Solvation Free Energy Simulation for Rosmarinic Acid Extraction from Orthosiphon stamineus
Abstract
:1. Introduction
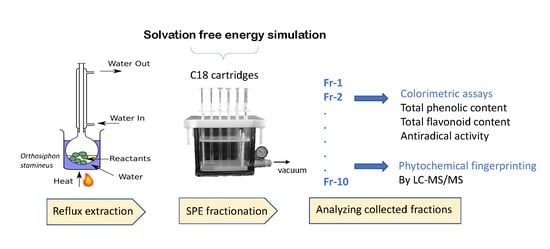
2. Experimental Design
2.1. Plant Material
2.2. Reflux Extraction
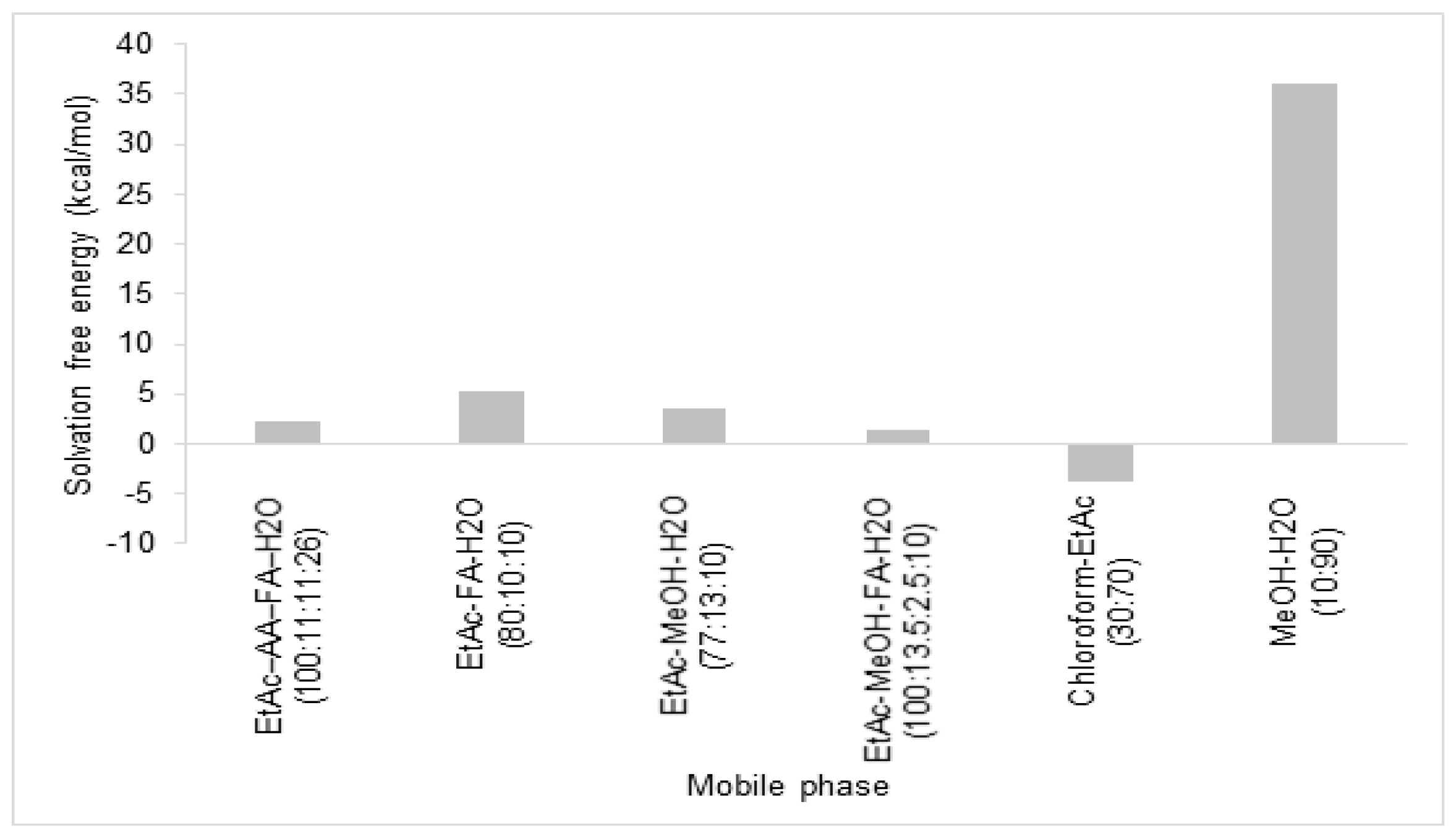
2.3. Prediction of Rosmarinic Acid Solubility by Solvation Free Energy
2.4. Solid Phase Extraction
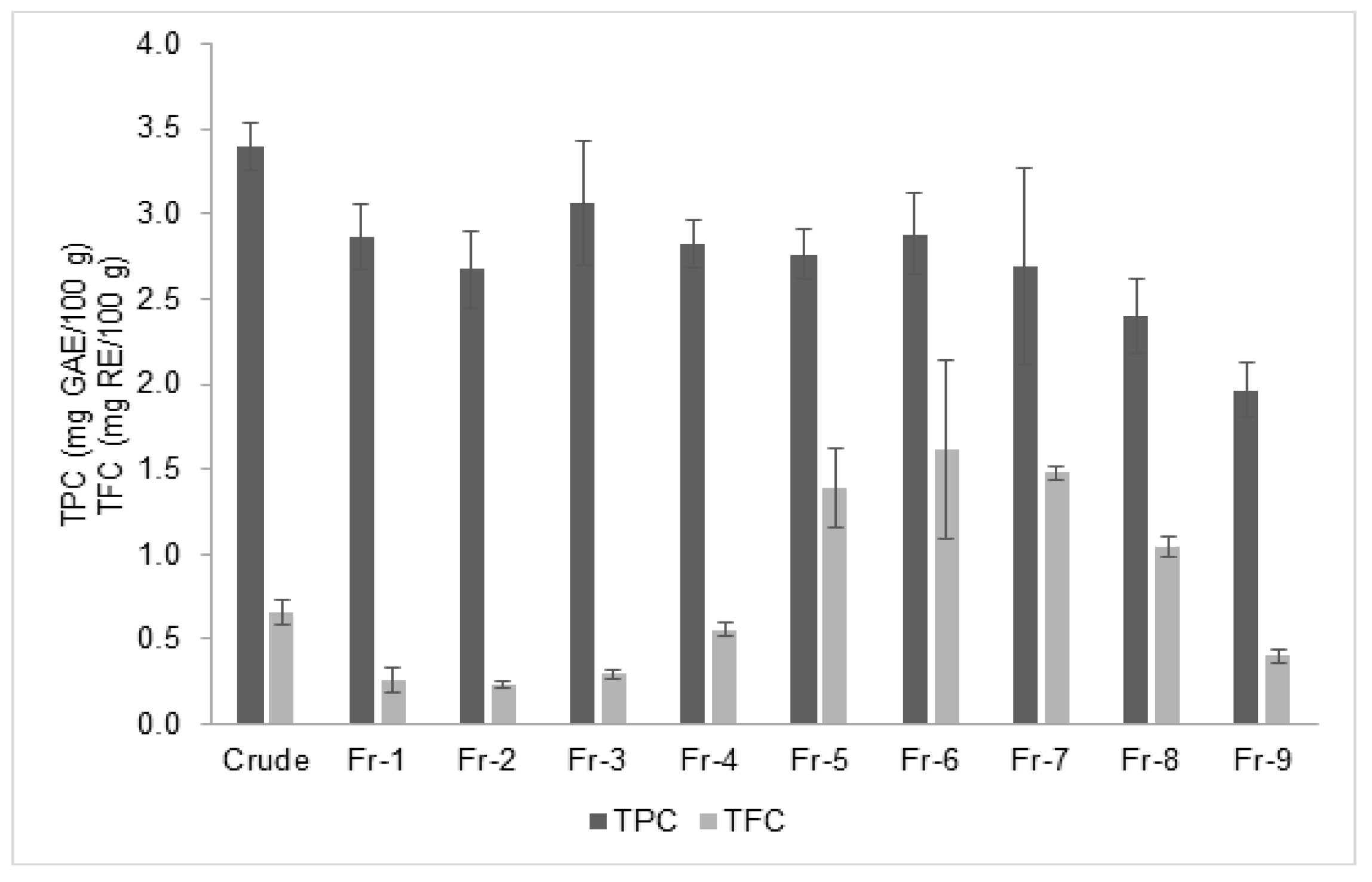
2.5. Determination of Total Phenolic and Flavonoid Content
2.6. Determination of Free Radical Scavenging Activity
2.7. Phytochemical Fingerprinting by Liquid Chromatography
2.8. Targeted Phytochemicals by Liquid Chromatography Tandem Mass Spectrometry
3. Results and Discussion
3.1. Prediction of Rosmarinic Acid Solubility by Simulation
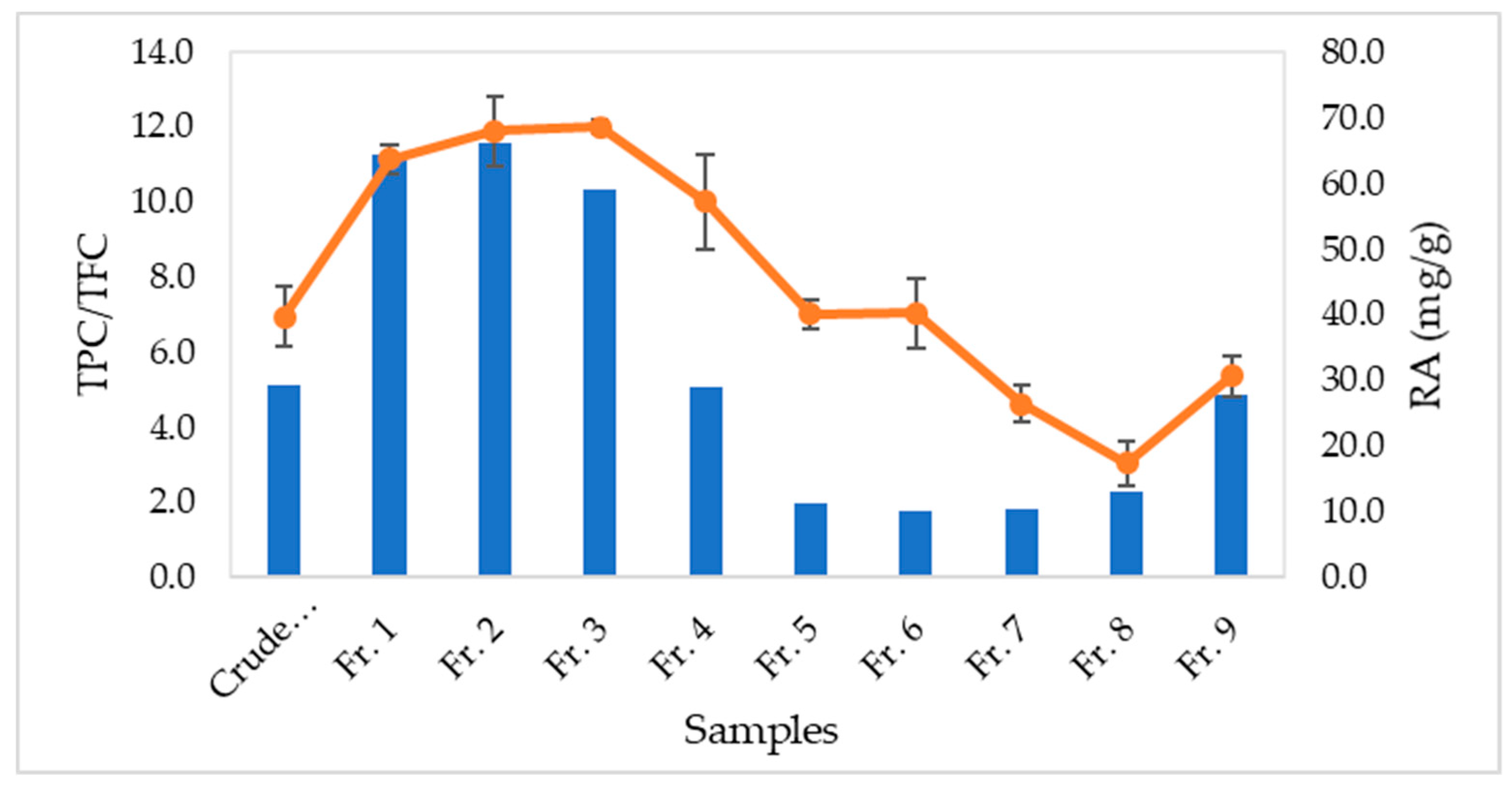
3.2. Relationship of Rosmarinic Acid and Scavenging Activity
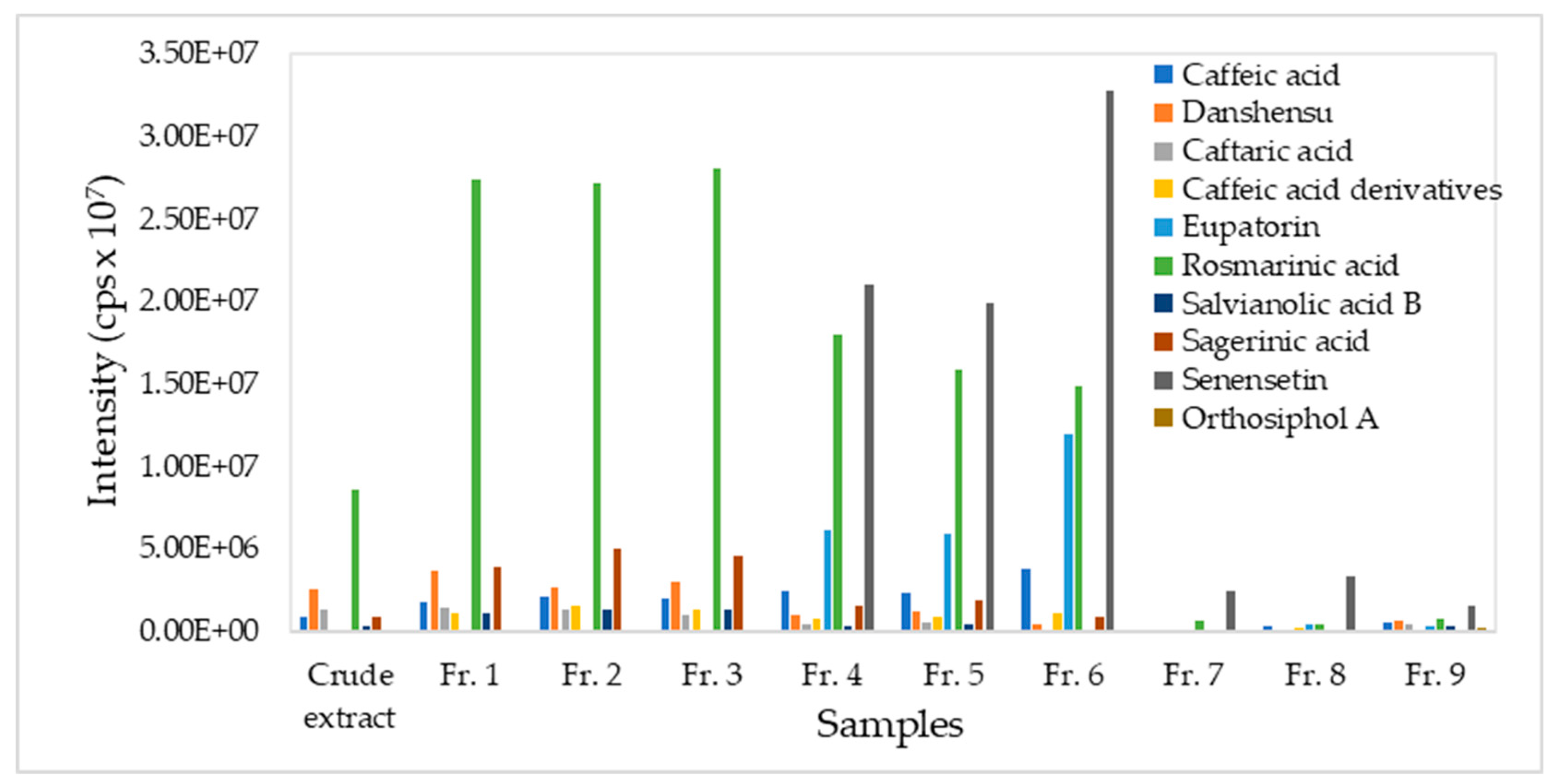
3.3. Major Phytochemicals in Plant Fractions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adam, Y.; Somchit, M.N.; Sulaiman, M.R.; Nasaruddin, A.A.; Zuraini, A.; Bustamam, A.A.; Zakaria, Z.A. Diuretic properties of Orthosiphon stamineus Benth. J. Ethnopharmacol. 2009, 124, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Tezuka, Y.; Tran, Q.L.; Kadota, S. Chemical constituents and biological activities of Vietnamese medicinal plants. Curr. Top. Med. Chem. 2003, 3, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Ameer, O.Z.; Salman, I.M.; Asmawi, M.Z.; Ibraheem, Z.O.; Yam, M.F. Orthosiphon stamineus: Traditional uses, phytochemistry, pharmacology, and toxicology. J. Med. Food 2012, 15, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W. Extraction and Isolation of Compounds from Herbal Medicines, Traditional Herbal Medicine Research Methods; John Wiley & Sons, Inc.: New York, NY, USA, 2011; pp. 81–138. [Google Scholar]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Zhari, I.; Norhayati, I.; Sadikun, A.; Khamsah, S.M. Sinensetin, eupatorin, 3′-hydroxy-5, 6, 7, 4′-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of Orthosiphon stamineus from Malaysia. Food Chem. 2004, 87, 559–566. [Google Scholar] [CrossRef]
- Lau, C.H.; Chua, L.S.; Lee, C.T.; Aziz, R. Optimization and kinetic modeling of rosmarinic acid extraction from Orthosiphon stamineus. Curr. Bioact. Compd. 2014, 10, 271–285. [Google Scholar] [CrossRef]
- Maleš, Z.; Medić-Šarić, M. Optimization of TLC analysis of flavonoids and phenolic acids of Helleborus atrorubens Waldst. et Kit. J. Pharm. BioMed. Anal. 2001, 24, 353–359. [Google Scholar] [CrossRef]
- Vundać, V.; Maleš, Z.; Plazibat, M.; Golja, P.; Cetina-Čižmek, B. HPTLC determination of flavonoids and phenolic acids in some Croatian Stachys taxa. J. Planar Chrom. Mod. TLC 2005, 18, 269–273. [Google Scholar] [CrossRef]
- Ly, T.N.; Shimoyamada, M.; Yamauchi, R. Isolation and characterization of rosmarinic acid oligomers in Celastrus hindsii Benth leaves and their antioxidative activity. J. Agric. Food Chem. 2006, 54, 3786–3793. [Google Scholar] [CrossRef] [PubMed]
- Arafat, O.M.; Tham, S.Y.; Sadikun, A.; Zhari, I.; Haughton, P.J.; Asmawi, M.Z. Studies on diuretic and hypouricemic effects of Orthosiphon stamineus methanol extracts in rats. J. Ethnopharmacol. 2008, 118, 354–360. [Google Scholar] [CrossRef] [PubMed]
- ISO 14502-1. Determination of Substances Characteristic of Green and Black Tea, Part 1: Content of Total Polyphenols in Tea, Colorimetric Method Using Folin-Ciocalteu Reagent; International Organization for Standardization: Geneva, Switzerland, 2005; Available online: https://www.iso.org/standard/31356.html (accessed on 15 May 2018).
- Chua, L.S.; Latiff, N.A.; Lee, S.Y.; Lee, C.T.; Sarmidi, M.R.; Aziz, R. Flavonoids and phenolic acids from Labisia pumila (Kacip Fatimah). Food Chem. 2011, 127, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Nuengchamnong, N.; Krittasilp, K.; Ingkaninan, K. Characterisation of phenolic antioxidants in aqueous extract of Orthosiphon grandiflorus tea by LC–ESI-MS/MS coupled to DPPH assay. Food Chem. 2011, 127, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; Piccolella, S.; Papale, F.; Nocera, P.; Lettieri, A.; Catauro, M. A polyphenol complex from Thymus vulgaris L. plants cultivated in the Campania Region (Italy): New perspectives against neuroblastoma. J. Funct. Foods 2016, 20, 253–266. [Google Scholar] [CrossRef]
- Moradkhani, S.; Kobarfard, F.; Ayatollahi, S.A.M. Phytochemical investigations on chemical constituents of Achillea tenuifolia Lam. Iran. J. Pharm. Res. 2014, 13, 1049–1054. [Google Scholar] [PubMed]
- Wang, D.; Lu, J.; Miao, A.; Xie, Z.; Yang, D. HPLC-DAD-ESI-MS/MS analysis of polyphenols and purine alkaloids in leaves of 22 tea cultivars in China. J. Food Compos. Anal. 2008, 21, 361–369. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Zhang, X.L.; Xu, Q.; Xue, X.Y.; Zhang, F.F.; Liang, X.M. UPLC/Q-TOFMS/MS as a powerful technique for rapid identification of polymethoxylated flavones in Fructus aurantii. J. Pharm. BioMed. Anal. 2009, 50, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Masuda, K.; Nakatani, N. Orthosiphol A, a highly oxygenated diterpene from the leaves of Orthosiphon stamineus. Tetrahedron Lett. 1992, 33, 945–946. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, Chapter 2: Solute-Solvent Interactions; Wiley-VCH Verlag GmbH & Co. KGaA: Weiheim, Germany, 2004; pp. 5–56. [Google Scholar]
- Accelrys. Material Studio: Forcite Module; Accelrys Inc.: San Diego, CA, USA, 2011. [Google Scholar]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. In Vitro 2017, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, H.; Gomes-Carneiro, M.R.; Poça, K.S.; De-Oliveira, A.C.A.X.; Afzan, A.; Sulaiman, S.A.; Ismail, Z.; Paumgartten, F.J.R. Evaluation of the genotoxicity of Orthosiphon stamineus aqueous extract. J. Ethnopharmacol. 2011, 133, 647–653. [Google Scholar] [CrossRef] [PubMed]

| No. | Compounds | Molecular Weight | Mode | Fragments | Reference |
|---|---|---|---|---|---|
| 1 | Caffeic acid | 180 | Negative | 179, 135 | [14] |
| 2 | Danshensu | 198 | Negative | 197, 179, 134, 123 | [14] |
| 3 | Caftaric acid | 312 | Negative | 311, 149, 179 | [14] |
| 4 | Caffeic acid derivative | 344 | Negative | 343, 161, 197, 181, 137, 135 | [14] |
| 5 | Eupatorin | 344 | Negative | 343, 328, 313, 298, 197, 161, 135 | [15] |
| 6 | 5-hydroxy-3’,4’,6,7-tetramethoxyflavone | 358 | Negative | 359, 357, 358, 343, 328, 313, 299, 285, 196, 181, 162, 153 | [16] |
| 7 | Rosmarinic acid | 360 | Negative | 359, 197, 179, 161, 135 | [14] |
| 8 | Salvianolic acid B (Lithospermic acid B) | 718 | Negative | 717, 519, 339 | [14] |
| 9 | Sagerinic acid | 720 | Negative | 719, 359, 538 | [14] |
| 10 | Caffeine | 194 | Positive | 195, 163, 138, 110 | [17] |
| 11 | Sinensetin | 372 | Positive | 373, 358, 343, 185 | [18] |
| 12 | Kaempferol-rutinoside | 594 | Positive | 593, 285 | [17] |
| 13 | Orthosiphol A | 676 | Positive | 677 | [19] |
| Sample | IC50 (μg/mL) |
|---|---|
| Rosmarinic acid | 15.05 ± 2.03 |
| Ascorbic acid | 15.54 ± 1.83 |
| Rutin | 49.27 ± 6.99 |
| Crude extract | 58.85 ± 7.11 |
| Fraction 1 | 37.30 ± 1.69 |
| Fraction 2 | 38.29 ± 0.48 |
| Fraction 3 | 39.13 ± 4.23 |
| Fraction 4 | 45.10 ± 4.74 |
| Fraction 5 | 71.38 ± 6.80 |
| Fraction 6 | 79.53 ± 0.57 |
| Fraction 7 | 82.58 ± 5.86 |
| Fraction 8 | 98.56 ± 5.63 |
| Fraction 9 | 74.14 ± 5.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, C.H.; Chua, L.S. Solvation Free Energy Simulation for Rosmarinic Acid Extraction from Orthosiphon stamineus. Methods Protoc. 2019, 2, 64. https://doi.org/10.3390/mps2030064
Lau CH, Chua LS. Solvation Free Energy Simulation for Rosmarinic Acid Extraction from Orthosiphon stamineus. Methods and Protocols. 2019; 2(3):64. https://doi.org/10.3390/mps2030064
Chicago/Turabian StyleLau, Cher Haan, and Lee Suan Chua. 2019. "Solvation Free Energy Simulation for Rosmarinic Acid Extraction from Orthosiphon stamineus" Methods and Protocols 2, no. 3: 64. https://doi.org/10.3390/mps2030064
APA StyleLau, C. H., & Chua, L. S. (2019). Solvation Free Energy Simulation for Rosmarinic Acid Extraction from Orthosiphon stamineus. Methods and Protocols, 2(3), 64. https://doi.org/10.3390/mps2030064