Newborn Screening Protocols and Positive Predictive Value for Congenital Adrenal Hyperplasia Vary across the United States
Abstract
1. Introduction
2. Materials and Methods
Statistical Methods
3. Results
3.1. Epidemiology
3.2. Assays and Cut-Off Points
3.3. Post-Analytical Procedures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CAH | Congenital adrenal hyperplasia |
| CAH-21 | CAH due to steroid 21-hydroxylase deficiency |
| 17OHP | 17-hydroxyprogesterone |
| DSD-TRN | Disorders/Differences of Sex Development Translational Research Network |
| PPV | Positive predictive value |
| IQR | Interquartile range |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
References
- Eunice Kennedy Shriver National Institute of Child Health and Human Development. Available online: https://www.nichd.nih.gov/health/topics/newborn/conditioninfo/infants-screened (accessed on 22 April 2020).
- Speiser, P.W.; Arlt, W.; Auchus, R.J.; Baskin, L.S.; Conway, G.S.; Merke, D.P.; Meyer-Bahlburg, H.F.L.; Miller, W.L.; Murad, M.H.; Oberfield, S.E.; et al. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 4043–4088. [Google Scholar] [CrossRef]
- Gidlof, S.; Wedell, A.; Guthenberg, C.; von, D.U.; Nordenstrom, A. Nationwide neonatal screening for congenital adrenal hyperplasia in sweden: A 26-year longitudinal prospective population-based study. JAMA Pediatr. 2014, 168, 567–574. [Google Scholar] [CrossRef]
- Gidlof, S.; Falhammar, H.; Thilen, A.; von, D.U.; Ritzen, M.; Wedell, A.; Nordenstrom, A. One hundred years of congenital adrenal hyperplasia in Sweden: A retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 2013, 1, 35–42. [Google Scholar] [CrossRef]
- van der Linde, A.A.A.; Schönbeck, Y.; van der Kamp, H.J.; van den Akker, E.L.T.; van Albada, M.E.; Boelen, A.; Finken, M.J.J.; Hannema, S.E.; Hoorweg-Nijman, G.; Odink, R.J.; et al. Evaluation of the Dutch neonatal screening for congenital adrenal hyperplasia. Arch. Dis. Child 2019, 104, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Guran, T.; Tezel, B.; Gurbuz, F.; Selver Eklioglu, B.; Hatipoglu, N.; Kara, C.; Simsek, E.; Cizmecioglu, F.; Ozon, A.; Bas, F.; et al. Neonatal screening for congenital adrenal hyperplasia in Turkey: A pilot study with 38,935 infants. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, P.G.; Brosnan, C.A.; Kemp, S.F.; Domek, D.B.; Jelley, D.H.; Blackett, P.R.; Riley, W.J. Effect of Newborn Screening for Congenital Adrenal Hyperplasia. Arch. Pediatr. Adolesc. Med. 1999, 153, 1272. [Google Scholar] [CrossRef] [PubMed]
- Thilen, A.; Nordenstrom, A.; Hagenfeldt, L.; von Dobeln, U.; Guthenberg, C.; Larsson, A. Benefits of Neonatal Screening for Congenital Adrenal Hyperplasia (21-Hydroxylase Deficiency) in Sweden. Pediatrics 1998, 101, e11. [Google Scholar] [CrossRef] [PubMed]
- Van der Kamp, H.J.; Noordam, K.; Elvers, B.; Van Baarle, M.; Otten, B.J.; Verkerk, P.H. Newborn screening for congenital adrenal hyperplasia in the Netherlands. Pediatrics 2001, 108, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Nakamura, A.; Fujikura, K.; Fukushi, M.; Hotsubo, T.; Miyata, J.; Ishizu, K.; Tajima, T. Results from 28 years of newborn screening for congenital adrenal hyperplasia in sapporo. Clin. Pediatr. Endocrinol. 2014, 23, 35–43. [Google Scholar] [CrossRef][Green Version]
- Nass, R.; Baker, S. Learning disabilities in children with congenital adrenal hyperplasia. J. Child Neurol. 1991, 6, 306–312. [Google Scholar] [CrossRef]
- Inozemtseva, O.; Matute, E.; Juárez, J. Learning disabilities spectrum and sexual dimorphic abilities in girls with congenital adrenal hyperplasia. J. Child Neurol. 2008, 23, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A.; Witchel, S.F. 46,XX patients with congenital adrenal hyperplasia: Initial assignment as male, reassigned female. J. Pediatr. Endocrinol. Metab. 2005, 18, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Apóstolos, R.A.C.; Canguçu-Campinho, A.K.; Lago, R.; Costa, A.C.S.; Oliveira, L.M.B.; Toralles, M.B.; Barroso, U. Gender Identity and Sexual Function in 46,XX Patients with Congenital Adrenal Hyperplasia Raised as Males. Arch. Sex. Behav. 2018, 47, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, K.; Elamin, M.B.; Smushkin, G.; Murad, M.H.; Lampropulos, J.F.; Elamin, K.B.; Abu Elnour, N.O.; Gallegos-Orozco, J.F.; Fatourechi, M.M.; Agrwal, N.; et al. Clinical review: Adult height in patients with congenital adrenal hyperplasia: A systematic review and metaanalysis. J. Clin. Endocrinol. Metab. 2010, 95, 4161–4172. [Google Scholar] [CrossRef]
- Wilson, R.C.; Mercado, A.B.; Cheng, K.C.; New, M.I. Steroid 21-hydroxylase deficiency: Genotype may not predict phenotype. J. Clin. Endocrinol. Metab. 1995, 80, 2322–2329. [Google Scholar]
- White, P.C. Optimizing newborn screening for congenital adrenal hyperplasia. J. Pediatr. 2013, 163, 10–12. [Google Scholar] [CrossRef]
- Held, P.K.; Shapira, S.K.; Hinton, C.F.; Jones, E.; Hannon, W.H.; Ojodu, J. Congenital adrenal hyperplasia cases identified by newborn screening in one- and two-screen states. Mol. Genet. Metab. 2015, 116, 133–138. [Google Scholar] [CrossRef]
- Pearce, M.; DeMartino, L.; McMahon, R.; Hamel, R.; Maloney, B.; Stansfield, D.M.; McGrath, E.C.; Occhionero, A.; Gearhart, A.; Caggana, M.; et al. Newborn screening for congenital adrenal hyperplasia in New York State. Mol. Genet. Metab. Rep. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Tsuji, A.; Konishi, K.; Hasegawa, S.; Anazawa, A.; Onishi, T.; Ono, M.; Morio, T.; Kitagawa, T.; Kashimada, K. Newborn screening for congenital adrenal hyperplasia in Tokyo, Japan from 1989 to 2013: A retrospective population-based study. BMC Pediatr. 2015, 15, 209. [Google Scholar] [CrossRef]
- Pode-Shakked, N.; Blau, A.; Pode-Shakked, B.; Tiosano, D.; Weintrob, N.; Eyal, O.; Zung, A.; Levy-Khademi, F.; Tenenbaum-Rakover, Y.; Zangen, D.; et al. Combined gestational age- and birth weight-adjusted cutoffs for newborn screening of congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2019, 104, 3172–3180. [Google Scholar] [CrossRef]
- Monostori, P.; Szabo, P.; Marginean, O.; Bereczki, C.; Karg, E. Concurrent confirmation and differential diagnosis of congenital adrenal hyperplasia from dried blood spots: Application of a second-tier LC-MS/MS assay in a cross-border cooperation for newborn screening. Horm. Res. Paediatr. 2015, 84, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Gaudl, A.; Kratzsch, J.; Ceglarek, U. Advancement in steroid hormone analysis by LC-MS/MS in clinical routine diagnostics-A three year recap from serum cortisol to dried blood 17α-hydroxyprogesterone. J. Steroid Biochem. Mol. Biol. 2019, 192, 105389. [Google Scholar] [CrossRef] [PubMed]
- Bialk, E.; Lasarev, M.R.; Held, P.K. Wisconsin’s screening algorithm for the identification of newborns with congenital adrenal hyperplasia. Int. J. Neonatal Screen. 2019, 5, 33. [Google Scholar] [CrossRef]
- Martínez-Morillo, E.; Prieto García, B.; Álvarez Menéndez, F.V. Challenges for Worldwide Harmonization of Newborn Screening Programs. Clin. Chem. 2016, 62, 689–698. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lloyd-Puryear, M.; Brower, A.; Berry, S.A.; Brosco, J.P.; Bowdish, B.; Watson, M.S. Foundation of the Newborn Screening Translational Research Network and its tools for research. Genet. Med. 2019, 21, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.L.; McFann, K.; Taylor, L.; Wright, D.; Zeitler, P.S.; Barker, J.M. Congenital adrenal hyperplasia and the second newborn screen. J. Pediatr. 2013, 163, 109–113.e101. [Google Scholar] [CrossRef] [PubMed]
- Sarafoglou, K.; Banks, K.; Gaviglio, A.; Hietala, A.; McCann, M.; Thomas, W. Comparison of one-tier and two-tier newborn screening metrics for congenital adrenal hyperplasia. Pediatrics 2012, 130, e1261–e1268. [Google Scholar] [CrossRef]
- Glidewell, J.; Grosse, S.D.; Riehle-Colarusso, T.; Pinto, N.; Hudson, J.; Daskalov, R.; Gaviglio, A.; Darby, E.; Singh, S.; Sontag, M. Actions in Support of Newborn Screening for Critical Congenital Heart Disease-United States, 2011–2018. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 107–111. [Google Scholar] [CrossRef]
- Sandberg, D.E.; Gardner, M.; Callens, N.; Mazur, T.; the DSD-TRN Psychosocial Workgroup; the DSD-TRN Advocacy Advisory Network; Accord Alliance. Interdisciplinary care in disorders/differences of sex development (DSD): The psychosocial component of the DSD-Translational research network. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 279–292. [Google Scholar] [CrossRef]
- Délot, E.C.; Papp, J.C.; Sandberg, D.E.; Vilain, E.; Workgroup, D.-T.G. Genetics of Disorders of Sex Development: The DSD-TRN Experience. Endocrinol. Metab. Clin. N. Am. 2017, 46, 519–537. [Google Scholar] [CrossRef]
- Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guidelines-Third Edition CLSI Document (2010), 3rd ed.; CLSI: Wayne, PA, USA, 2010; Volume EP28-A3c.
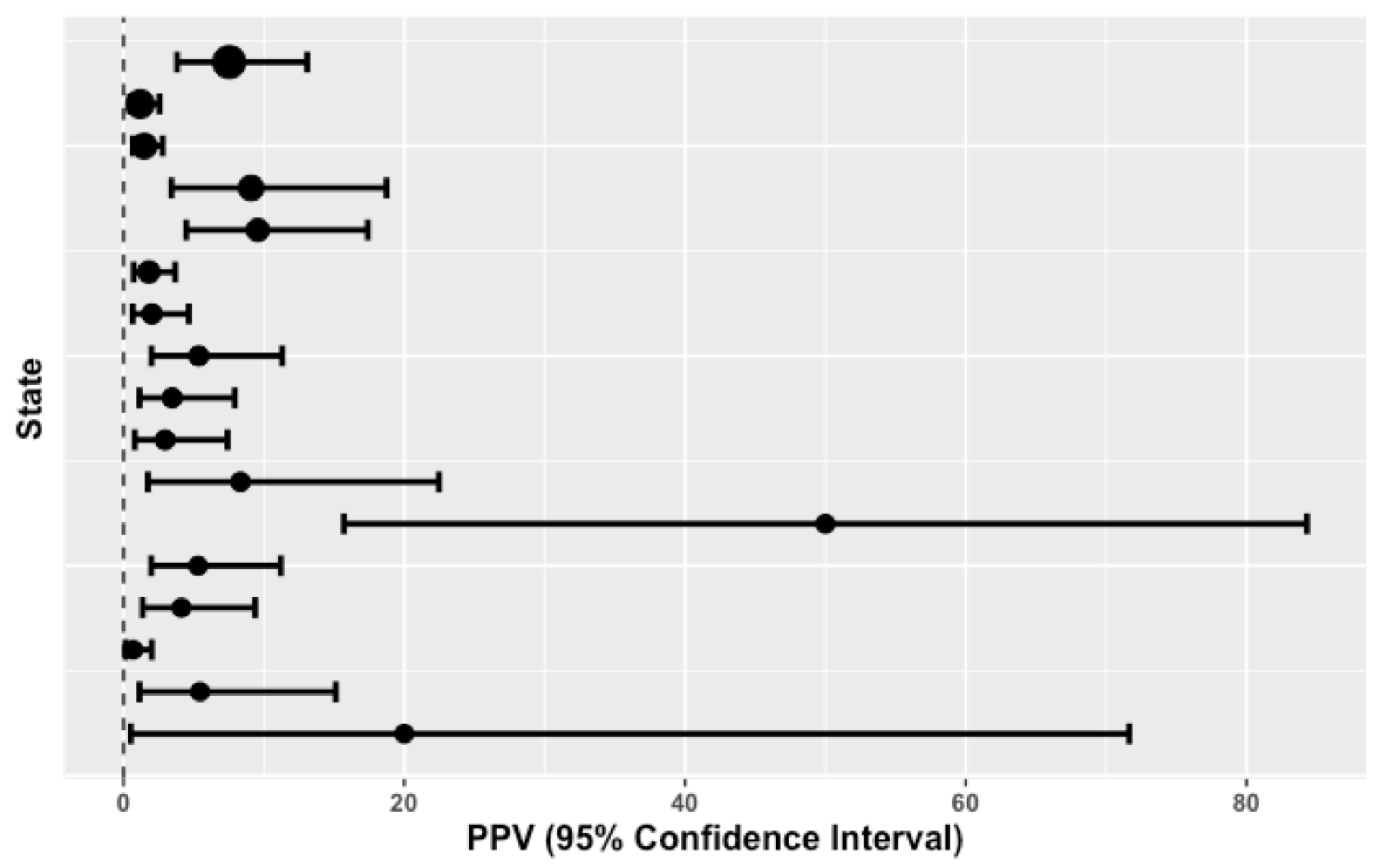
| #Screened (Total = 1,564,756) | #Referred Positive (Total = 3217) | #Confirmed CAH (Total = 93) | Prevalence (Weighted Mean = 1:16,825) | PPV (95% CI) IQR = [2.02 to 8.33] | Birth Weight Cut-Off Point (Grams) | 17OHP Cut-Off Point (ng/mL) (Mean = 41.2) | Two-Screens Mandate |
|---|---|---|---|---|---|---|---|
| 230,431 | 146 | 11 | 1:20,948 | 7.53 (3.82–13.08) | >2251 | 35 | N |
| 171,964 | 506 | 6 | 1:28,661 | 1.19 (0.44–2.56) | >2500 | 55 | N |
| 138,226 | 608 | 9 | 1:15,358 | 1.48 (0.68–2.79) | >2500 | 70 | N |
| 135,590 | 66 | 6 | 1:22,598 | 9.09 (3.41–18.74) | >2500 | 30 | N |
| 109,740 | 94 | 9 | 1:12,193 | 9.57 (4.47–17.4) | >2500 | 65 | N |
| 104,000 | 387 | 7 | 1:14,857 | 1.81 (0.73–3.69) | >2250 | 25 | N |
| 84,000 | 247 | 5 | 1:16,800 | 2.02 (0.66–4.66) | >2500 | 30 | N |
| 81,117 | 112 | 6 | 1:13,520 | 5.36 (1.99–11.30) | >2500 | 50 | Y |
| 79,948 | 144 | 5 | 1:15,990 | 3.47 (1.14–7.92) | >2249 | 37 | N |
| 79,000 | 135 | 4 | 1:19,750 | 2.96 (0.81–7.41) | ≥2500 | 35 | Y |
| 72,440 | 36 | 3 | 1:24,147 | 8.33 (1.75–22.47) | >2251 | 75 | N |
| 61,500 | 8 | 4 | 1:15,375 | 50.00 (15.70–84.30) | >2500 | 25 | Y |
| 59,643 | 113 | 6 | 1:9941 | 5.31 (1.97–11.2) | No values given | N | |
| 55,935 | 121 | 5 | 1:11,187 | 4.13 (1.36–9.38) | >2500 | 25 | N |
| 53,361 | 434 | 3 | 1:17,787 | 0.69 (0.14–2.01) | >2500 | 30 | N |
| 36,361 | 55 | 3 | 1:12,120 | 5.45 (1.14–15.12) | ≥2500 | 38.3 | N |
| 11,500 | 5 | 1 | 1:11,500 | 20.00 (0.51–71.64) | ≥2300 | 35 | Y |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speiser, P.W.; Chawla, R.; Chen, M.; Diaz-Thomas, A.; Finlayson, C.; Rutter, M.M.; Sandberg, D.E.; Shimy, K.; Talib, R.; Cerise, J.; et al. Newborn Screening Protocols and Positive Predictive Value for Congenital Adrenal Hyperplasia Vary across the United States. Int. J. Neonatal Screen. 2020, 6, 37. https://doi.org/10.3390/ijns6020037
Speiser PW, Chawla R, Chen M, Diaz-Thomas A, Finlayson C, Rutter MM, Sandberg DE, Shimy K, Talib R, Cerise J, et al. Newborn Screening Protocols and Positive Predictive Value for Congenital Adrenal Hyperplasia Vary across the United States. International Journal of Neonatal Screening. 2020; 6(2):37. https://doi.org/10.3390/ijns6020037
Chicago/Turabian StyleSpeiser, Phyllis W., Reeti Chawla, Ming Chen, Alicia Diaz-Thomas, Courtney Finlayson, Meilan M. Rutter, David E. Sandberg, Kim Shimy, Rashida Talib, Jane Cerise, and et al. 2020. "Newborn Screening Protocols and Positive Predictive Value for Congenital Adrenal Hyperplasia Vary across the United States" International Journal of Neonatal Screening 6, no. 2: 37. https://doi.org/10.3390/ijns6020037
APA StyleSpeiser, P. W., Chawla, R., Chen, M., Diaz-Thomas, A., Finlayson, C., Rutter, M. M., Sandberg, D. E., Shimy, K., Talib, R., Cerise, J., Vilain, E., Délot, E. C., & , on behalf of the Disorders/Differences of Sex Development-Translational Research Network (DSD-TRN). (2020). Newborn Screening Protocols and Positive Predictive Value for Congenital Adrenal Hyperplasia Vary across the United States. International Journal of Neonatal Screening, 6(2), 37. https://doi.org/10.3390/ijns6020037






