The Combined Impact of CLIR Post-Analytical Tools and Second Tier Testing on the Performance of Newborn Screening for Disorders of Propionate, Methionine, and Cobalamin Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Methods
2.2. Reference Ranges
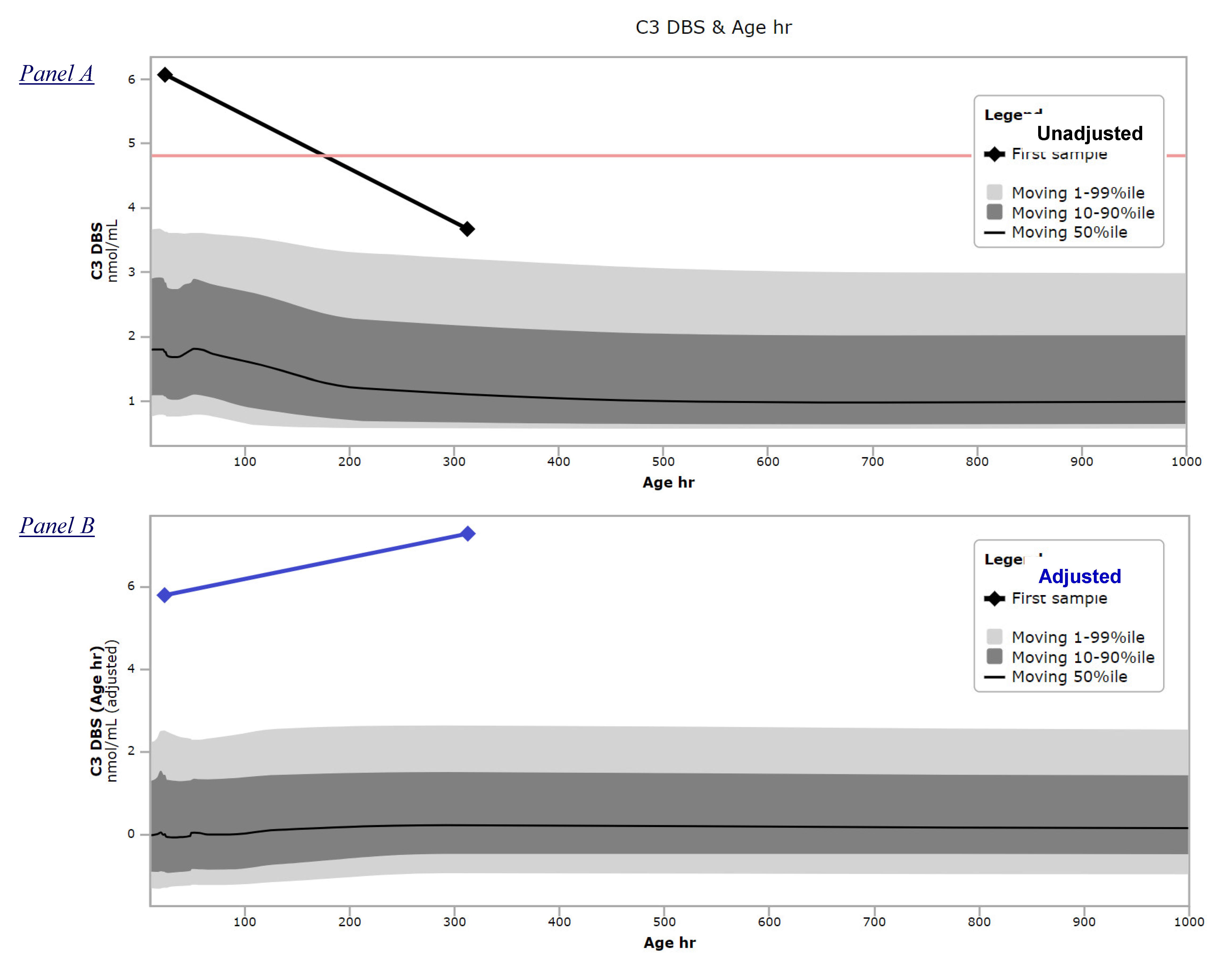
2.3. Covariate Adjustments
2.4. Disease Ranges
2.5. CLIR Tools
3. Results
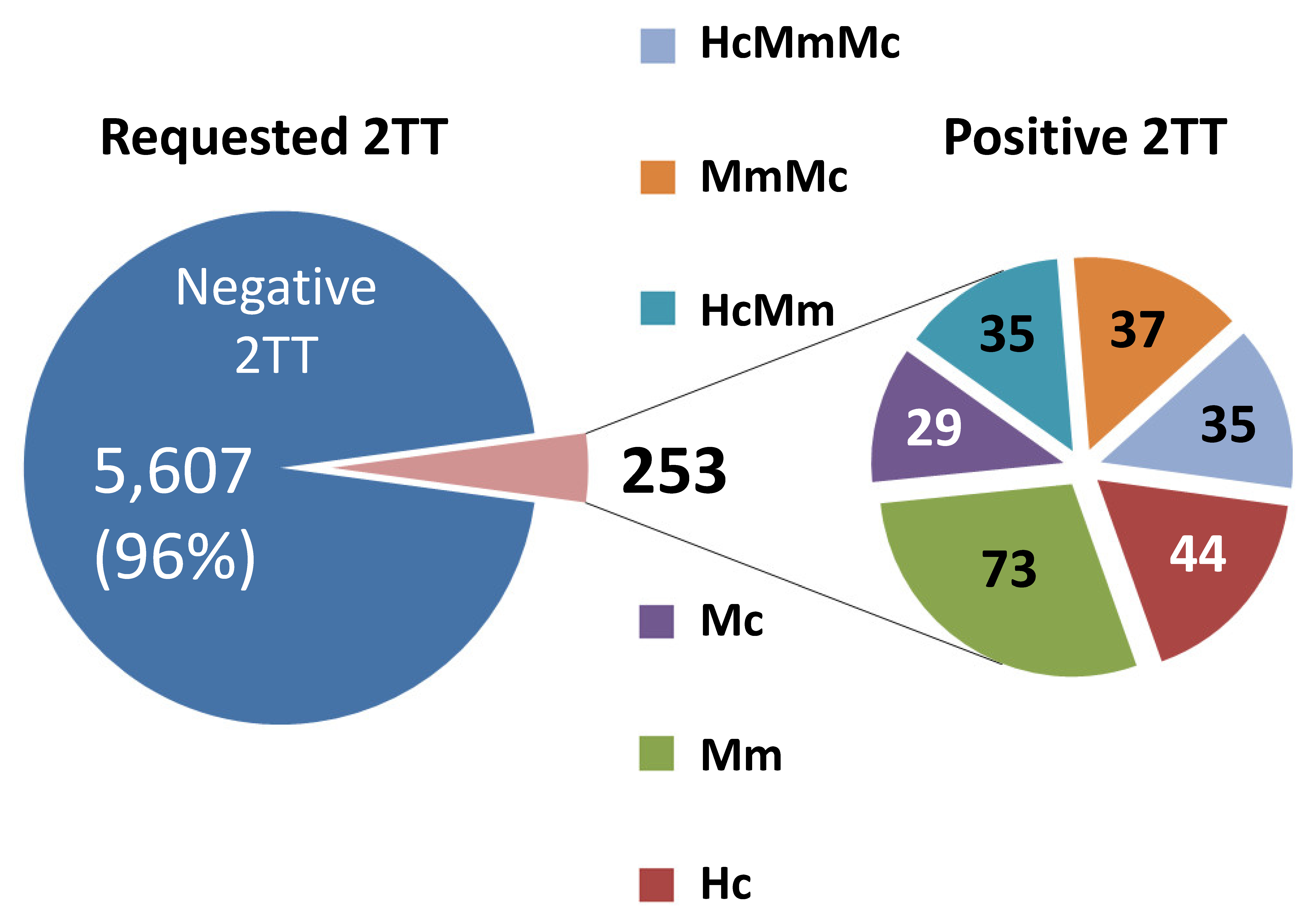
3.1. Outcome of Second Tier Test
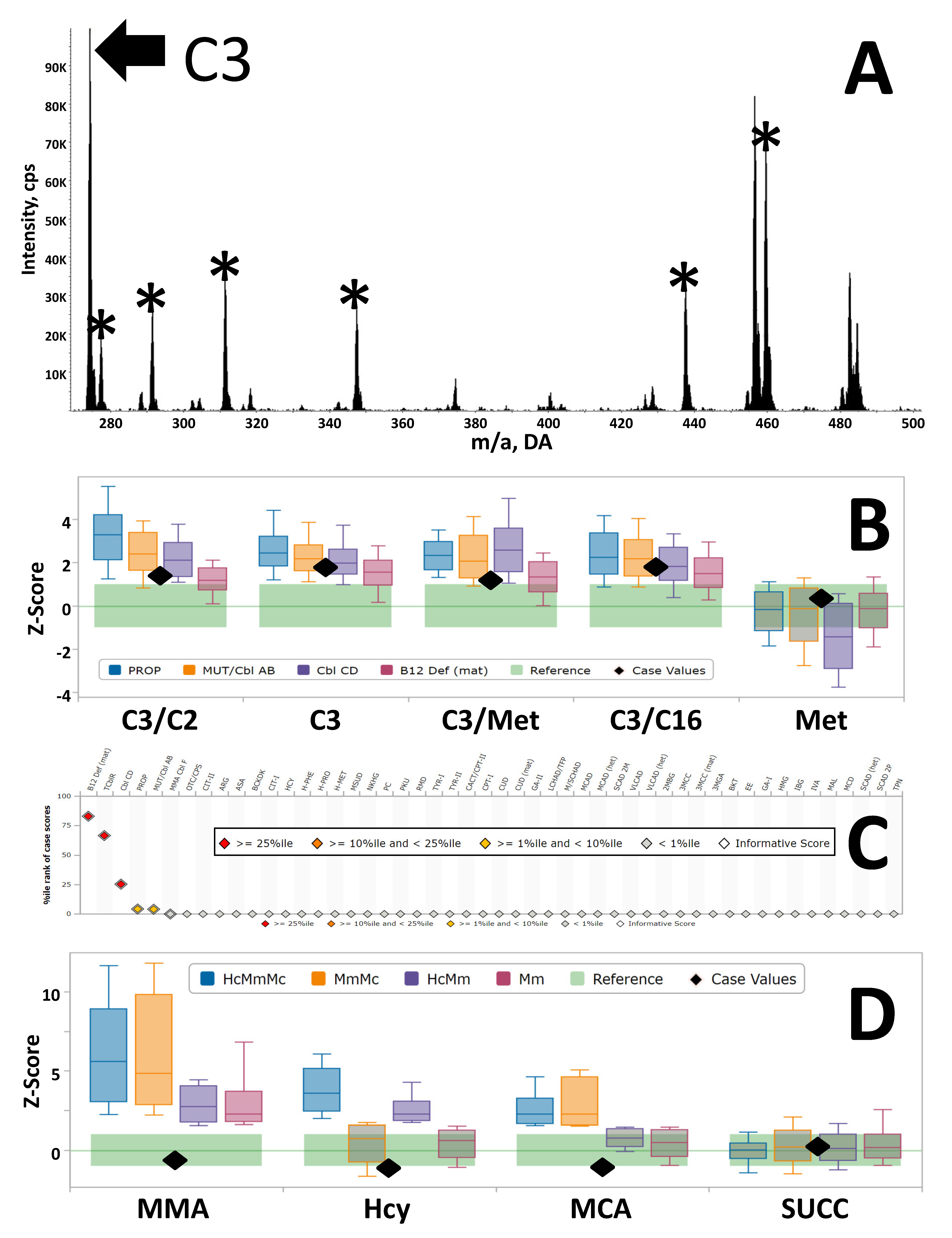
3.2. Example of Clinical Utility
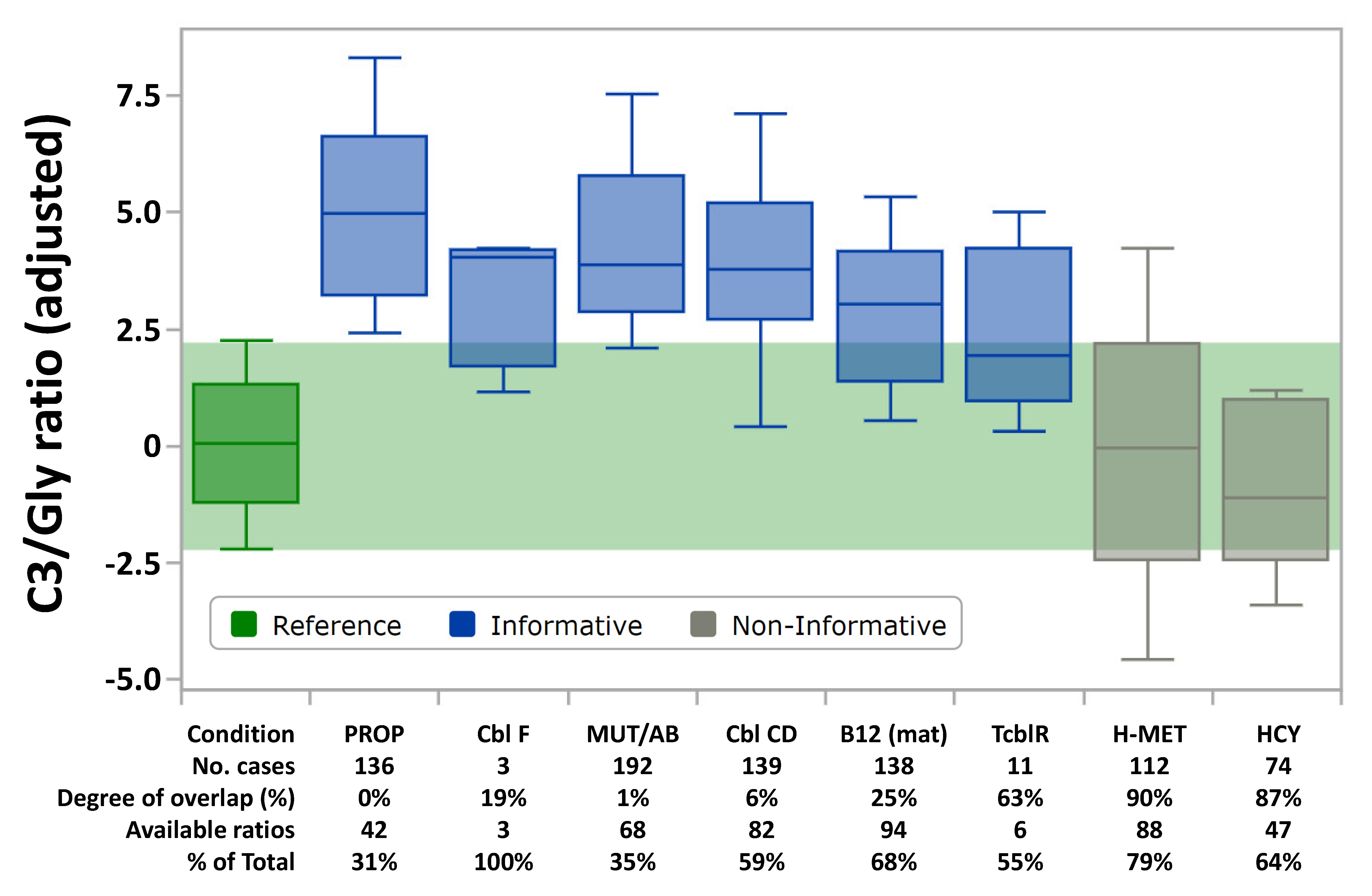
3.3. Distribution of Abnormal Cases
3.4. Actual and Potential Cost Savings
3.5. The Price of Being Wrong
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chace, D.H.; Kalas, T.A.; Naylor, E.W. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin. Chem. 2003, 49, 1797–1817. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.S.; Mann, M.Y.; Lloyd-Puryear, M.A.; Rinaldo, P.; Howell, R.R. Newborn screening: Toward a uniform screening panel and system [Executive summary]. Genet. Med. 2006, 8, 1S–11S. [Google Scholar] [CrossRef] [PubMed]
- HRSA Advisory Committee for Heritable Disorders. Available online: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders (accessed on 31 January 2020).
- McHugh, D.M.; Cameron, C.A.; Abdenur, J.E.; Abdulrahman, M.; Adair, O.; Al Nuaimi, S.A.; Ahlman, H.; Allen, J.J.; Antonozzi, I.; Archer, S.; et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: A worldwide collaborative project. Genet. Med. 2011, 13, 230–254. [Google Scholar] [CrossRef] [PubMed]
- Tarini, B.A.; Christakis, D.A.; Welch, H.G. State newborn screening in the tandem mass spectrometry era: More tests, more false-positive results. Pediatrics 2006, 118, 448–456. [Google Scholar] [CrossRef]
- Peterschmitt, M.J.; Simmons, J.R.; Levy, H.L. Reduction of false negative results in screening of newborns for homocystinuria. N. Engl. J. Med. 1999, 341, 1572–1576. [Google Scholar] [CrossRef]
- Keller, R.; Chrastina, P.; Pavlíková, M.; Gouveia, S.; Ribes, A.; Kölker, S.; Blom, H.J.; Baumgartner, M.R.; Bártl, J.; Dionisi-Vici, C.; et al. Newborn screening for homocystinurias: Recent recommendations versus current practice. J. Inherit. Metab. Dis. 2019, 42, 128–139. [Google Scholar] [CrossRef]
- Magera, M.J.; Gunawardena, N.D.; Hahn, S.H.; Tortorelli, S.; Mitchell, G.A.; Goodman, S.I.; Rinaldo, P.; Matern, D. Rapid quantitative determination of succinylacetone in dried blood spots by liquid chromatography tandem mass spectrometry. Mol. Genet. Metab. 2006, 88, 16–21. [Google Scholar] [CrossRef]
- Matern, D.; Tortorelli, S.; Oglesbee, D.; Gavrilov, D.; Rinaldo, P. Reduction of the false positive rate in newborn screening by implementation of MS/MS-based second tier tests: The Mayo Clinic experience (2004–2007). J. Inherit. Metab. Dis. 2007, 30, 585–592. [Google Scholar] [CrossRef]
- Turgeon, C.T.; Magera, M.J.; Allard, P.; Tortorelli, S.; Gavrilov, D.; Oglesbee, D.; Raymond, K.; Rinaldo, P.; Matern, D. Combined newborn screening for succinylacetone, amino acids, and acylcarnitines in dried blood spots. Clin. Chem. 2008, 54, 657–664. [Google Scholar] [CrossRef]
- Huemer, M.; Baumgartner, M.R. The clinical presentation of cobalamin-related disorders: From acquired deficiencies to inborn errors of absorption and intracellular pathways. J. Inherit. Metab. Dis. 2019, 42, 686–705. [Google Scholar] [CrossRef]
- Mudd, S.H. Hypermethioninemias of genetic and non-genetic origin: A review. Am. J. Med. Genet. C Semin. Med. Genet. 2011, 157C, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Tortorelli, S.; Bishop, L.; Sellars, E.A.; Schimmenti, L.A.; Gallant, N.; Prada, C.E.; Hopkin, R.J.; Leslie, N.D.; Berry, S.A.; et al. Outcomes of four patients with homocysteine remethylation disorders detected by newborn screening. Genet. Med. 2016, 18, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Tortorelli, S.; Turgeon, C.T.; Lim, J.S.; Baumgart, S.; Day-Salvatore, D.L.; Abdenur, J.; Bernstein, J.A.; Lorey, F.; Lichter-Konecki, U.; Oglesbee, D.; et al. Two-tier approach to the newborn screening of methylenetetrahydrofolate reductase deficiency and other remethylation disorders with tandem mass spectrometry. J. Pediatr. 2010, 157, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Waisbren, S.E.; Albers, S.; Amato, S.; Ampola, M.; Brewster, T.G.; Demmer, L.; Eaton, R.B.; Greenstein, R.; Korson, M.; Larson, C.; et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA 2003, 290, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, P.; Zafari, S.; Tortorelli, S.; Matern, D. Making the case for objective performance metrics in newborn screening by tandem mass spectrometry. Ment. Retard. Dev. Disabil. Res. Rev. 2006, 12, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, G.; Currier, R.; McHugh, D.M.; Gavrilov, D.; Magera, M.J.; Matern, D.; Oglesbee, D.; Raymond, K.; Rinaldo, P.; Smith, E.H.; et al. Enhanced interpretation of newborn screening results without analyte cutoff values. Genet. Med. 2012, 14, 648–655. [Google Scholar] [CrossRef]
- Hall, P.L.; Marquardt, G.; McHugh, D.M.; Currier, R.J.; Tang, H.; Stoway, S.D.; Rinaldo, P. Postanalytical tools improve performance of newborn screening by tandem mass spectrometry. Genet. Med. 2014, 16, 889–895. [Google Scholar] [CrossRef]
- Minter Baerg, M.M.; Stoway, S.D.; Hart, J.; Mott, L.; Peck, D.S.; Nett, S.L.; Eckerman, J.S.; Lacey, J.M.; Turgeon, C.T.; Gavrilov, D.; et al. Precision newborn screening for lysosomal disorders. Genet. Med. 2018, 20, 847–854. [Google Scholar] [CrossRef]
- Turgeon, C.T.; Magera, M.J.; Cuthbert, C.D.; Loken, P.; Gavrilov, D.K.; Tortorelli, S.; Raymond, K.; Oglesbee, D.; Rinaldo, P.; Matern, D. Simultaneous determination of total homocysteine, methylmalonic acid, and 2-methylcitric acid in dried blood spots by tandem mass spectrometry. Clin. Chem. 2010, 56, 1686–1695. [Google Scholar] [CrossRef]
- Mørkrid, L.; Rowe, A.D.; Elgstoen, K.B.P.; Olesen, J.H.; Ruijter, G.; Hall, P.L.; Tortorelli, S.; Schulze, A.; Kyriakopoulou, L.; Wamelink, M.M.C.; et al. Continuous age- and gender-adjusted reference intervals of urinary markers for cerebral creatine deficiency syndromes: A novel approach to the definition of reference intervals. Clin. Chem. 2015, 61, 760–768. [Google Scholar]
- Peng, G.; Shen, P.; Gandotra, N.; Le, A.; Fung, E.; Jelliffe-Pawlowski, L.; Davis, R.W.; Enns, G.M.; Zhao, H.; Cowan, T.M.; et al. Combining newborn metabolic and DNA analysis for second-tier testing of methylmalonic acidemia. Genet. Med. 2019, 21, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.M.; van Dyck, P.C.; Rinaldo, P.; McDonald, C.; Howell, R.R.; Zuckerman, A.; Downing, G. Improving newborn screening laboratory test ordering and result reporting using health information exchange. J. Am. Med. Inform. Assoc. 2010, 17, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Peck, D.S.; Lacey, J.M.; White, A.L.; Pino, G.; Studinski, A.; Fisher, R.; Ahmad, A.; Spencer, L.; Viall, S.; Shallow, N.; et al. Incorporation of second tier biomarker testing improves the specificity of newborn screening for Mucopolysaccharidosis type I. Int. J. Neonatal Screen. 2020, 6, 10. [Google Scholar] [CrossRef]
- American College of Medical Genetics and Genomics. ACT Sheets and Confirmatory Algorithms; American College of Medical Genetics: Bethesda, MD, USA, 2001. Available online: http://www.ncbi.nlm.nih.gov/books/NBK55832/PubMed (accessed on 8 April 2020).
- Gabler, E. The Price of Being Wrong. Milwaukee J. Sentin. 2016. Available online: http://projects.jsonline.com/news/2016/12/11/the-price-of-being-wrong.html (accessed on 4 February 2020).
- La Marca, G.; Malvagia, S.; Pasquini, E.; Innocenti, M.; Donati, M.A.; Zammarchi, E. Rapid 2nd-tier test for measurement of 3-OH-propionic and methylmalonic acids on dried blood spots: Reducing the false-positive rate for propionylcarnitine during expanded newborn screening by liquid chromatography-tandem mass spectrometry. Clin. Chem. 2007, 53, 1364–1369. [Google Scholar] [CrossRef]
- Shigematsu, Y.; Hata, I.; Tajima, G. Useful second-tier tests in expanded newborn screening of isovaleric acidemia and methylmalonic aciduria. J. Inherit. Metab. Dis. 2010, 33 (Suppl. 2), S283–S288. [Google Scholar] [CrossRef]
- Fu, X.; Xu, Y.K.; Chan, P.; Pattengale, P.K. Simple, fast, and simultaneous detection of plasma total homocysteine, methylmalonic acid, methionine, and 2-methylcitric acid using liquid chromatography and mass spectrometry (LC/MS/MS). JIMD Rep. 2013, 10, 69–78. [Google Scholar]
- Scolamiero, E.; Villani, G.R.; Ingenito, L.; Pecce, R.; Albano, L.; Caterino, M.; di Girolamo, M.G.; Di Stefano, C.; Franzese, I.; Gallo, G.; et al. Maternal vitamin B12 deficiency detected in expanded newborn screening. Clin. Biochem. 2014, 47, 312–317. [Google Scholar] [CrossRef]
- Al-Dirbashi, O.Y.; McIntosh, N.; McRoberts, C.; Fisher, L.; Rashed, M.S.; Makhseed, N.; Geraghty, M.T.; Santa, T.; Chakraborty, P. Analysis of methylcitrate in dried blood spots by liquid chromatography-tandem mass spectrometry. JIMD Rep. 2014, 16, 65–73. [Google Scholar]
- Gramer, G.; Fang-Hoffmann, J.; Feyh, P.; Klinke, G.; Monostori, P.; Mütze, U.; Posset, R.; Weiss, K.H.; Hoffmann, G.F.; Okun, J.G. Newborn screening for vitamin B12 deficiency in Germany—Strategies, results, and public health implications. J. Pediatr. 2020, 216, 165–172. [Google Scholar] [CrossRef]
- Maase, R.; Skrinska, V.; Younes, N.; Hassan, L.; Mitri, R.; Matern, D.; Rinaldo, P.; Turgeon, C. A rapid screening method for the measurement of neonatal total homocysteine in dried blood spots by liquid chromatography-tandem mass spectrometry. Int. J. Neonatal Screen. 2017, 3, 32. [Google Scholar] [CrossRef]
- Oglesbee, D.; Sanders, K.A.; Lacey, J.M.; Magera, M.J.; Casetta, B.; Strauss, K.A.; Tortorelli, S.; Rinaldo, P.; Matern, D. 2nd-tier test for quantification of alloisoleucine and branched-chain amino acids in dried blood spots to improve newborn screening for maple syrup urine disease (MSUD). Clin. Chem. 2008, 54, 542–549. [Google Scholar] [CrossRef]
- Forni, S.; Fu, X.; Palmer, S.E.; Sweetman, L. Rapid determination of C4-acylcarnitine and C5-acylcarnitine isomers in plasma and dried blood spots by UPLC-MS/MS as a second tier test following flow-injection MS/MS acylcarnitine profile analysis. Mol. Genet. Metab. 2010, 101, 25–32. [Google Scholar] [CrossRef]
- Gelb, M.H. Newborn screening for lysosomal storage diseases: Methodologies, screen positive rates, normalization of datasets, second-tier tests, and post-analysis Tools. Int. J. Neonatal Screen. 2018, 4, 23. [Google Scholar] [CrossRef]
- Tortorelli, S.; Eckerman, J.S.; Orsini, J.J.; Stevens, C.; Hart, J.; Hall, P.L.; Alexander, J.J.; Gavrilov, D.; Oglesbee, D.; Raymond, K.; et al. Moonlighting newborn screening markers: The incidental discovery of a second tier test for Pompe disease. Genet. Med. 2018, 20, 840–846. [Google Scholar] [CrossRef]
- Guenzel, A.J.; Turgeon, C.T.; Nickander, K.K.; White, A.L.; Peck, D.S.; Pino, G.B.; Studinski, A.L.; Prasad, V.K.; Kurtzberg, J.; Escolar, M.L.; et al. The critical role of psychosine in screening, diagnosis, and monitoring of Krabbe disease. Genet. Med. 2020. [Google Scholar] [CrossRef]
- Gallagher, R.C.; Currier, R.J.; Tang, H.; Puck, J.M. Genomic analysis of historical cases with positive newborn screens for short chain acyl-CoA dehydrogenase shows that a validated second tier biochemical test can replace future sequencing. Int. J. Neonatal Screen. CLIR Special Issue.
- Narravula, A.; Garber, K.B.; Askree, S.H.; Hegde, M.; Hall, P.L. Variants of uncertain significance in newborn screening disorders: Implications for large-scale genomic sequencing. Genet. Med. 2017, 19, 77–82. [Google Scholar] [CrossRef]
| Disorder | Compl. Group | OMIM # | Gene | 1st Tier Markers | CLIR | 2nd Tier Markers | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| C3 | Met | * No. Cases | MS/MS Tool | Hcy | MMA | MCA | ||||
| Propionic acidemia | n/a | 606054 | PCA, PCB | High | N | 136 | PROP | N | N | High |
| Isolated Methylmalonic acidemia | mut0 mut- | 251000 | MCM | 192 | MUT/Cbl AB | N | High | High | ||
| Cbl A | 251100 | MMAA | ||||||||
| Cbl B | 251100 | MMAB | ||||||||
| Methylmalonic acidemia and Homocystinuria | Cbl C | 277400 | MMACHC | Low | 139 | Cbl CD | High | High | N to High | |
| Cbl D | 277410 | MMACHC | ||||||||
| Cbl F | 277380 | LMBRD1 | 3 | Cbl F | ||||||
| Cbl J | 614857 | ABCD4 | - | - | ||||||
| Cbl X | 309541 | HCFC1 | - | - | ||||||
| Intrinsic factor deficiency | n/a | 261000 | GIF | N | - | - | High | High | N to High | |
| Megaloblastic anemia-1 | 261100 | CUBN, AMN | - | - | ||||||
| Transcobalamin II deficiency | 275350 | TCN2 | - | - | ||||||
| Transcobalamin receptor defect | 613646 | CD320 | 11 | TCblR | ||||||
| Maternal Vitamin B12 deficiency | - | - | Low | 138 | B12 (mat) | |||||
| Homocystinuria (CBS deficiency) | n/a | 236200 | CBS | N | High | 74 | HCY | High | N | N |
| Homocystinuria and megaloblastic anemia | Cbl G | 250940 | MTR | Low | 11 | RMD | ||||
| Cbl E | 236270 | MTRR | ||||||||
| MTHFR deficiency | n/a | 236250 | MTHFR | |||||||
| Methionine adenosyltransferase def. | 250850 | MAT 1A | High | 112 | H-MET | N | N | N | ||
| Adenosine kinase deficiency | 180960 | ADK | ||||||||
| Glycine N-methyltransferase def. | 606664 | GNMT | ||||||||
| S-adenosylhomocysteine hydrolase def. | 613752 | AHCY | ||||||||
| FP C3 | n/a | n/a | High | N | 124 | FP C3 | N | N | N | |
| TPN | n/a | n/a | N | High | 2816 | TPN | N | N | N | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilov, D.K.; Piazza, A.L.; Pino, G.; Turgeon, C.; Matern, D.; Oglesbee, D.; Raymond, K.; Tortorelli, S.; Rinaldo, P. The Combined Impact of CLIR Post-Analytical Tools and Second Tier Testing on the Performance of Newborn Screening for Disorders of Propionate, Methionine, and Cobalamin Metabolism. Int. J. Neonatal Screen. 2020, 6, 33. https://doi.org/10.3390/ijns6020033
Gavrilov DK, Piazza AL, Pino G, Turgeon C, Matern D, Oglesbee D, Raymond K, Tortorelli S, Rinaldo P. The Combined Impact of CLIR Post-Analytical Tools and Second Tier Testing on the Performance of Newborn Screening for Disorders of Propionate, Methionine, and Cobalamin Metabolism. International Journal of Neonatal Screening. 2020; 6(2):33. https://doi.org/10.3390/ijns6020033
Chicago/Turabian StyleGavrilov, Dimitar K., Amy L. Piazza, Gisele Pino, Coleman Turgeon, Dietrich Matern, Devin Oglesbee, Kimiyo Raymond, Silvia Tortorelli, and Piero Rinaldo. 2020. "The Combined Impact of CLIR Post-Analytical Tools and Second Tier Testing on the Performance of Newborn Screening for Disorders of Propionate, Methionine, and Cobalamin Metabolism" International Journal of Neonatal Screening 6, no. 2: 33. https://doi.org/10.3390/ijns6020033
APA StyleGavrilov, D. K., Piazza, A. L., Pino, G., Turgeon, C., Matern, D., Oglesbee, D., Raymond, K., Tortorelli, S., & Rinaldo, P. (2020). The Combined Impact of CLIR Post-Analytical Tools and Second Tier Testing on the Performance of Newborn Screening for Disorders of Propionate, Methionine, and Cobalamin Metabolism. International Journal of Neonatal Screening, 6(2), 33. https://doi.org/10.3390/ijns6020033





