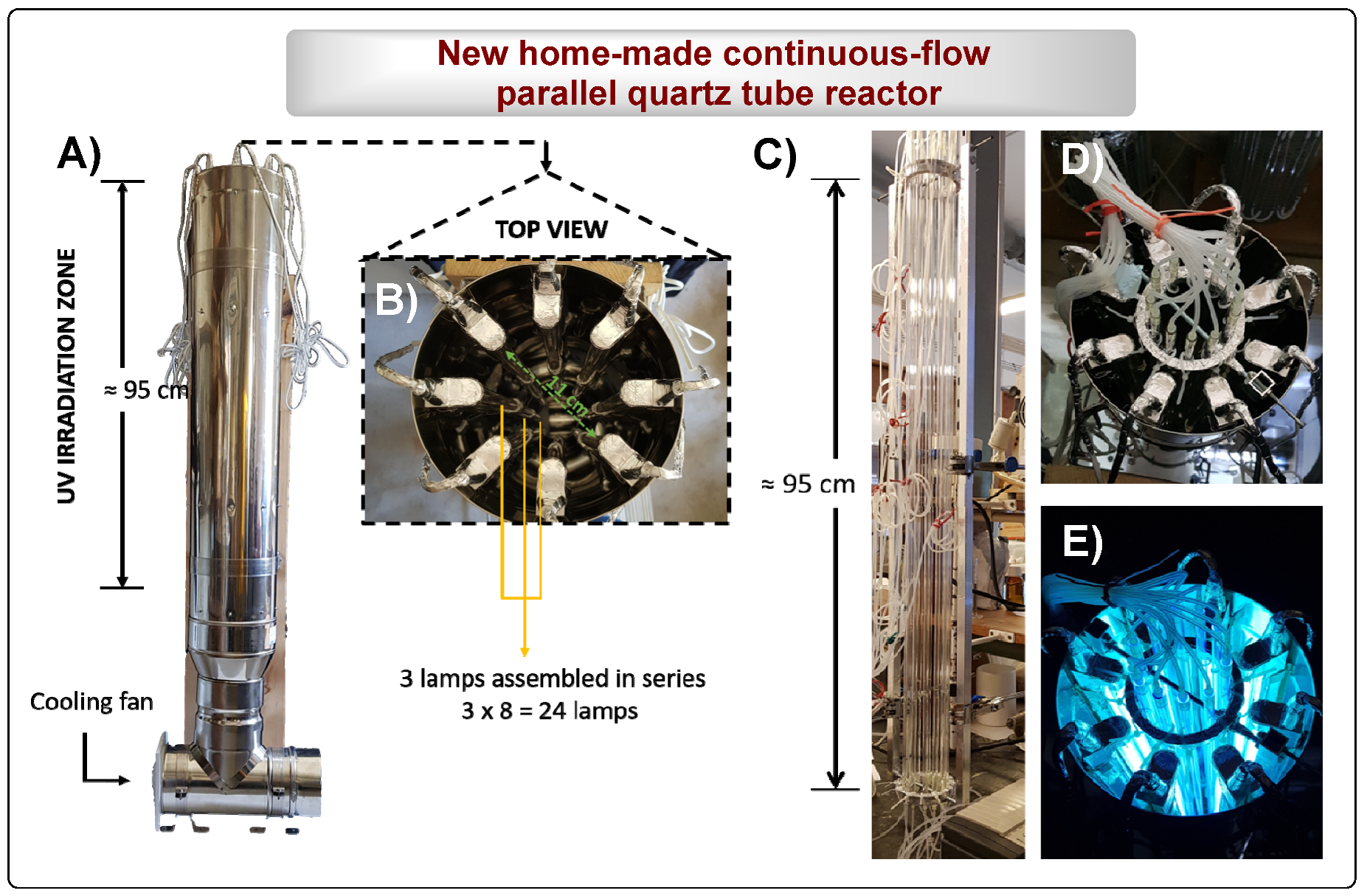
To overcome these limitations, herein is presented the development of a home-made UV reactor and a PQT reactor capable of operating under continuous-flow. The reactors were designed for higher scale production of 2a via photochemical irradiation of 1a (Scheme 2 and Figure 2).
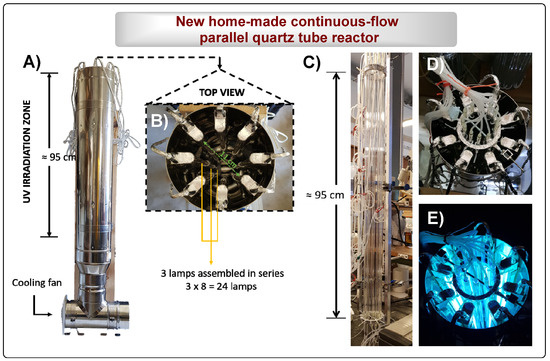
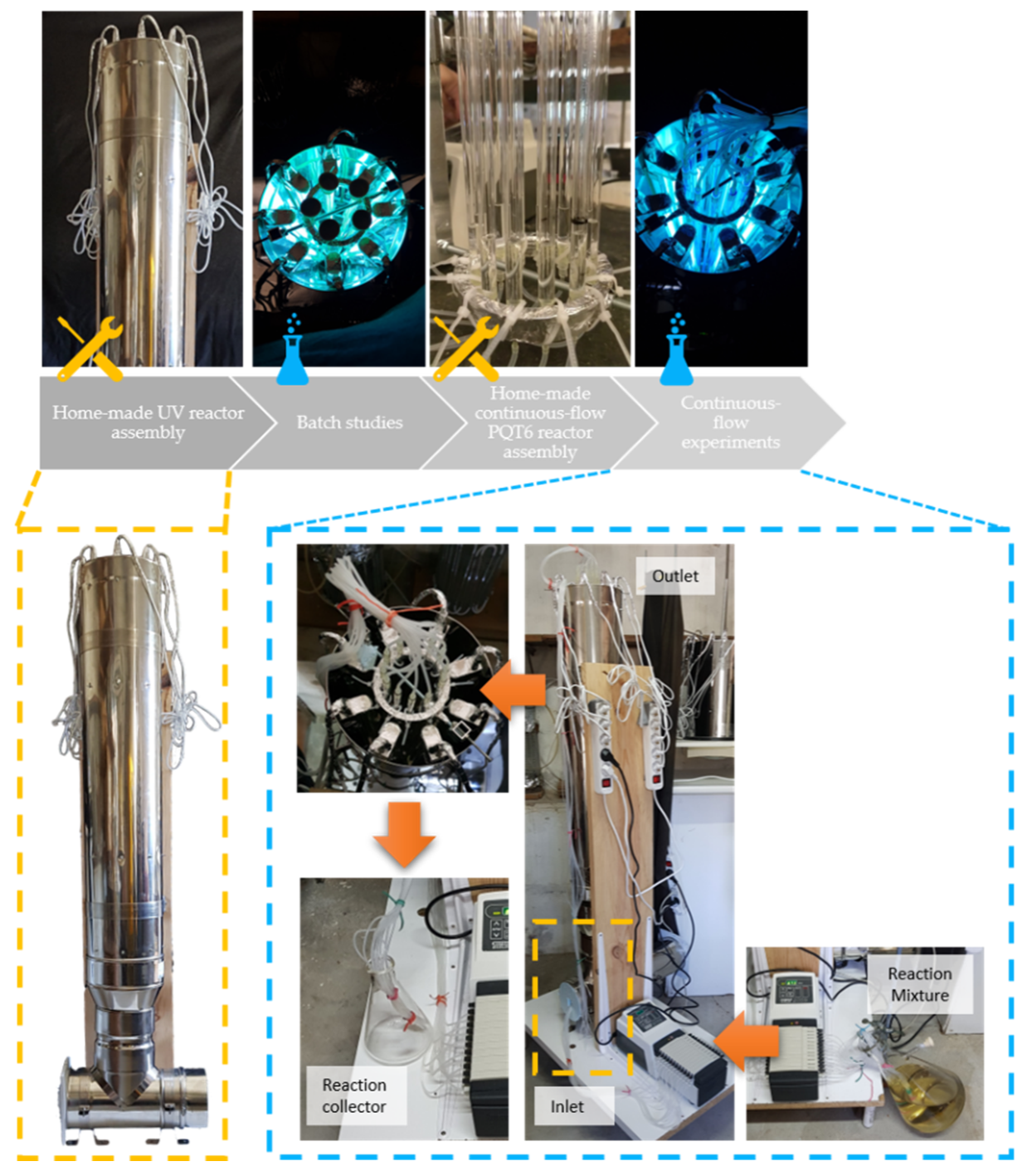
Figure 2.
New home-made UV reactor and continuous-flow parallel quartz tube reactor. (A) Overview of the new home-made UV reactor; (B) UV reactor top-view; (C) 12 assembled parallel quartz tubes reactor (PQT6); (D) Reactors top-view; (E) Top-view of the reactors under photochemical irradiation.
2. Experimental Design
The workflow for the process intensification of 6-allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol (2a) via photochemical irradiation of 1-allylpyridinium bromide (1a) is divided into four stages: (i) home-made UV reactor assembly, (ii) batch studies, (iii) home-made continuous-flow PQT reactor assembly and (iv) continuous-flow experiments, as shown in Figure 3.
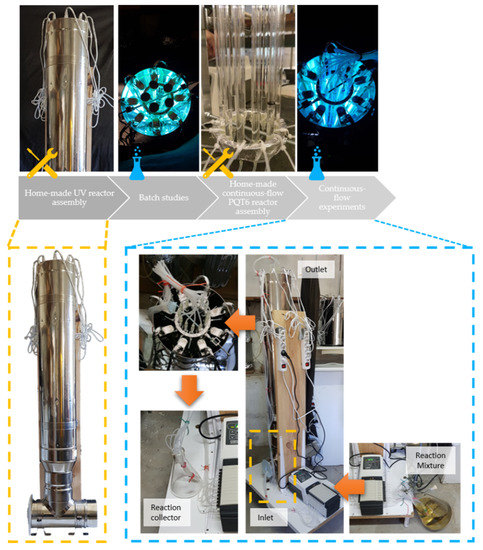
Figure 3.
Workflow overview.
2.1. Materials
- Pyridine (Carlo Erba, CAS no. 110-86-1, Product Sku 469622)
- Allyl bromide (Sigma-Aldrich (97%), CAS no. 106-95-6)
- Potassium carbonate (Sigma-Aldrich (99%), CAS no. 584-08-7)
- Dichloromethane (Valentim Ribeiro 99–100%, Ref. VR0370)
2.2. Equipment
- Home-made UV reactor containing 24 PURITEC HNS Germicidal Lamps Ref. HNS 8W G5 G5 (8W at 254 nm) with 144 cm length and 95 cm of irradiation zone (3 lamps assembled in line).
- Quartz tubes (QT): [total length (l), length under irradiation zone (l) and internal diameter (d)];
QT2: [±100 cm (l), 95 cm under irradiation (l) × 0.2 cm (d)];
QT4: [±100 cm (l), 95 cm under irradiation (l) × 0.4 cm (d)];
QT6: [±100 cm (l), 95 cm under irradiation (l) × 0.6 cm (d)].
- Quartz tubes’ support for the home-made UV reactor.
- Home-made continuous-flow parallel quartz tube reactor (PQT6): [12 tubes: 95 cm under irradiation (l) × 0.6 cm (d)].
- Multichannel cassette pumps Watson Marlow 205S.
- 24 silicon tubes 1 × 3 mm, of ±2 m each, Ref. 350013.
3. Procedure
3.1. General Methods
1H NMR spectra were obtained on a Bruker Fourier 300 spectrometer. NMR experiments were performed in D2O at room temperature. Chemical shifts are given in parts per million (ppm), the symbols m, s, d, t, and q represent multiplet, singlet, doublet, triplet, and quartet, respectively and the coupling constants (J) are given in Hertz (Hz). 1-allylpyridinium bromide (1a) was prepared as previously described by us [7].
3.2. General Procedure for Batch Photochemical Transformation of 1a to 2a
With the quartz tubes (QT2, QT4, QT6) in the support, an aqueous solution of 1a with potassium carbonate (1.2 mol equiv.) in a specific concentration (20, 40, 60, or 80 mM) was added. The support was placed inside the photochemical home-made UV reactor and was irradiated up to 10 h at room temperature (the measured temperature inside the top of the reactor was below 34 ºC). At selected times, samples were collected from the reaction mixture, concentrated under vacuum and analyzed by 1H NMR (D2O). All data are available in the Supporting Information.
3.3. Optimization of the Reaction Conditions for Continuous-Flow Photochemical Transformation of 1a to 2a
3.3.1. Using PQT6 with 12 QTs under Continuous-Flow Conditions
PQT6 reactor was placed inside the UV photochemical reactor. A 600 mL of an aqueous solution of 1a (20 mM) and potassium carbonate (1.2 molar equiv.) was prepared. The solution was pumped (multichannel cassette pumps Watson Marlow 205S, using 12 silicon tubes 1 × 3 mm) onto PQT6, at 8.75 rpm which correspond to a 0.35 mL/min flow rate. After 1.3 h of irradiation, a sample was collected, concentrated and analyzed by 1H NMR (D2O).

- PAUSE STEP In order to save resources from the laboratory, the experiences described in Section 3.3.2 used 11 QTs under recirculating continuous-flow conditions, with the solution already irradiated in Section 3.3.1. These 11 tubes were not used for analysis of the reaction, they were only used to mimic the continuous operation conditions using 12 tubes.
In addition, 1 QT was removed from PQT6 reactor, cleaned and placed back in the support. This clean QT was used under continuous-flow conditions and allowed determination of the reaction conversion by 1H NMR.
3.3.2. Using PQT6 with 1 QT under Continuous-Flow and 11 QTs under Recirculating Continuous-Flow
With PQT6 reactor already in the UV reactor, a 100 mL of fresh solution of 1a (20 mM) and potassium carbonate (1.2 molar equiv.) was pumped towards 1 QT, using a multichannel cassette peristaltic pump (Watson Marlow 205S) at 5 rpm (0.21 mL/min flow rate, residence time 2.3 h) and in the second experience at 3.5 rpm (0.14 mL/min flow rate, residence time 3.3 h). After the respective residence time, samples were analyzed by 1H NMR (D2O).
As stated above, the remaining 11 QTs were also pumped at the same flow rate, 0.21 or 0.14 mL/min, corresponding to 5 or 3.5 rpm respectively, using the solution previous irradiated in Section 3.3.1. As these tubes are under recirculating continuous-flow conditions, they were not used for analysis.
3.4. General Procedure for Continuous-Flow Photochemical Transformation of 1a to 2a
The PQT6 reactor consisting of 12 QT6 tubes was placed inside the home-made UV reactor. To fill the PQT6, a 350 mL of an aqueous solution of 1a (20 mM) and potassium carbonate (1.2 molar equiv.) was pumped using the multichannel cassette pumps Watson Marlow 205S (12 silicon tubes 1 × 3 mm). Then, the pump was set to 3 rpm (0.12 mL/min flow rate) and the irradiation was turned on. A 3150 mL of an aqueous solution of 1a (20 mM) and potassium carbonate (1.2 molar equiv.) was pumped (0.12 mL/min flow rate). The reaction was irradiated during 27 h. Samples were collected at selected times, concentrated under vacuum and analyzed by 1H NMR (D2O).
To isolate 6-allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol (2a), 1360 mL of collected water corresponding to the period of 4 to 19 h of irradiation, was evaporated under vacuum and the obtained solid was dissolved in dichloromethane (3 × 250 mL), stirred for 15 min, and filtered. The solvent was evaporated to give 1.94g (52%) of 2a as a brown oil.
6-allyl-6-azabicyclo[3.1.0]hex-3-en-2-ol (2a) 1H NMR (300 MHz, CDCl3) δ 6.32–6.29 (m, 1H), 5.95–5.86 (m, 2H), 5.23–5.19 (m, 2H), 4.12 (q, J = 7.1 Hz, 1H), 3.02–3.27 (m, 2H), 2.56–2.50 (m, 2H). Spectral data was in accordance with the literature [7].
4. Results
4.1. Batch Results
In order to achieve the photochemical transformation of 1a to 2a under continuous operation, we first established the best reaction conditions under batch. In this line, diverse parameters were evaluated (Table 1), such as reaction concentration (20, 40, 60, and 80 mM), residence time (up to 10 h), and the internal diameter of the QTs (0.2, 0.4 and 0.6 cm).

Table 1.
Selected results of photoreaction of 1a to 2a under batch 1.
By using QT2, QT4 and QT6, with the same concentration (60 mM) of 1a (Table 1—Entries 1, 2, 5), we observed that QT2, QT4 rapidly gave full conversion (2 and 4 h, respectively). This is due to the greater surface-to-volume ratio and to the shorter photoreactor diameter that circumvent nonuniform energy profiles [9]. However, for QT6 we obtained 82% conversion after 4 h of irradiation which means that the photon penetration in the solution is less effective. However, when looking for the productivity in mg h−1, the QT6 gave the best result (47.5 mg h−1 QT6 vs. 24.6 mg h−1 QT4 vs. 12.3 mg h−1 QT2; Table 1, entries 5, 2 and 1). This result is attributed to the larger internal diameter of QT6, allowing the production of 2a in larger amount. We have chosen QT6 to continue our studies, based on the productivity results.
Since in a photoreaction the absorption of photons is correlated to the reaction concentration by the Bouguer−Lambert−Beer law [9,10,11], we then focused our attention on evaluating different concentrations of 1a using QT6 (Table 1—Entries 3–6). As expected, the increase of concentration affected the photon penetration which led to the need of longer irradiation time to obtain a higher or full conversion. To our delight, we observed full conversion for 20 mM of 1a, after 1 h of irradiation, and the best productivity result of 73.7 mg h−1 (Table 1—Entry 3). This was the best batch result and afforded the selected conditions to implement in the continuous-flow experiments.
4.2. Continuous-Flow Results
The batch studies shown QT6 as the selected tube for the continuous-flow experiments. Based on this, we set up the parallel quartz tube continuous-flow reactor using 12 quartz tubes assembled in the support (Figure S4 and Figure 2C). The new reactor was designated as PQT6: [12 tubes, 95 cm under irradiation (l) × 0.6 cm (d)], and has a surface-to-volume (S/V ratio) of 667 m2/m3 (Figure S4). With PQT6 reactor in hands, we started the flow experiments (Table 2—Entry 1), mimicking the best batch conditions (20 mM of 1a, 1 h of residence time, Table 1—Entry 3).

Table 2.
Optimization of photochemical transformation of 1a to 2a under continuous-flow.
We programmed the peristaltic pump at 8.75 rpm (Table 2—Entry 1), which corresponds to a 0.35 mL/min flow rate and a 1.3 h residence time. However, after this time only 59% conversion was observed by 1H NMR analysis (Figure 4 and Table 2—Entry 1). The continuous process did not reproduce the batch experiment. Probably the 12 QTs of PTQ6 affected the photon flux in the system and consequently, less photons were available for the photochemical transformation. However, this situation is not new, as we observed the same behavior for the phototransformation of 1-butylpyridinium salt 1b at 60 mM, under batch (FEP, S/V ratio = 1000 m2/m3; conversion—92%, irradiation time—8 h) and continuous-flow (FEP4 reactor, S/V ratio = 960 m2/m3, conversion—56%, irradiation time—8 h). We attributed this results to the geometry of FEP4, which could limit the photon flux [7].
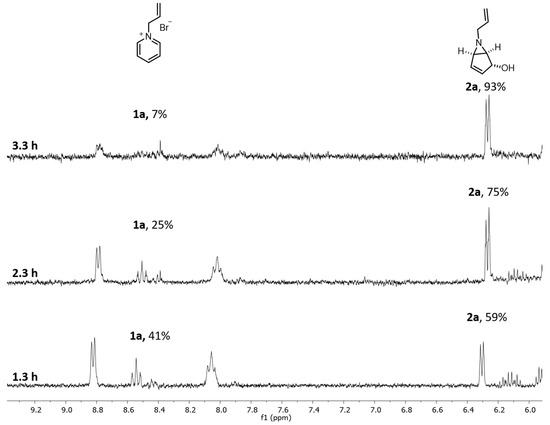
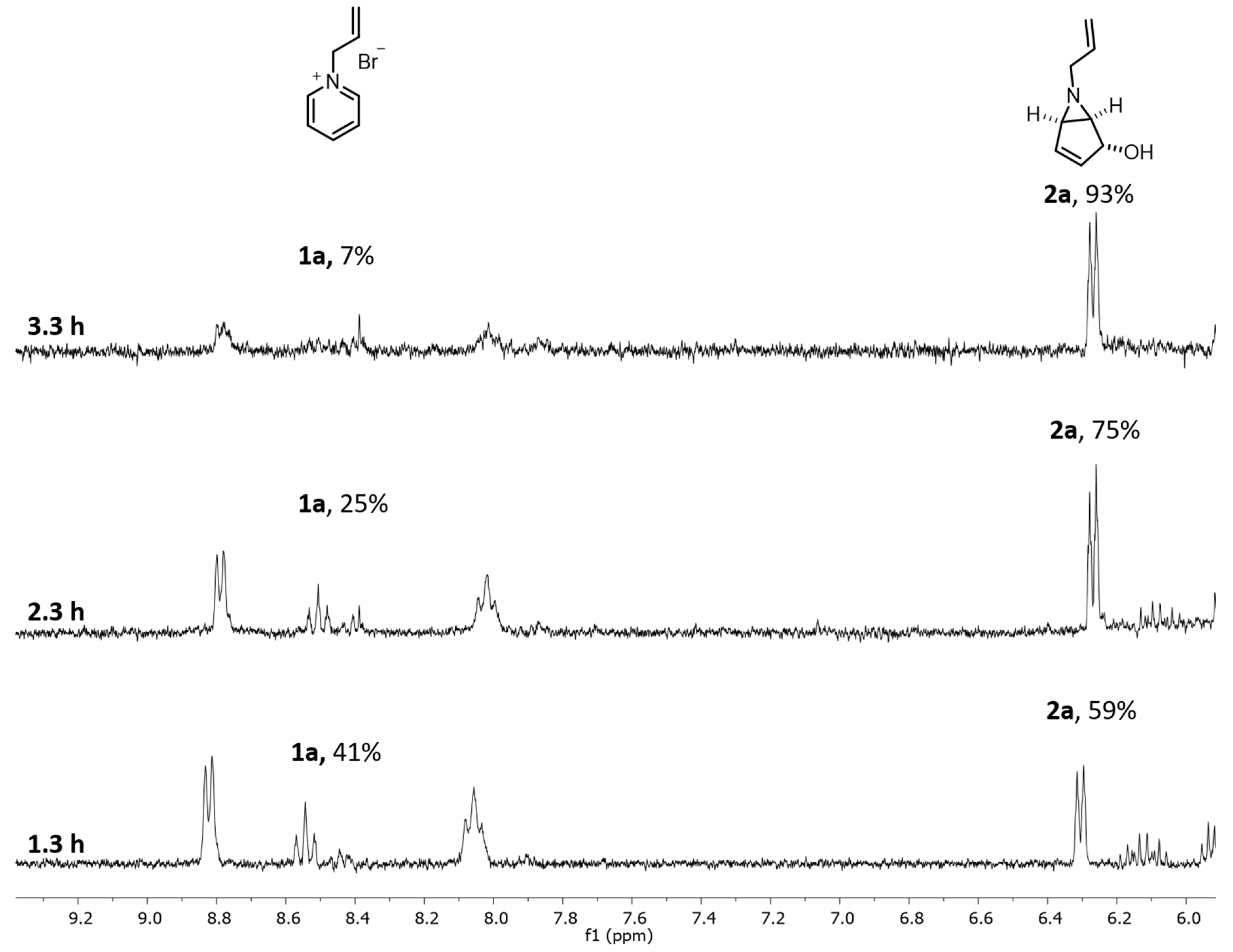
Figure 4.
1H NMR profile for continuous-flow experiments optimization of 1a to 2a at 20 mM, with 1.3 h, 2.3 h and 3.3 h of residence time.
In order to obtain a higher conversion of 1a to 2a, we decided to increase the residence time (Table 2—Entries 2, 3 and Figure 4). After 2.3 h (5 rpm, 0.21 mL/min) we obtained 75% conversion by 1H NMR (Figure 4) and for 3.3 h (3.5 rpm, 0.14 mL/min) a 93% conversion was achieved. With this result, we envisaged that 4 h of residence time would allow a full conversion of 1a (0.12 mL/min flow rate; 3 rpm).
We started the continuous-flow experience for process intensification of 2a, via photoreaction of 1a (Table 3) using 20 mM and 4 h of residence time (0.12 mL/min flow rate; 3 rpm). To start the experience, we filled the PQT6 with 350 mL of solution (Cycle 0) and then turned on the irradiation (Table 3, Entry 1). To our delight, the first collected samples with 4 h of residence time showed full conversion (Figure 5 and Table S8, Entries 2, 3). High conversions were observed on both Cycle 1, between 4–8 h of irradiation time (92%, Figure 5 and Table 3, Entry 2) and Cycle 2, between 8–13 h of irradiation time, (82%, Figure 5 and Table 3, Entry 3).

Table 3.
Photochemical transformation of 1a to 2a under continuous-flow 1.
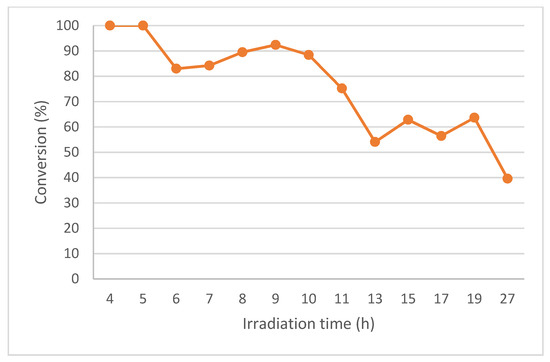
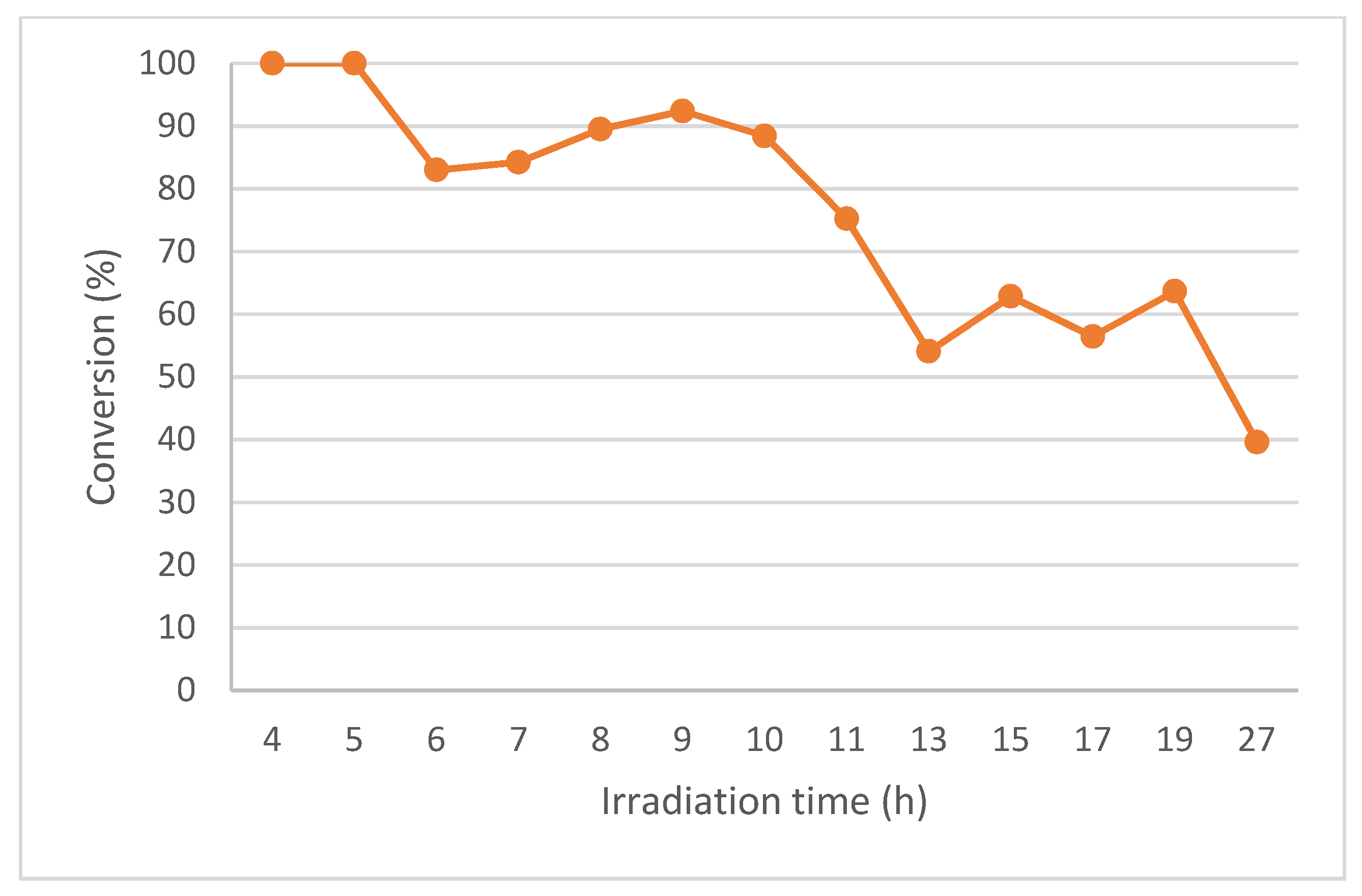
Figure 5.
Conversion (%) for the continuous-photoflow of 1a at 20 mM with 4 h of residence time using the PQT6 reactor.
However, for Cycle 3 (13–19 h irradiation time), the conversion started to drop (66%, Figure 5 and Table 3, Entry 4) and continued to decrease for Cycle 4 (19–27 h irradiation time; 41%, Figure 5 and Table 3, Entry 5). This is probably due to the yellow polymer formed on the surface of the QTs during the irradiation (Figures S5C and S7) which reduce the photon flux. The formation of this yellow polymer was already observed previously by us [7].
For Cycle 1–3, which correspond to 15 h of irradiation time and 1.3 L of recovered volume, we isolated 1.94 g of 2a (52% yield), allowing a space-time yield (STYs) of 129.3 mg h−1. This result is three times better than our previous one, obtained using PQT4 under recirculation continuous-flow condition, e.g., STYs of 40.9 mg h−1 (1.2 g of isolated aziridine in 29.3 h of irradiation) [7]. In the same work, we only performed continuous-flow for FEP4 reactor and to prepare 6-butyl-6-azabicyclo[3.1.0]hex-3-en-2-ol, 2b. This was due to the high dead volume of the PQT4 reactor, which limits its use under continuous operation, and also the difficulty of cleaning the FEP4 reactor caused by the formation of a yellow polymer during the irradiation time [7]. Herein, this is the first example of continuous-flow for the synthesis of 2a.
5. Conclusions
We conducted the process intensification of 2a under continuous-photoflow, using a home-made 12 parallel quartz tubes reactor (PTQ6) on a home-made UV reactor. The obtained space-time yield (129.3 mg h−1) improved 3 times when compared to our previous recirculating approach. In addition, we were able to prepare, for the first time under continuous flow, this valuable compound.
Supplementary Materials
The following data are available online at https://www.mdpi.com/2409-9279/2/3/67/s1: details of home-made UV reactor, home-made-continuous-flow reactors and obtained results, copies of 1H NMR spectra of photochemical crude reaction mixtures and synthesized compounds.
Author Contributions
Conceptualization, supervision, F.S. and C.A.M.A.; investigation, M.A.G.F., C.P.L. and F.S.; data curation, formal analysis and writing, M.A.G.F., F.S. and C.A.M.A.
Funding
This research was funded by Fundação para a Ciência e a Tecnologia (FCT) (ref. PTDC/QUI-QOR/32008/2017, UID/DTP/04138/2013, PESSOA 2018/2019 (Proc. 441.00 França and PHC PESSOA 2018 No 40875QJ)) and European Research Area Network; ERANet LAC (ref. ELAC2014/BEE-0341).
Acknowledgments
Jaime A. S. Coelho is acknowledged for useful discussions, and all the help provided for the assembly and disassembly of the continuous-flow experiences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Damiano, T.; Morton, D.; Nelson, A. Photochemical transformations of pyridinium salts: Mechanistic studies and applications in synthesis. Org. Biomol. Chem. 2007, 5, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Mariano, P.S. The synthetic potential of pyridinium salt photochemistry. Photochem. Photobiol. Sci. 2008, 7, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Mariano, P.S.; Lam, Y.F. A concise synthesis of the (-)-allosamizoline aminocyclopentitol based on pyridinium salt photochemistry. Tetrahedron Lett. 2001, 42, 4755–4757. [Google Scholar] [CrossRef]
- Ling, R.; Mariano, P.S. A Demonstration of the Synthetic Potential of Pyridinium Salt Photochemistry by Its Application to a Stereocontrolled Synthesis of (+)-Mannostatin A1. J. Org. Chem. 1998, 63, 6072–6076. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Pinto, B.M.; Bernardi, A.; Bennet, A.J. Synthesis and evaluation of influenza A viral neuraminidase candidate inhibitors based on a bicyclo[3.1.0]hexane scaffold. Org. Biomol. Chem. 2016, 14, 6539–6553. [Google Scholar] [CrossRef] [PubMed]
- Hook, B.D.A.; Dohle, W.; Hirst, P.R.; Pickworth, M.; Berry, M.B.; Booker-Milburn, K.I. A Practical Flow Reactor for Continuous Organic Photochemistry. J. Org. Chem. 2005, 70, 7558–7564. [Google Scholar] [CrossRef] [PubMed]
- Siopa, F.; António, J.P.M.; Afonso, C.A.M. Flow-Assisted Synthesis of Bicyclic Aziridines via Photochemical Transformation of Pyridinium Salts. Org. Process Res. Dev. 2018, 22, 551–556. [Google Scholar] [CrossRef]
- Snieckus, V.; Allais, C. Flow Photochemical Synthesis of Bicyclic Aziridines from Pyridinium Salts. Synfacts 2018, 14, 0903. [Google Scholar]
- Cambie, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noel, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Straathof, N.J.W.; Hessel, V.; Noel, T. Photochemical Transformations Accelerated in Continuous-Flow Reactors: Basic Concepts and Applications. Chem. Eur. J. 2014, 20, 10562–10589. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.P.; Elliott, L.D.; Booker-Milburn, K.I. Flow photochemistry: Old light through new windows. Beilstein J. Org. Chem. 2012, 8, 2025–2052. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).