Alberta Spinal Muscular Atrophy Newborn Screening—Results from Year 1 Pilot Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.1.1. Validation Study
2.1.2. Alberta Pilot Project February 2022–February 2023
2.2. DBS Punching and DNA Isolation
2.3. SMN1 Multiplex qPCR Assay
2.4. Validation Study
2.5. Alberta Pilot Project
2.6. MLPA
3. Results
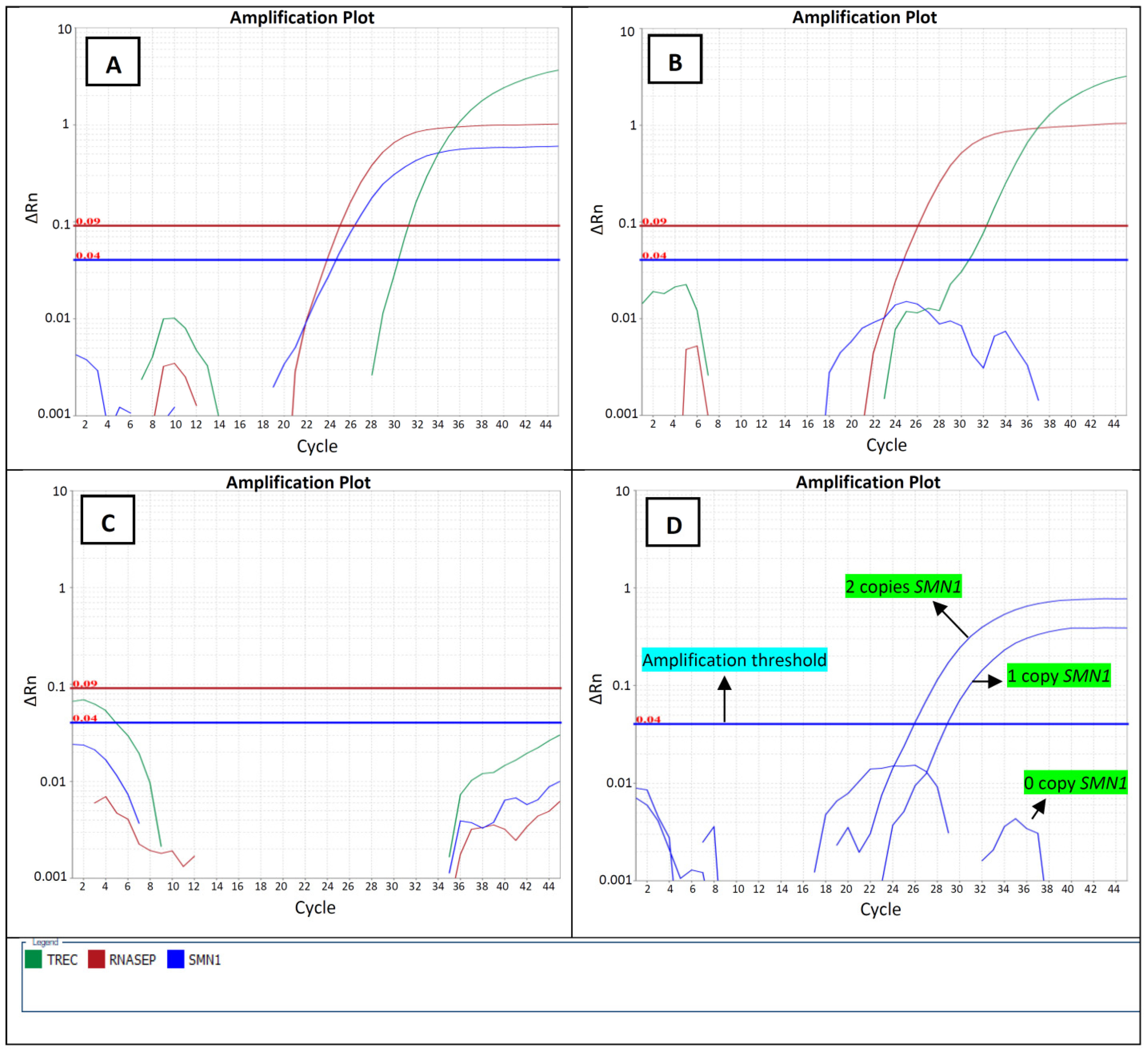
3.1. Determination of the Cut-Offs
3.2. Analytical Validation and Evaluation of the qPCR Assays
- -
- Analytical sensitivity and specificity: the assay was able to correctly identify the tested de-identified previously genotyped control samples, including 19 homozygous absence of exon 7 and 35 normal controls, which were comprised of 20 samples with heterozygous absence of exon 7 and 15 samples with 2 copies of SMN1 (Supplementary Table S2). The data show that the assay analytical specificity and sensitivity are 100%, which makes it suitable for our screening purposes (Table 2).
- -
- Repeatability: The assessment of intra-run repeatability involved comparing 14 de-identified DBS samples and 10 controls with known SMN1 copy numbers in quadruplicate within a single run (Supplementary Table S3). All samples were correctly identified in all of the quadruple runs with 100% intra-run concordance, demonstrating the good repeatability of both SMN1 and the RPP30 assays. The intra-assay coefficient of variability (%CV) for SMN1 CT values ranged from 0.54% to 3.71%, with an average of 0.65%. The %CV for RPP30 CT values ranged from 2.0% to 4.9%, with an average of 0.71%. These values demonstrate the acceptable repeatability of the assay.
- -
- Intermediate precision: The assessment of inter-run repeatability was performed by two technologists testing the same 96 de-identified DBS samples and the control samples with known SMN1 copy numbers (Supplementary Table S3). For all the runs 100% of agreement between runs was observed and the CT values were in the expected ranges. The inter-assay %CV for SMN1 CT values ranged from 0.65% to 6.51%, with an average of 2.39%. The %CV for RPP30 CT values ranged from 0.68% to 4.1%, with an average of 1.89%. These values demonstrate the acceptable inter-run repeatability of the assay.
- -
- Reproducibility: To monitor interlaboratory reproducibility and conduct proficiency testing, 10 External Quality Assessment (EQA) samples with known SMN1 copy numbers, specifically designed for SMA analysis, were analyzed. The results of this analysis can be found in Supplementary Table S3. The analysis revealed that all of the samples were identified correctly, indicating a 100% agreement in interlaboratory concordance.
- -
- Robustness: The robustness of the assay was evaluated by comparing the impact of master mix age on the CT values. The experiment involved running the same de-identified DBS samples while storing the master mix at 4 °C. The results indicated that the master mix can be stored at 4 °C for up to 10 days without significantly affecting the CT values (Supplementary Table S3).
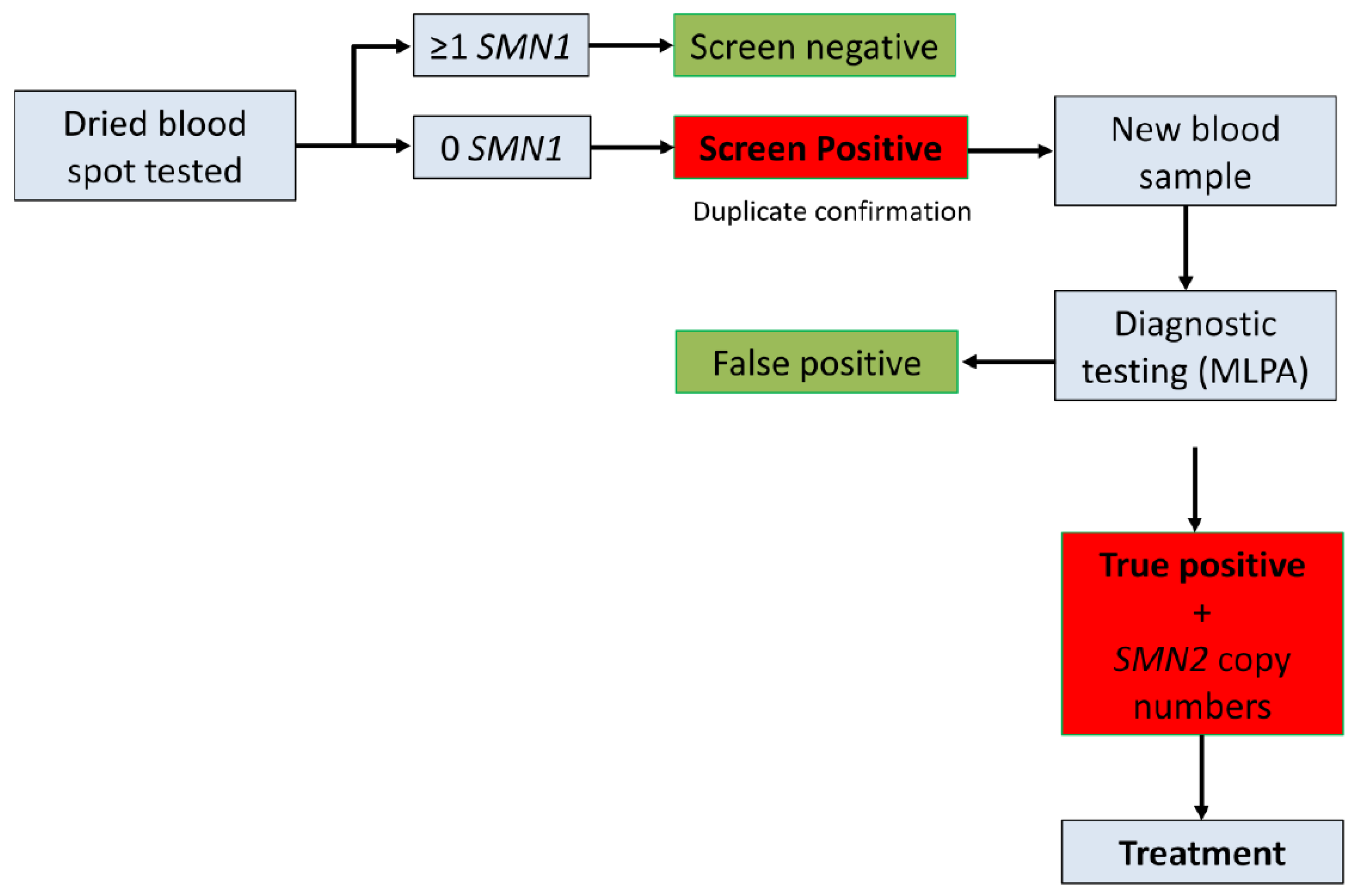
3.3. Alberta Pilot Project
3.4. Clinical Outcomes and Treatment
4. Discussion
False Positive Case
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mercuri, E.; Sumner, C.J.; Muntoni, F.; Darras, B.T.; Finkel, R.S. Spinal Muscular Atrophy. Nat. Rev. Dis. Primers 2022, 8, 52. [Google Scholar] [CrossRef]
- Chaytow, H.; Huang, Y.-T.; Gillingwater, T.H.; Faller, K.M.E. The Role of Survival Motor Neuron Protein (SMN) in Protein Homeostasis. Cell Mol. Life Sci. 2018, 75, 3877–3894. [Google Scholar] [CrossRef]
- Wirth, B. Spinal Muscular Atrophy: In the Challenge Lies a Solution. Trends Neurosci. 2021, 44, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Calucho, M.; Bernal, S.; Alías, L.; March, F.; Venceslá, A.; Rodríguez-Álvarez, F.J.; Aller, E.; Fernández, R.M.; Borrego, S.; Millán, J.M.; et al. Correlation between SMA Type and SMN2 Copy Number Revisited: An Analysis of 625 Unrelated Spanish Patients and a Compilation of 2834 Reported Cases. Neuromuscul. Disord. 2018, 28, 208–215. [Google Scholar] [CrossRef]
- Feldkötter, M.; Schwarzer, V.; Wirth, R.; Wienker, T.F.; Wirth, B. Quantitative Analyses of SMN1 and SMN2 Based on Real-Time LightCycler PCR: Fast and Highly Reliable Carrier Testing and Prediction of Severity of Spinal Muscular Atrophy. Am. J. Hum. Genet. 2002, 70, 358–368. [Google Scholar] [CrossRef]
- Wirth, B.; Brichta, L.; Schrank, B.; Lochmüller, H.; Blick, S.; Baasner, A.; Heller, R. Mildly Affected Patients with Spinal Muscular Atrophy Are Partially Protected by an Increased SMN2 Copy Number. Hum. Genet. 2006, 119, 422–428. [Google Scholar] [CrossRef]
- Van der Steege, G.; Grootscholten, P.M.; Cobben, J.M.; Zappata, S.; Scheffer, H.; den Dunnen, J.T.; van Ommen, G.B.; Brahe, C.; Buys, C.H.C.M. Apparent Gene Conversions Involving the SMN Gene in the Region of the Spinal Muscular Atrophy Locus on Chromosome 5. Am. J. Hum. Genet. 1996, 59, 834–838. [Google Scholar]
- Mercuri, E.; Pera, M.C.; Scoto, M.; Finkel, R.; Muntoni, F. Spinal Muscular Atrophy—Insights and Challenges in the Treatment Era. Nat. Rev. Neurol. 2020, 16, 706–715. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef]
- Finkel, R.S.; Chiriboga, C.A.; Vajsar, J.; Day, J.W.; Montes, J.; De Vivo, D.C.; Yamashita, M.; Rigo, F.; Hung, G.; Schneider, E.; et al. Treatment of Infantile-Onset Spinal Muscular Atrophy with Nusinersen: A Phase 2, Open-Label, Dose-Escalation Study. Lancet 2016, 388, 3017–3026. [Google Scholar] [CrossRef]
- Finkel, R.S.; Mercuri, E.; Darras, B.T.; Connolly, A.M.; Kuntz, N.L.; Kirschner, J.; Chiriboga, C.A.; Saito, K.; Servais, L.; Tizzano, E.; et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2017, 377, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.L.; Saito, K.; et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.-L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen Initiated in Infants during the Presymptomatic Stage of Spinal Muscular Atrophy: Interim Efficacy and Safety Results from the Phase 2 NURTURE Study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef]
- Darras, B.T.; Chiriboga, C.A.; Iannaccone, S.T.; Swoboda, K.J.; Montes, J.; Mignon, L.; Xia, S.; Bennett, C.F.; Bishop, K.M.; Shefner, J.M.; et al. Nusinersen in Later-Onset Spinal Muscular Atrophy: Long-Term Results from the Phase 1/2 Studies. Neurology 2019, 92, e2492–e2506. [Google Scholar] [CrossRef] [PubMed]
- Al-Zaidy, S.; Pickard, A.S.; Kotha, K.; Alfano, L.N.; Lowes, L.; Paul, G.; Church, K.; Lehman, K.; Sproule, D.M.; Dabbous, O.; et al. Health Outcomes in Spinal Muscular Atrophy Type 1 Following AVXS-101 Gene Replacement Therapy. Pediatr. Pulmonol. 2019, 54, 179–185. [Google Scholar] [CrossRef]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef]
- Dhillon, S. Risdiplam: First Approval. Drugs 2020, 80, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Ramdas, S.; Servais, L. New Treatments in Spinal Muscular Atrophy: An Overview of Currently Available Data. Expert Opin. Pharmacother. 2020, 21, 307–315. [Google Scholar] [CrossRef]
- Dangouloff, T.; Servais, L. Clinical Evidence Supporting Early Treatment of Patients with Spinal Muscular Atrophy: Current Perspectives. TCRM 2019, 15, 1153–1161. [Google Scholar] [CrossRef]
- McMillan, H.J.; Kernohan, K.D.; Yeh, E.; Amburgey, K.; Boyd, J.; Campbell, C.; Dowling, J.J.; Gonorazky, H.; Marcadier, J.; Tarnopolsky, M.A.; et al. Newborn Screening for Spinal Muscular Atrophy: Ontario Testing and Follow-up Recommendations. Can. J. Neurol. Sci. 2021, 48, 504–511. [Google Scholar] [CrossRef]
- Baker, M.W.; Mochal, S.T.; Dawe, S.J.; Wiberley-Bradford, A.E.; Cogley, M.F.; Zeitler, B.R.; Piro, Z.D.; Harmelink, M.M.; Kwon, J.M. Newborn Screening for Spinal Muscular Atrophy: The Wisconsin First Year Experience. Neuromuscul. Disord. 2022, 32, 135–141. [Google Scholar] [CrossRef]
- Alberta Health—Drug Benefit List. Available online: https://idbl.ab.bluecross.ca/idbl/lookupDinPinDetail.do?productID=0000084687 (accessed on 18 May 2023).
- Onasemnogene Abeparvovec|CADTH. Available online: https://www.cadth.ca/onasemnogene-abeparvovec (accessed on 18 May 2023).
- Verhaart, I.E.C.; Robertson, A.; Leary, R.; McMacken, G.; König, K.; Kirschner, J.; Jones, C.C.; Cook, S.F.; Lochmüller, H. A Multi-Source Approach to Determine SMA Incidence and Research Ready Population. J. Neurol. 2017, 264, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Dangouloff, T.; Vrščaj, E.; Servais, L.; Osredkar, D.; Adoukonou, T.; Aryani, O.; Barisic, N.; Bashiri, F.; Bastaki, L.; Benitto, A.; et al. Newborn Screening Programs for Spinal Muscular Atrophy Worldwide: Where We Stand and Where to Go. Neuromuscul. Disord. 2021, 31, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Kernohan, K.D.; McMillan, H.J.; Yeh, E.; Lacaria, M.; Kowalski, M.; Campbell, C.; Dowling, J.J.; Gonorazky, H.; Marcadier, J.; Tarnopolsky, M.A.; et al. Ontario Newborn Screening for Spinal Muscular Atrophy: The First Year. Can. J. Neurol. Sci. 2022, 49, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, B.C.; Donohoe, C.; Akmaev, V.R.; Sugarman, E.A.; Labrousse, P.; Boguslavskiy, L.; Flynn, K.; Rohlfs, E.M.; Walker, A.; Allitto, B.; et al. Differences in SMN1 Allele Frequencies among Ethnic Groups within North America. J. Med. Genet. 2009, 46, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Vill, K.; Schwartz, O.; Blaschek, A.; Gläser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Röschinger, W.; Becker, M.; Czibere, L.; et al. Newborn Screening for Spinal Muscular Atrophy in Germany: Clinical Results after 2 Years. Orphanet J. Rare Dis. 2021, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Dangouloff, T.; Burghes, A.; Tizzano, E.F.; Servais, L.; Dangouloff, T.; Burghes, A.; Bertini, E.; Boemer, F.; Hiligsmann, M.; Mueller-Felber, W.; et al. 244th ENMC International Workshop: Newborn Screening in Spinal Muscular Atrophy May 10–12, 2019, Hoofdorp, The Netherlands. Neuromuscul. Disord. 2020, 30, 93–103. [Google Scholar] [CrossRef]
- Fang, P.; Li, L.; Zeng, J.; Zhou, W.-J.; Wu, W.-Q.; Zhong, Z.-Y.; Yan, T.-Z.; Xie, J.-S.; Huang, J.; Lin, L.; et al. Molecular Characterization and Copy Number of SMN1, SMN2 and NAIP in Chinese Patients with Spinal Muscular Atrophy and Unrelated Healthy Controls. BMC Musculoskelet. Disord. 2015, 16, 11. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Zhang, Y.; Li, L.; Tang, X.; Wang, L.; Guo, J.; Jin, C.; Tighe, S.; Zhang, Y.; et al. The Analysis of the Association between the Copy Numbers of Survival Motor Neuron Gene 2 and Neuronal Apoptosis Inhibitory Protein Genes and the Clinical Phenotypes in 40 Patients with Spinal Muscular Atrophy: Observational Study. Medicine 2020, 99, e18809. [Google Scholar] [CrossRef]
- Qu, Y.; Ge, X.; Bai, J.; Wang, L.; Cao, Y.; Lu, Y.; Jin, Y.; Wang, H.; Song, F. Association of Copy Numbers of Survival Motor Neuron Gene 2 and Neuronal Apoptosis Inhibitory Protein Gene with the Natural History in a Chinese Spinal Muscular Atrophy Cohort. J. Child. Neurol. 2015, 30, 429–436. [Google Scholar] [CrossRef]
- Chen, X.; Sanchis-Juan, A.; French, C.E.; Connell, A.J.; Delon, I.; Kingsbury, Z.; Chawla, A.; Halpern, A.L.; Taft, R.J.; Bentley, D.R.; et al. Spinal Muscular Atrophy Diagnosis and Carrier Screening from Genome Sequencing Data. Genet. Med. 2020, 22, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Ogino, S.; Gao, S.; Leonard, D.G.B.; Paessler, M.; Wilson, R.B. Inverse Correlation between SMN1 and SMN2 Copy Numbers: Evidence for Gene Conversion from SMN2 to SMN1. Eur. J. Hum. Genet. 2003, 11, 275–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jędrzejowska, M.; Gos, M.; Zimowski, J.G.; Kostera-Pruszczyk, A.; Ryniewicz, B.; Hausmanowa-Petrusewicz, I. Novel Point Mutations in Survival Motor Neuron 1 Gene Expand the Spectrum of Phenotypes Observed in Spinal Muscular Atrophy Patients. Neuromuscul. Disord. 2014, 24, 617–623. [Google Scholar] [CrossRef] [PubMed]
| Assay Performance Parameters | |
|---|---|
| Population study | 3200 de-identified DBS collected >4 years ago |
| Limit of blank | 45 DBS interspersed with 45 filter blanks |
| Sensitivity, Specificity, Accuracy | 20 positive, 20 carriers, and 15 normal DBS |
| Repeatability | 24 known samples run in quadruple |
| Intermediate Precision | Same 96 DBS samples run in 4 different runs |
| Reproducibility | Proficiency samples tested |
| Robustness | Durability of prepared mastermix tested |
| Genotype | Samples |
|---|---|
| True Negative | 35 |
| True Positive | 19 |
| False Negative | 0 |
| False Positive | 0 |
| Total valid | 54 |
| Sensitivity | 100% |
| Specificity | 100% |
| Accuracy | 100% |
| SMA Case | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age (in days) when NBS sample was collected | 1 | 1 | 1 | 1 | 1 |
| Age (in days) when sample was received age | 3 | 1 | 2 | 2 | 3 |
| Age (in days) when positive screen reported | 8 | 7 | 6 | 6 | 7 |
| Age (in days) when parents were contacted | 8 | 7 | 6 | 6 | 7 |
| Age (in days) when seen in clinic and confirmatory lab sent | 9 | 9 | 7 | 8 | 7 |
| Age (in days) when confirmatory results reported | 17 | 27 | 15 | 15 | 13 |
| SMN1 copies | 0 | 0 | 0 | 0 | 0 |
| SMN2 copies | 3 | 3 | 3 | 3 | 3 |
| Age (in days) when 1st treatment given | 29 | 72 | 28 | 30 | 25 |
| Age (in days) when 2nd treatment given | N/A | 111 | N/A | N/A | N/A |
| Symptomatic before 1st treatment? Yes/No | No | Yes | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niri, F.; Nicholls, J.; Baptista Wyatt, K.; Walker, C.; Price, T.; Kelln, R.; Hume, S.; Parboosingh, J.; Lilley, M.; Kolski, H.; et al. Alberta Spinal Muscular Atrophy Newborn Screening—Results from Year 1 Pilot Project. Int. J. Neonatal Screen. 2023, 9, 42. https://doi.org/10.3390/ijns9030042
Niri F, Nicholls J, Baptista Wyatt K, Walker C, Price T, Kelln R, Hume S, Parboosingh J, Lilley M, Kolski H, et al. Alberta Spinal Muscular Atrophy Newborn Screening—Results from Year 1 Pilot Project. International Journal of Neonatal Screening. 2023; 9(3):42. https://doi.org/10.3390/ijns9030042
Chicago/Turabian StyleNiri, Farshad, Jessie Nicholls, Kelly Baptista Wyatt, Christine Walker, Tiffany Price, Rhonda Kelln, Stacey Hume, Jillian Parboosingh, Margaret Lilley, Hanna Kolski, and et al. 2023. "Alberta Spinal Muscular Atrophy Newborn Screening—Results from Year 1 Pilot Project" International Journal of Neonatal Screening 9, no. 3: 42. https://doi.org/10.3390/ijns9030042
APA StyleNiri, F., Nicholls, J., Baptista Wyatt, K., Walker, C., Price, T., Kelln, R., Hume, S., Parboosingh, J., Lilley, M., Kolski, H., Ridsdale, R., Muranyi, A., Mah, J. K., & Bulman, D. E. (2023). Alberta Spinal Muscular Atrophy Newborn Screening—Results from Year 1 Pilot Project. International Journal of Neonatal Screening, 9(3), 42. https://doi.org/10.3390/ijns9030042






