Evaluation of a Two-Tier Screening Pathway for Congenital Adrenal Hyperplasia in the New South Wales Newborn Screening Programme
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Immunoassay
2.3. LC-MS/MS
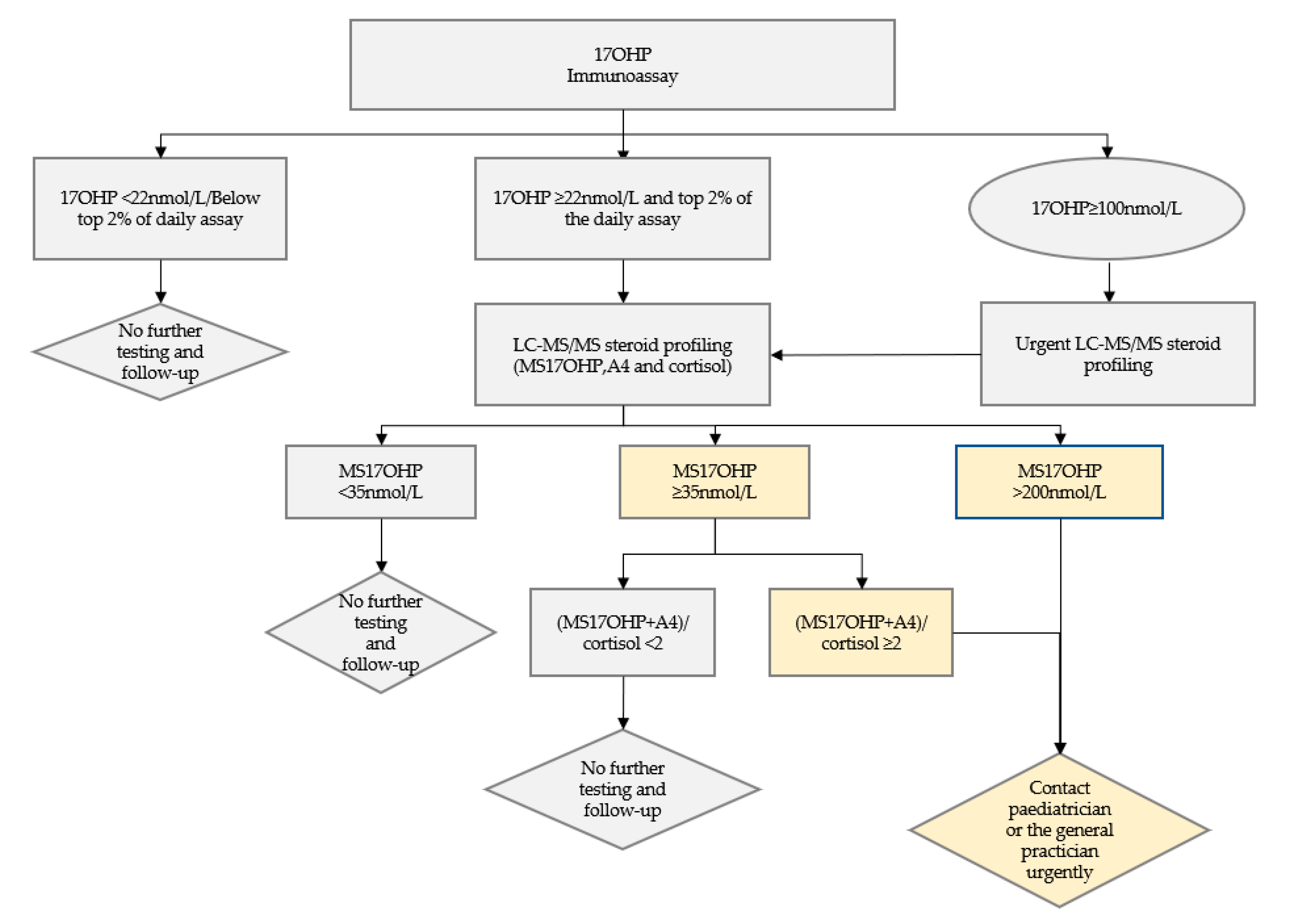
2.4. Criteria for and Follow-Up of an Abnormal Screen
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F.; Azziz, R. Congenital adrenal hyperplasia. J. Pediatric Adolesc. Gynecol. 2011, 24, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Hannah-Shmouni, F.; Chen, W.; Merke, D.P. Genetics of congenital adrenal hyperplasia. Endocrinol. Metab. Clin. N. Am. 2017, 46, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.; Hughes, I. Congenital adrenal hyperplasia. Medicine 2017, 45, 502–505. [Google Scholar] [CrossRef]
- Speiser, P.W.; White, P.C. Congenital adrenal hyperplasia. N. Engl. J. Med. 2003, 349, 776–788. [Google Scholar] [CrossRef]
- Wass, J.A.H.; Stewart, P.M. Oxford Textbook of Endocrinology and Diabetes; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Nimkarn, S.G.P.; Yau, M.; New, M.I. 21-Hydroxylase Deficiency Congenital Adrenal Hyperplasia; University of Washington: Seattle, WA, USA, 2016. [Google Scholar]
- Nimkarn, S.; Lin-Su, K.; New, M.I. Steroid 21 hydroxylase deficiency congenital adrenal hyperplasia. Pediatr. Clin. N. Am. 2011, 58, 1281–1300. [Google Scholar] [CrossRef]
- Pang, S.Y. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 1988, 81, 866. [Google Scholar] [CrossRef]
- Pang, S.; Hotchkiss, J.; Drash, A.L.; Levine, L.S.; New, M.I. Microfilter paper method for 17α-hydroxyprogesterone radioimmunoassay: Its application for rapid screening for congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 1977, 45, 1003–1008. [Google Scholar] [CrossRef]
- Gong, L.-F.; Gao, X.; Yang, N.; Zhao, J.-Q.; Yang, H.-H.; Kong, Y.-Y. A pilot study on newborn screening for congenital adrenal hyperplasia in Beijing. J. Pediatr. Endocrinol. Metab. 2019, 32, 253–258. [Google Scholar] [CrossRef]
- Heather, N.L.; Seneviratne, S.N.; Webster, D.; Derraik, J.G.B.; Jefferies, C.; Carll, J.; Jiang, Y.; Cutfield, W.S.; Hofman, P.L. Newborn screening for congenital adrenal hyperplasia in New Zealand, 1994–2013. J. Clin. Endocrinol. Metab. 2015, 100, 1002–1008. [Google Scholar] [CrossRef]
- Kumar, R.K.; Das, H.; Kini, P. Newborn screening for congenital adrenal hyperplasia in India: What do we need to watch out for? J. Obstet. Gynecol. India 2016, 66, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Kopacek, C.; de Castro, S.M.; Prado, M.J.; da Silva, C.M.D.; Beltrão, L.A.; Spritzer, P.M. Neonatal screening for congenital adrenal hyperplasia in Southern Brazil: A population based study with 108,409 infants. BMC Pediatr. 2017, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Murphey, W.; Levine, L.S.; Spence, D.A.; Leon, A.; LaFranchi, S.; Surve, A.S.; New, M.I. A pilot newborn screening for congenital adrenal hyperplasia in Alaska. J. Clin. Endocrinol. Metab. 1982, 55, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.; DeMartino, L.; McMahon, R.; Hamel, R.; Maloney, B.; Stansfield, D.-M.; McGrath, E.C.; Occhionero, A.; Gearhart, A.; Caggana, M.; et al. Newborn screening for congenital adrenal hyperplasia in New York State. Mol. Genet. Metab. Rep. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Perrin, C.W. Neonatal screening for congenital adrenal hyperplasia. Nat. Rev. Endocrinol. 2009, 5, 490. [Google Scholar]
- Sarafoglou, K.; Gaviglio, A.; Hietala, A.; Frogner, G.; Banks, K.; McCann, M.; Thomas, W. Comparison of newborn screening protocols for congenital adrenal hyperplasia in preterm infants. J. Pediatr. 2014, 164, 1136–1140. [Google Scholar] [CrossRef]
- Therrell, B.L.; Adams, J. Newborn screening in North America. J. Inherit. Metab. Dis. 2007, 30, 447–465. [Google Scholar] [CrossRef]
- Tsuji-Hosokawa, A.; Konishi, K.; Hasegawa, S.; Anazawa, A.; Onishi, T.; Ono, M.; Morio, T.; Kitagawa, T.; Kashimada, K. Newborn screening for congenital adrenal hyperplasia in Tokyo, Japan from 1989 to 2013: A retrospective population-based study. BMC Pediatr. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Van Der Kamp, H.J.; Noordam, C.; Elvers, B.; Van Baarle, M.; Otten, B.J.; Verkerk, P.H. Newborn screening for congenital adrenal hyperplasia in The Netherlands. Pediatrics 2001, 108, 1320–1324. [Google Scholar] [CrossRef]
- Van der Linde, A.A.A.; Schönbeck, Y.; van der Kamp, H.J.; Akker, E.L.V.D.; van Albada, M.E.; Boelen, A.; Finken, M.J.J.; Hannema, S.E.; Hoorweg-Nijman, G.; Odink, R.J.; et al. Evaluation of the Dutch neonatal screening for congenital adrenal hyperplasia. Arch. Dis. Child. 2019, 104, 653–657. [Google Scholar] [CrossRef]
- Pang, S.; Clark, A.; Neto, E.C.; Giugliani, R.; Dean, H.; Winter, J.; Dhondt, J.-L.; Farriaux, J.; Graters, A.; Cacciari, E.; et al. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: Newborn screening and its relationship to the diagnosis and treatment of the disorder. Screening 1993, 2, 105–139. [Google Scholar] [CrossRef]
- Lacey, J.M.; Minutti, C.Z.; Magera, M.J.; Tauscher, A.L.; Casetta, B.; McCann, M.; Lymp, J.; Hahn, S.H.; Rinaldo, P.; Matern, D. Improved specificity of newborn screening for congenital adrenal hyperplasia by second-tier steroid profiling using tandem mass spectrometry. Clin. Chem. 2004, 50, 621–625. [Google Scholar] [CrossRef] [PubMed]
- 12118_Newborn Bloodspot Framework_V4_WEB.PDF. Available online: http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/C79A7D94CB73C56CCA257CEE0000EF35/$File/12118_Newborn%20Bloodspot%20Framework_V4_WEB.PDF (accessed on 29 June 2020).
- Wudy, S.; Schuler, G.; Guijo, A.S.; Hartmann, M. The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J. Steroid Biochem. Mol. Boil. 2018, 179, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B.; Wiley, V. Fifty years of newborn screening. J. Paediatr. Child Heal. 2015, 51, 103–107. [Google Scholar] [CrossRef]
- Wilcken, B.; Wiley, V. Newborn screening. Pathology 2008, 40, 104–115. [Google Scholar] [CrossRef]
- Gleeson, H.K.; Wiley, V.; Wilcken, B.; Elliott, E.J.; Cowell, C.; Thonsett, M.; Byrne, G.; Ambler, G. Two-year pilot study of newborn screening for congenital adrenal hyperlasia in New South Wales compared with nationwide case surveillance in Australia. J. Paediatr. Child Heal. 2008, 44, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Warne, G.L.; Armstrong, K.L.; Faunce, T.; Wilcken, B.M.; Boneh, A.; Geelhoed, E.; Craig, M.E. The case for newborn screening for congenital adrenal hyperplasia in Australia. Med. J. Aust. 2010, 192, 107. [Google Scholar] [CrossRef]
- Rossi, C.; Calton, L.; Brown, H.A.; Gillingwater, S.; Wallace, A.M.; Petrucci, F.; Ciavardelli, D.; Urbani, A.; Sacchetta, P.; Morris, M.R. Confirmation of congenital adrenal hyperplasia by adrenal steroid profiling of filter paper dried blood samples using ultra-performance liquid chromatography-tandem mass spectrometry. Clin. Chem. Lab. Med. 2011, 49, 677–684. [Google Scholar] [CrossRef]
- White, P.C. Optimizing newborn screening for congenital adrenal hyperplasia. J. Pediatr. 2013, 163, 10–12. [Google Scholar] [CrossRef]
- Turcu, A.F.; Auchus, R.J. The next 150 years of congenital adrenal hyperplasia. J. Steroid Biochem. Mol. Boil. 2015, 153, 63–71. [Google Scholar] [CrossRef]
- Hird, B.E.; Tetlow, L.; Tobi, S.; Patel, L.; Clayton, R. No evidence of an increase in early infant mortality from congenital adrenal hyperplasia in the absence of screening. Arch. Dis. Child. 2014, 99, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, G.; Czernichow, P. Screening for neonatal endocrinopathies: Rationale, methods and results. Semin. Neonatol. 2004, 9, 75–85. [Google Scholar] [CrossRef]
- Speiser, P.W.; Azziz, R.; Baskin, L.S.; Ghizzoni, L.; Hensle, T.W.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Montori, V.M.; Oberfield, S.E.; et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010, 95, 4133–4160. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, G.Y.; Carvalho, D.F.; De Miranda, M.C.; Faure, C.; Vallejos, C.; Brito, V.N.; Rodrigues, A.D.S.; Madureira, G.; Mendonca, B.B.; Bachega, T.A. Neonatal 17-hydroxyprogesterone levels adjusted according to age at sample collection and birthweight improve the efficacy of congenital adrenal hyperplasia newborn screening. Clin. Endocrinol. 2017, 86, 480–487. [Google Scholar] [CrossRef]
- Chan, C.L.; McFann, K.; Taylor, L.; Wright, D.; Zeitler, P.; Barker, J. Congenital adrenal hyperplasia and the second newborn screen. J. Pediatr. 2013, 163, 109–113. [Google Scholar] [CrossRef]
- Votava, F.; Novotna, D.; Kracmar, P.; Vinohradska, H.; Stahlova-Hrabincova, E.; Vrzalová, Z.; Neumann, D.; Malikova, J.; Lebl, J.; Matern, D. Lessons learned from 5 years of newborn screening for congenital adrenal hyperplasia in the Czech Republic: 17-hydroxyprogesterone, genotypes, and screening performance. Eur. J. Nucl. Med. Mol. Imaging 2012, 171, 935–940. [Google Scholar] [CrossRef]
- Dörr, H.G.; Odenwald, B.; Nennstiel-Ratzel, U. Early diagnosis of children with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency by newborn screening. Int. J. Neonatal Screen. 2015, 1, 36–44. [Google Scholar] [CrossRef]
- Gidlöf, S.; Wedell, A.; Guthenberg, C.; von Döbeln, U.; Nordenström, A. nationwide neonatal screening for congenital adrenal hyperplasia in Sweden. JAMA Pediatr. 2014, 168, 567. [Google Scholar] [CrossRef]
- Van der Kamp, H.J.; Oudshoorn, C.G.M.; Elvers, B.H.; Van Baarle, M.; Otten, B.J.; Wit, J.; Verkerk, P.H. Cutoff levels of 17-α-hydroxyprogesterone in neonatal screening for congenital adrenal hyperplasia should be based on gestational age rather than on birth weight. J. Clin. Endocrinol. Metab. 2005, 90, 3904–3907. [Google Scholar] [CrossRef]
- Congenital adrenal hyperplasia (CAH) condition assessment summary—March 2019.pdf. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiH7MSEzpTrAhUqyosBHbNVBCQQFjAAegQIAhAB&url=http%3A%2F%2Fwww.cancerscreening.gov.au%2Finternet%2Fscreening%2Fpublishing.nsf%2FContent%2FC79A7D94CB73C56CCA257CEE0000EF35%2F%24File%2FCongenital%2520adrenal%2520hyperplasia%2520(CAH)%2520condition%2520assessment%2520summary%2520-%2520March%25202019.pdf&usg=AOvVaw0lv0qPSTCr6j5LW7p2zRCQ (accessed on 29 June 2020).
- Guran, T.; Tezel, B.; Gürbüz, F.; Eklioğlu, B.S.; Hatipoğlu, N.; Kara, C.; Şimşek, E.; Çizmecioğlu, F.M.; Ozon, A.; Baş, F.; et al. Neonatal screening for congenital adrenal hyperplasia in Turkey: A pilot study with 38,935 infants. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 13–23. [Google Scholar] [CrossRef]
- Choi, R.; Park, H.-D.; Oh, H.J.; Lee, K.; Song, J.; Lee, S. Dried blood spot multiplexed steroid profiling using liquid chromatography tandem mass spectrometry in Korean neonates. Ann. Lab. Med. 2019, 39, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bialk, E.R.; Lasarev, M.R.; Held, P.K. Wisconsin’s screening algorithm for the identification of newborns with congenital adrenal hyperplasia. Int. J. Neonatal Screen. 2019, 5, 33. [Google Scholar] [CrossRef]
- Tieh, P.Y.; Yee, J.K.; Hicks, R.; Mao, C.S.-M.; Lee, W.-N. Utility of a precursor-to-product ratio in the evaluation of presumptive positives in newborn screening of congenital adrenal hyperplasia. J. Perinatol. 2017, 37, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Boelen, A.; Ruiter, A.F. Determination of a steroid profile in heel prick blood using LC-MSMS. Bioanalysis 2016, 8, 375–384. [Google Scholar] [CrossRef]
- Janzen, N.; Peter, M.; Steuerwald, U.; Terhardt, M.; Holtkamp, U.; Sander, S. Newborn screening for congenital adrenal hyperplasia: Additional steroid profile using liquid chromatography-tandem mass spectrometry. J. Clin. Endocrinol. Metab. 2007, 92, 2581–2589. [Google Scholar] [CrossRef]
- Hicks, R.A.; Yee, J.K.; Mao, C.S.; Graham, S.; Kharrazi, M.; Lorey, F.; Lee, W.P. Precursor-to-product ratios reflect biochemical phenotype in congenital adrenal hyperplasia. Metabolomics 2014, 10, 123–131. [Google Scholar] [CrossRef]
- El-Maouche, D.; Arlt, W.; Merke, D.P. Congenital adrenal hyperplasia. Lancet 2017, 390, 2194–2210. [Google Scholar] [CrossRef]
- Pignatelli, D.; Carvalho, B.L.; Palmeiro, A.; Barros, A.; Guerreiro, S.G.; Maçut, D. The complexities in genotyping of congenital adrenal hyperplasia: 21-hydroxylase deficiency. Front. Endocrinol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Sarafoglou, K.; Lorentz, C.P.; Otten, N.; Oetting, W.S.; Grebe, S.K.G. Molecular testing in congenital adrenal hyperplasia due to 21?-hydroxylase deficiency in the era of newborn screening. Clin. Genet. 2012, 82, 64–70. [Google Scholar] [CrossRef]
- Turan, I.; Tastan, M.; Boga, D.D.; Gurbuz, F.; Kotan, L.D.; Tuli, A.; Yuksel, B. 21-Hydroxylase deficiency: Mutational spectrum and genotype–phenotype relations analyses by next-generation sequencing and multiplex ligation-dependent probe amplification. Eur. J. Med. Genet. 2019, 63, 103782. [Google Scholar] [CrossRef]

| Immuno-Assay | Results from Steroid Profiling | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case number | Sex | Birth Weight (kg) | Gestational Age (Days) | Initial DBS Sample Collection (*) | Initial DBS Sample Received Date (*) | 17OHP | MS17OHP | A4 | CORTISOL | Ratio | Initial Day of Notification (*) |
| 1 | I | 3.51 | 287 | 2 | 5 | >220 | 97 | 46 | 47 | 3 | 5 |
| 2 | F | 3.3 | 280 | 2 | 4 | >220 | >250 | 150 | 18 | >22 | 4 |
| 3 | F/tw2 | 1.66 | 238 | 2 | 7 | >220 | 173 | 11 | 73 | 3 | 9 |
| 4 | M | 4.37 | 284 | 2 | 6 | >220 | 228 | 148 | 23 | 16 | 6 |
| 5 | F | 2.91 | 266 | 2 | 6 | >220 | 208 | 64 | 49 | 6 | 6 |
| 6 | M | 1.58 | 216 | 3 | 6 | >220 | 403 | 30 | 31 | 14 | 6 |
| 7 | M | 3.97 | 266 | 2 | 4 | >220 | 455 | 139 | 810 | 1 | 4 |
| 8 | M | 1.58 | 280 | 3 | 6 | >220 | 403 | 30 | 31 | 14 | 6 |
| 9 | F | 0.68 | 175 | 2 | 5 | 100.3 | 104 | 51 | 63 | 2 | 5 |
| 10 | M | 4.5 | 287 | 3 | 8 | >220 | 136 | 49 | 8 | 23 | 8 |
| 11 | M | 4.35 | 280 | 3 | 6 | >220 | 364 | 297 | 26 | 25 | 6 |
| 12 | F | 2.55 | 238 | 2 | 6 | 90.7 | 46.1 | 7.1 | 22.7 | 2 | 6 |
| 13 | M | 2.03 | 252 | 2 | 7 | 45 | 34.8 | 4 | 10.5 | 4 | 7 |
| 14 | M | 0.49 | 175 | 3 | 6 | 180 | 55.2 | 13 | 24 | 3 | 6 |
| Case Number | Na * | K * | Glucose * | 17OHP ** | A4 ** | CORTISOL ** | TESTOSTERONE ** | Family History | Symptoms | Diagnosis Suspected before Notification | Final Diagnosis | Treatment Commencement Day (*) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 141 | 4.9 | 234 | 320 | 5.8 | N | virilisation | Y | SW CAH | 5 | ||
| 2 | 119 | 7.3 | 3.8 | 680 | 130 | 61 | N | poor weight gain | N | SW CAH | 11 | |
| 3 | 130 | 6.1 | 30.3 | N | N | SW CAH | 9 | |||||
| 4 | 136 | 5.8 | 4.2 | 212 | >40 | 86 | 26.7 | N | N | SW CAH | 7 | |
| 5 | 132 | 8 | 4.6 | 175 | 25 | 87 | 2.2 | N | hypotension (associated with acute respiratory illness) virilisation | Y | SW CAH | 7 |
| 6 | 133 | 5.7 | 3.8 | >460 | >37 | 104 | 51.4 | N | poor feeding, preterm | N | SW CAH | 8 |
| 7 | 136 | 5.3 | 652 | 88 | Y | mild scrotal-transient, excess pigmentation | Y | SV CAH | 2 | |||
| 8 | 133 | 5.7 | 3.8 | >460 | >37 | 104 | 51 | N | poor feeding, preterm | N | SW CAH | 8 |
| 9 | NFT | |||||||||||
| 10 | 135 | 5.5 | 5 | 340 | >38 | 31 | 3.5 | N | N | SW CAH | 10 | |
| 11 | 132 | 7 | 498 | 64 | N | lethargy | N | SW CAH | 20 | |||
| 12 | 135 | 5.6 | 6.5 | 13 | 258 | NFT | ||||||
| 13 | NFT | |||||||||||
| 14 | NFT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, F.; Srinivasan, S.; Wiley, V. Evaluation of a Two-Tier Screening Pathway for Congenital Adrenal Hyperplasia in the New South Wales Newborn Screening Programme. Int. J. Neonatal Screen. 2020, 6, 63. https://doi.org/10.3390/ijns6030063
Lai F, Srinivasan S, Wiley V. Evaluation of a Two-Tier Screening Pathway for Congenital Adrenal Hyperplasia in the New South Wales Newborn Screening Programme. International Journal of Neonatal Screening. 2020; 6(3):63. https://doi.org/10.3390/ijns6030063
Chicago/Turabian StyleLai, Fei, Shubha Srinivasan, and Veronica Wiley. 2020. "Evaluation of a Two-Tier Screening Pathway for Congenital Adrenal Hyperplasia in the New South Wales Newborn Screening Programme" International Journal of Neonatal Screening 6, no. 3: 63. https://doi.org/10.3390/ijns6030063
APA StyleLai, F., Srinivasan, S., & Wiley, V. (2020). Evaluation of a Two-Tier Screening Pathway for Congenital Adrenal Hyperplasia in the New South Wales Newborn Screening Programme. International Journal of Neonatal Screening, 6(3), 63. https://doi.org/10.3390/ijns6030063




