Determining Reference Ranges for Total T4 in Dried Blood Samples for Newborn Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. tT4 Stability in Dried Blood Samples
2.2. Determination of tT4 Ranges
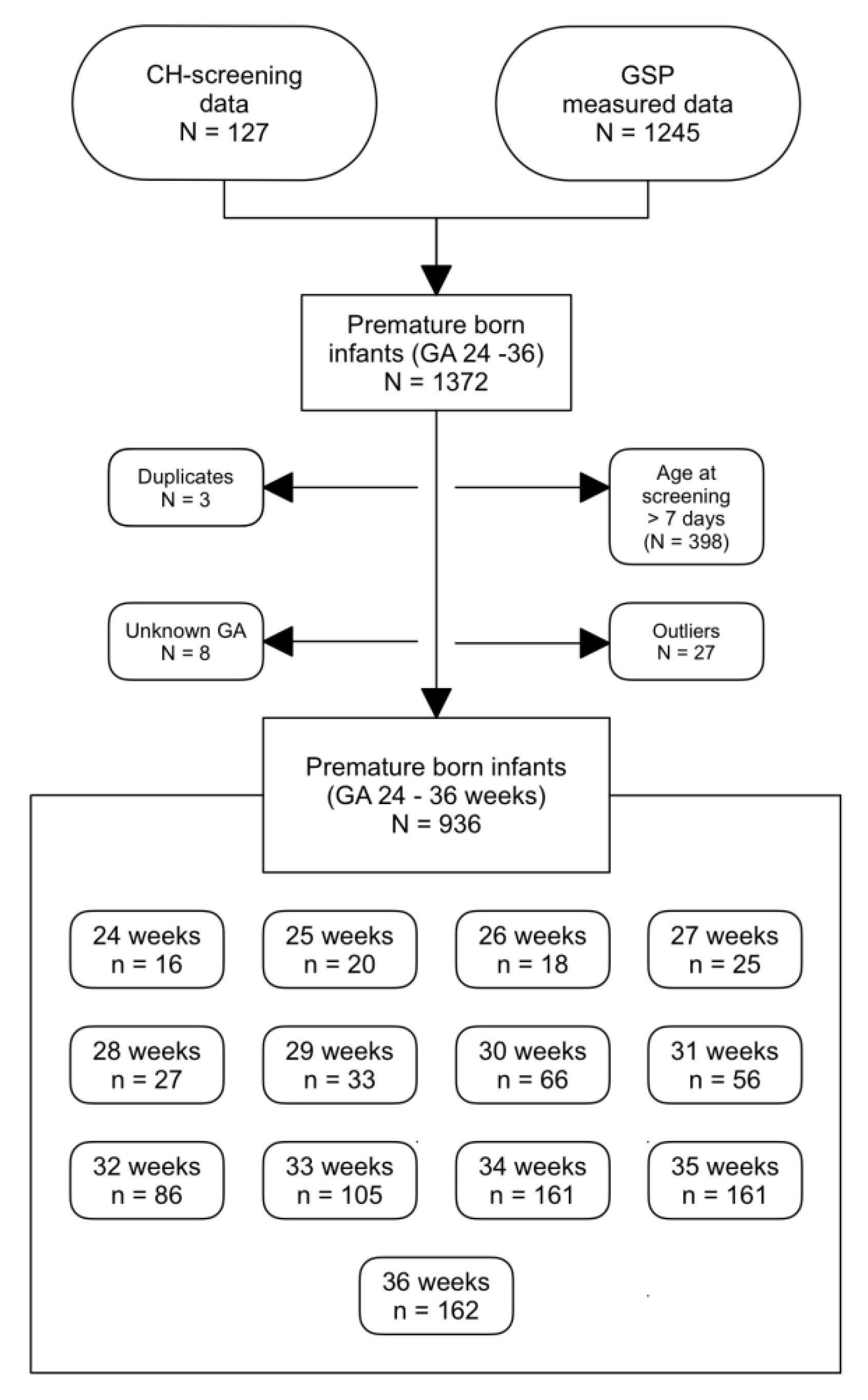
2.2.1. tT4 Values of Preterm-Born Infants
2.2.2. tT4 Concentrations of Term-Born Infants
3. Results
3.1. Stability of tT4 in Dried Blood Samples
3.2. Determination of the tT4 Ranges
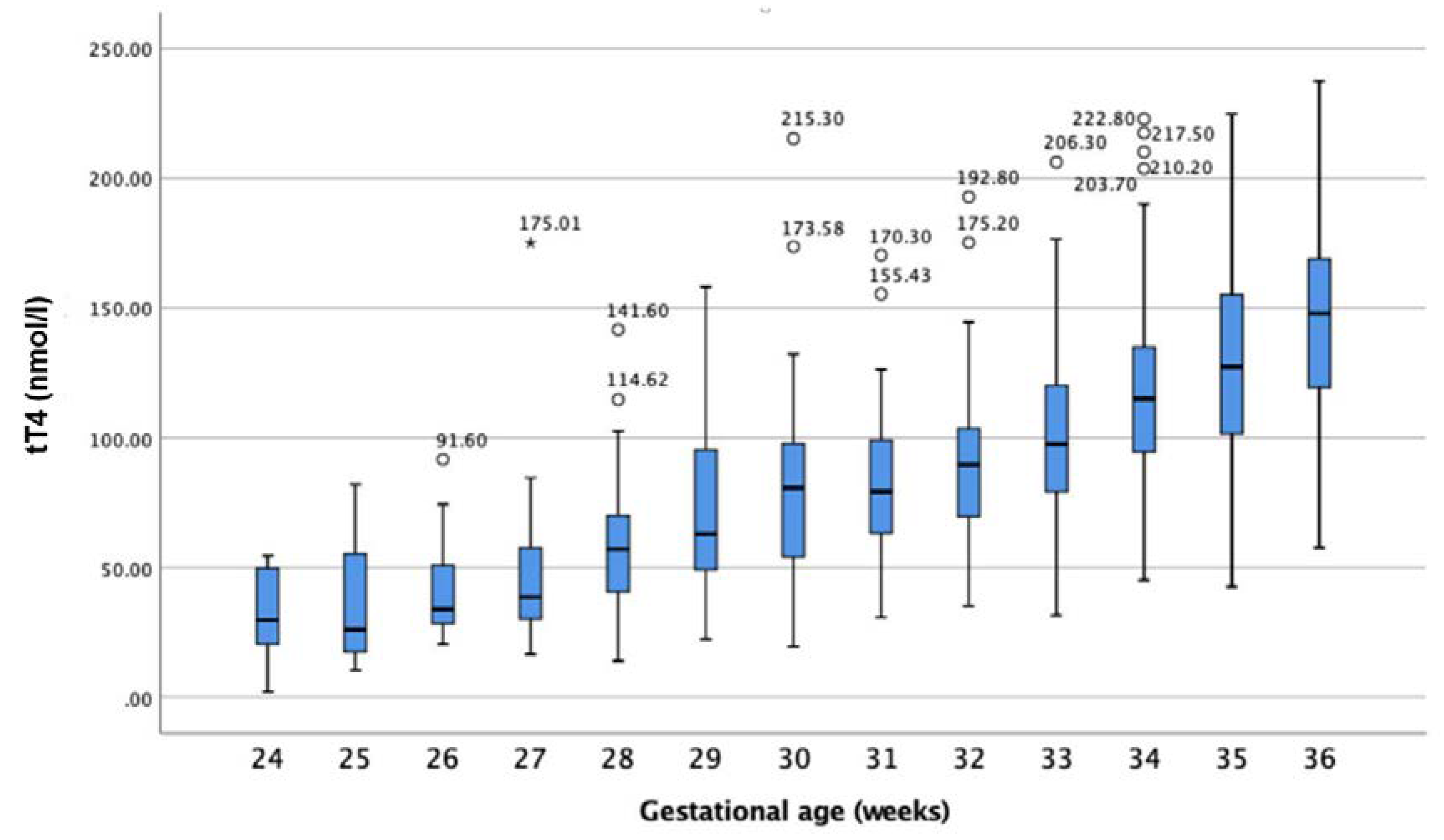
3.2.1. tT4 Values of Preterm-Born Infants
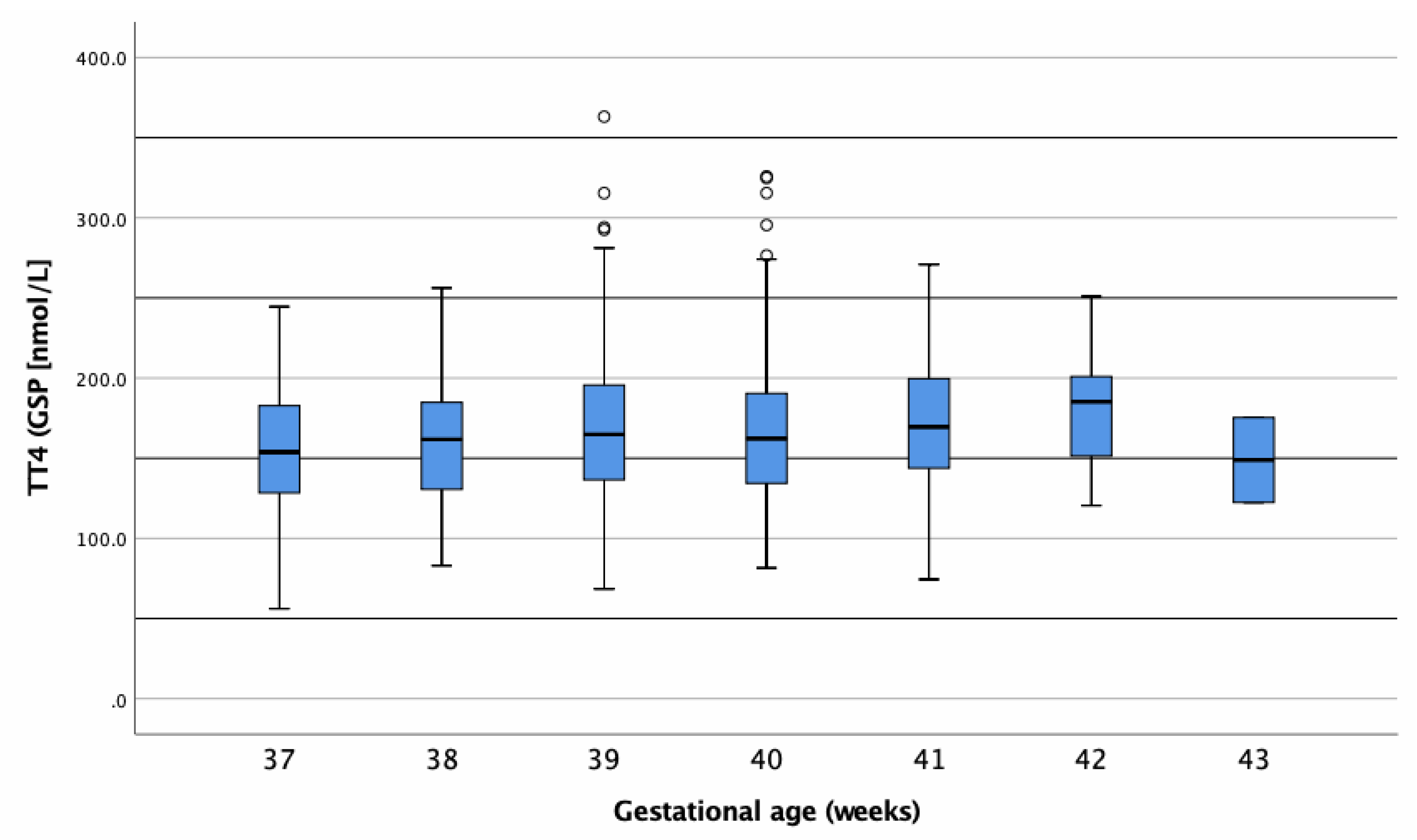
3.2.2. tT4 Concentrations of Term-Born Infants
3.2.3. tT4 Differences between Genders
3.3. Ethics
4. Discussion
4.1. Thyroxine Stability Testing
4.2. Reference Intervals for Newborn and Premature-Born Infants
4.3. Premature-Born Infants (24–36 Weeks)
4.3.1. Reference Individuals
4.3.2. Differences in Thyroid Hormone Regulation Post-Partum between Premature- and Term-Born Infants
4.3.3. Reasons for Hypothyroxinaemia in Premature Infants
4.3.4. Consequences of Hypothyroxinaemia in Premature-Born Infants
4.3.5. Thyroxine Reference Ranges for Premature Infants
4.4. Term-Born Infants (37–43 Weeks)
4.4.1. Reference Ranges for Term-Born Infants
4.4.2. Central Congenital Hypothyroidism
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DBS | Dried blood sample |
| GA | Gestational age |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| tT4 | Total thyroxine |
| fT4 | Free thyroxine |
| TSH | Thyroid stimulating hormone |
| THOP | Transitory hypothyroxinaemia of prematurity |
| LBW | Low birthweight |
| VLBW | Very low birthweight |
References
- Shanholtz, H.J. Congenital Hypothyroidism. J. Pediatric. Nurs. 2013, 28, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Ford, G.; Lafranchi, S.H. Screening for congenital hypothyroidism: A worldwide view of strategies. Best Pract. Res. 2014, 28, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.A.; Klein, A.H. Thyroid Development and Disorders of Thyroid Function in the Newborn. N. Engl. J. Med. 1981, 304, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, A.; Ruiz-Cuevas, P.; Potau, N.; Almar, J.; Salcedo, S.; Clemente, M.; Yeste, D. Thyroid Function in Seventy-Five Healthy Preterm Infants Thirty to Thirty-Five Weeks of Gestational Age: A Prospective and Longitudinal Study During the First Year of Life. Thyroid 2004, 14, 435–442. [Google Scholar] [CrossRef] [PubMed]
- McGrath, N.; Hawkes, C.P.; McDonnell, C.M.; Cody, D.; O’Connell, S.M.; Mayne, P.D.; Murphy, N.P. Incidence of Congenital Hypothyroidism Over 37 Years in Ireland. Pediatrics 2018, 142, e20181199. [Google Scholar] [CrossRef]
- Persani, L. Central Hypothyroidism: Pathogenic, Diagnostic, and Therapeutic Challenges. J. Clin. Endocrinol. Metab. 2012, 97, 3068–3078. [Google Scholar] [CrossRef]
- Zwaveling-Soonawala, N.; van Trotsenburg, A.S.P.; Verkerk, P.H. TSH and FT4 Concentrations in Congenital Central Hypothyroidism and Mild Congenital Thyroidal Hypothyroidism. J. Clin. Endocrinol. Metab. 2018, 103, 1342–1348. [Google Scholar] [CrossRef]
- LaFranchi, S.H. Newborn screening strategies for congenital hypothyroidism: An update. J. Inherit. Metab. Dis. 2010, 33, 225–233. [Google Scholar] [CrossRef]
- Available online: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitszustand/gesundheit-neugeborenen.html (accessed on 1 March 2020).
- Lando, V.S.; Batista, M.C.; Nakamura, I.T.; Mazi, C.R.; Mendonca, B.B.; Brito, V.N. Effects of long-term storage of filter paper blood samples on neonatal thyroid stimulating hormone, thyroxin and 17-alpha-hydroxyprogesterone measurements. J. Med. Screen. 2008, 15, 109–111. [Google Scholar] [CrossRef]
- Waite, K.V.; Maberly, G.F.; Eastman, C.J. Storage conditions and stability of thyrotropin and thyroid hormones on filter paper. Clin. Chem. 1987, 33, 853. [Google Scholar] [CrossRef]
- Davis, G.; Poholek, R. Stability of dried blood spots on paper, as used in screening neonates for hypothyroidism. Clin. Chem. 1979, 25, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Adeli, K. Clinical laboratory reference intervals in pediatrics: Current gaps and recent advance. Clin. Biochem. 2011, 44, S7–S8. [Google Scholar] [CrossRef]
- Ishaku, A.A.; Shabbal, D.M.; Isichei, C. Reference Interval of Thyroxine and Thyrotropin of Healthy Term Nigerian Newborns in Jos University of Teaching Hospital. Jos J. Med. 2017, 11, 26–30. [Google Scholar]
- Jacobsen, B.B.; Andersen, H.J.; Peitersen, B.; Dige-Petersen, H.; Hummer, L. Serum Levels of Thyrotropin, Thyroxine and Triiodothyronine in Fullterm, Small-for-gestational Age and Preterm Newborn Babies. Acta Paediatr. 1977, 66, 681–687. [Google Scholar] [CrossRef]
- Larson, C.; Hermos, R.; Delaney, A.; Daley, D.; Mitchell, M. Risk factors associated with delayed thyrotropin elevations in congenital hypothyroidism. J. Pediatr. 2003, 143, 587–591. [Google Scholar] [CrossRef]
- Smith, L. Updated AAP guidelines on newborn screening and therapy for congenital hypothyroidism. Am. Fam. Physician 2007, 76, 439. [Google Scholar] [CrossRef]
- Mandel, S.J.; Hermos, R.J.; Larson, C.A.; Prigozhin, A.B.; Rojas, D.A.; Mitchell, M.L. Atypical Hypothyroidism and the Very Low Birthweight Infant. Thyroid 2000, 10, 693–695. [Google Scholar] [CrossRef]
- Hunter, M.K.; Mandel, S.H.; Sesser, D.E.; Miyabira, R.S.; Rien, L.; Skeels, M.R.; LaFranchi, S.H. Follow-up of newborns with low thyroxine and nonelevated thyroid-stimulating hormone–screening concentrations: Results of the 20-year experience in the Northwest Regional Newborn Screening Program. J. Pediatrics 1998, 132, 70–74. [Google Scholar] [CrossRef]
- Ozarda, Y. Reference intervals: Current status, recent developments and future considerations. Biochem. Med. 2016, 26, 5–16. [Google Scholar] [CrossRef]
- Williams, F.L.R.; Simpson, J.; Delahunty, C.; Ogston, S.A.; Bongers-Schokking, J.J.; Murphy, N.; van Toor, H.; Wu, S.-Y.; Visser, T.J.; Hume, R. Developmental Trends in Cord and Postpartum Serum Thyroid Hormones in Preterm Infants. J. Clin. Endocrinol. Metab. 2004, 89, 5314–5320. [Google Scholar] [CrossRef]
- Chung, H.R.; Shin, C.H.; Yang, S.W.; Choi, C.W.; Kim, B.I.; Kim, E.K.; Kim, H.S.; Choi, J.H. High incidence of thyroid dysfunction in preterm infants. J. Korean. Med. Sci. 2009, 24, 627–631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Büyükgebiz, A. Newborn screening for congenital hypothyroidism. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Reuss, M.L.; Paneth, N.; Pinto-Martin, J.A.; Lorenz, J.M.; Susser, M. The Relation of Transient Hypothyroxinemia in Preterm Infants to Neurologic Development at Two Years of Age. N. Engl. J. Med. 1996, 334, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Meijer, W.J.; Verloove-Vanhorick, S.P.; Brand, R.; van den Brande, J.L. Transient hypothyroxinaemia associated with developmental delay in very preterm infants. Arch. Dis. Child. 1992, 67, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Hume, R.; van Toor, H.; Matthews, T.G.; Ogston, S.A.; Wu, S.-Y.; Visser, T.J.; Williams, F.L.R. The Hypothalamic-Pituitary-Thyroid Axis in Preterm Infants; Changes in the First 24 Hours of Postnatal Life. J. Clin. Endocrinol. Metab. 2004, 89, 2824–2831. [Google Scholar] [CrossRef]
- Clark, S.J.; Deming, D.D.; Emery, J.R.; Adams, L.M.; Carlton, E.I.; Nelson, J.C. Reference Ranges for Thyroid Function Tests in Premature Infants Beyond the First Week of Life. J. Perinatol. 2001, 21, 531–536. [Google Scholar] [CrossRef][Green Version]
- Hollanders, J.J.; Israëls, J.; van der Pal, S.M.; Verkerk, P.H.; Rotteveel, J.; Finken, M.J.J.; Dutch, P.-C.S.G. No Association between Transient Hypothyroxinemia of Prematurity and Neurodevelopmental Outcome in Young Adulthood. J. Clin. Endocrinol. Metab. 2015, 100, 4648–4653. [Google Scholar] [CrossRef][Green Version]
- La Gamma, E.F.; Korzeniewski, S.J.; Ballabh, P.; Paneth, N. Transient Hypothyroxinemia of Prematurity. NeoReviews 2016, 17, e394–e402. [Google Scholar] [CrossRef]
- Van Wassenaer, A.G.; Kok, J.H.; de Vijlder, J.J.M.; Briët, J.M.; Smit, B.J.; Tamminga, P.; van Baar, A.; Dekker, F.W.; Vulsma, T. Effects of Thyroxine Supplementation on Neurologic Development in Infants Born at Less Than 30 Weeks’ Gestation. N. Engl. J. Med. 1997, 336, 21–26. [Google Scholar] [CrossRef]
- Adams, L.M.; Emery, J.R.; Clark, S.J.; Carlton, E.I.; Nelson, J.C. Reference ranges for newer thyroid function tests in premature infants. J. Pediatr. 1995, 126, 122–127. [Google Scholar] [CrossRef]
- Braslavsky, D.; Méndez, M.V.; Prieto, L.; Keselman, A.; Enacan, R.; Gruñeiro-Papendieck, L.; Jullien, N.; Savenau, A.; Reynaud, R.; Brue, T.; et al. Pilot Neonatal Screening Program for Central Congenital Hypothyroidism: Evidence of Significant Detection. Horm. Res. Paediatr. 2017, 88, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Zwaveling-Soonawala, N.; van Trotsenburg, A.S.P.; Verkerk, P.H. The Severity of Congenital Hypothyroidism of Central Origin Should Not Be Underestimated. J. Clin. Endocrinol. Metab. 2015, 100, E297–E300. [Google Scholar] [CrossRef] [PubMed]
- Lanting, C.I.; van Tijn, D.A.; Loeber, J.G.; Vulsma, T.; de Vijlder, J.J.M.; Verkerk, P.H. Clinical Effectiveness and Cost-Effectiveness of the Use of the Thyroxine/Thyroxine-Binding Globulin Ratio to Detect Congenital Hypothyroidism of Thyroidal and Central Origin in a Neonatal Screening Program. Pediatrics 2005, 116, 168. [Google Scholar] [CrossRef] [PubMed]
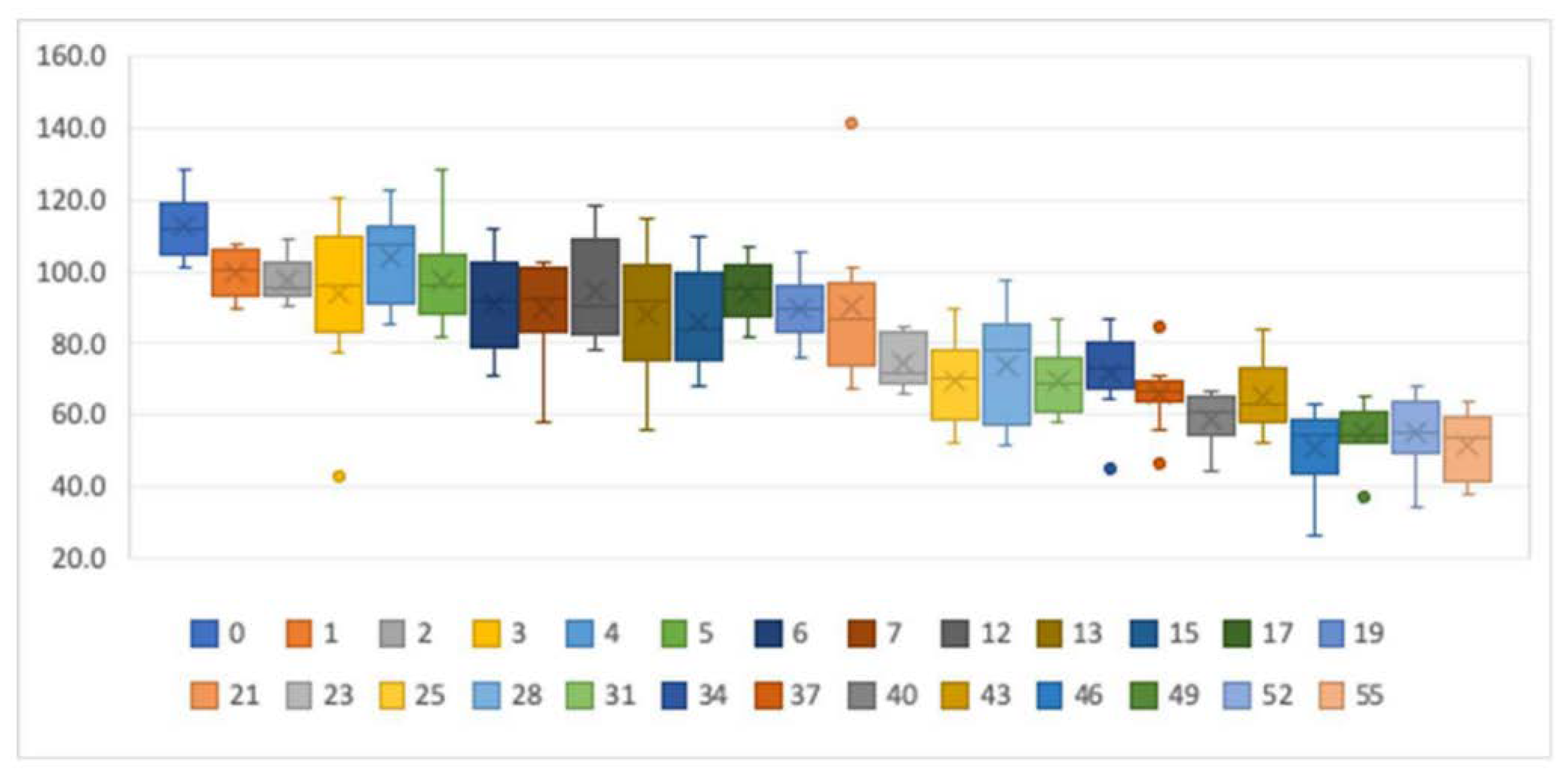

| Storage Time | n | tT4 (nmol/L) | p-Value | |||
|---|---|---|---|---|---|---|
| Baseline | Post Storage | |||||
| Mean | 95% CI | Mean | 95% CI | |||
| 0 weeks | 10 | 116.0 | 88.15–143.98 | 128.6 | 102.2–155.06 | 0.000 |
| 1 week | 10 | 108.4 | 87.25–129.63 | 106.9 | 89.88–123.90 | 0.572 |
| 2 weeks | 10 | 128.1 | 109.35–146.85 | 124.2 | 107.08–141.36 | 0.136 |
| 3 weeks | 10 | 114.4 | 90.52–138.36 | 106.9 | 81.64–132.08 | 0.345 |
| 4 weeks | 10 | 109.5 | 94.31–124.71 | 113.8 | 95.74–131.88 | 0.346 |
| 5 weeks | 9 | 110.6 | 84.02–137.23 | 104.0 | 79.66–128.34 | 0.075 |
| 6 weeks | 10 | 113.8 | 89.94–137.56 | 100.5 | 87.74–113.22 | 0.059 |
| 7 weeks | 11 | 111.2 | 87.47–134.93 | 98.4 | 74.66–122.20 | 0.020 |
| 12 weeks | 10 | 105.1 | 92.24–117.90 | 99.4 | 84.53–114.21 | 0.287 |
| 13 weeks | 10 | 103.4 | 86.76–119.96 | 90.2 | 72.90–107.40 | 0.068 |
| 15 weeks | 10 | 112.0 | 95.03–128.87 | 95.9 | 81.13–110.61 | 0.025 |
| 17 weeks | 10 | 108.9 | 95.30–122.56 | 103.0 | 86.46–119.58 | 0.084 |
| 19 weeks | 9 | 118.4 | 102.01–134.77 | 108.9 | 90.08–127.81 | 0.007 |
| 21 weeks | 9 | 106.5 | 86.17–126.90 | 91.3 | 67.76–114.80 | 0.003 |
| 23 weeks | 10 | 134.4 | 108.22–160.60 | 99.3 | 81.78–116.82 | 0.000 |
| 25 weeks | 11 | 118.1 | 96.31–139.91 | 82.4 | 61.34–103.50 | 0.000 |
| Storage Time | n | tT4 (nmol/L) | p-Value | |||
|---|---|---|---|---|---|---|
| Baseline | Post Storage and Post Correction | |||||
| Mean | 95% CI | Mean | 95% CI | |||
| 6 weeks | 10 | 113.8 | 89.94–137.56 | 110.53 | 96.51–124.54 | 0.594 |
| 7 weeks | 11 | 111.2 | 87.47–134.93 | 108.27 | 82.12–134.41 | 0.570 |
| 12 weeks | 10 | 105.1 | 92.24–117.90 | 109.31 | 92.98–125.63 | 0.455 |
| 13 weeks | 10 | 103.4 | 86.76–119.96 | 99.16 | 80.18–118.14 | 0.551 |
| 15 weeks | 10 | 112.0 | 95.03–128.87 | 105.46 | 89.25–121.67 | 0.324 |
| 17 weeks | 10 | 108.9 | 95.30–122.56 | 113.32 | 95.11–131.54 | 0.245 |
| 19 weeks | 9 | 118.4 | 102.01–134.77 | 119.84 | 103.49–143.31 | 0.660 |
| 21 weeks | 9 | 106.5 | 86.17–126.90 | 100.41 | 78.91–124.78 | 0.181 |
| 23 weeks | 10 | 134.4 | 108.22–160.60 | 109.23 | 89.96–128.50 | 0.000 |
| 25 weeks | 11 | 118.1 | 96.31–139.91 | 90.66 | 67.47–113.85 | 0.000 |
| GA at Birth (Weeks) | Number Studied (n) | Mean ± SD | Percentiles or (Min–Max) * | Unit | |
|---|---|---|---|---|---|
| 2.5 | 97.5 | ||||
| 24 | 16 | 32.52 ± 17.1 | (1.98–54.45) | nmol/L | |
| 2.5 ± 1.3 | (0.15–4.23) | µg/dL | |||
| 25 | 20 | 39.7 ± 25.8 | (10.50–87.30) | nmol/L | |
| 3.1 ± 2.0 | (0.81–6.78) | µg/dL | |||
| 26 | 18 | 36.7 ± 16.7 | (15.40–74.30 | nmol/L | |
| 2.8 ± 1.3 | (1.19–5.77) | µg/dL | |||
| 27 | 25 | 43.2 ± 17.1 | (16.60–84.48) | nmol/L | |
| 3.3 ± 1.3 | (1.29–6.56) | µg/dL | |||
| 28 | 27 | 57.9 ± 24.8 | (15.40–114.62) | nmol/L | |
| 4.4 ± 1.9 | (1.20–8.90) | µg/dL | |||
| 29 | 33 | 76.6 ± 37.6 | (22.33–158.18) | nmol/L | |
| 5.9 ± 2.9 | (1.73–12.29) | µg/dL | |||
| 30 | 66 | 77.6 ± 32.9 | 11.90 | 161.43 | nmol/L |
| 6.0 ± 2.5 | 0.92 | 12.51 | µg/dL | ||
| 31 | 56 | 82.0 ± 31.3 | 33.52 | 163.98 | nmol/L |
| 6.3 ± 2.4 | 2.60 | 12.71 | µg/dL | ||
| 32 | 86 | 86.1 ± 24.5 | 43.97 | 142.40 | nmol/L |
| 6.6 ± 1.9 | 3.41 | 11.04 | µg/dL | ||
| 33 | 105 | 102.4 ± 32.6 | 44.44 | 172.57 | nmol/L |
| 7.9 ± 2.5 | 3.44 | 13.38 | µg/dL | ||
| 34 | 161 | 115.5 ± 29.2 | 64.12 | 179.63 | nmol/L |
| 8.9 ± 2.5 | 4.97 | 13.92 | µg/dL | ||
| 35 | 161 | 129.9 ± 39.5 | 52.26 | 211.16 | nmol/L |
| 10.0 ± 3.0 | 4.05 | 16.37 | µg/dL | ||
| 36 | 162 | 144.4 ± 36.8 | 72.00 | 215.19 | nmol/L |
| 11.1 ± 2.8 | 5.58 | 16.68 | µg/dL | ||
| GA at Birth (Weeks) | Number Studied (n) | Mean ± SD | Percentiles or (Min–Max) * | Unit | |
|---|---|---|---|---|---|
| 2.5 | 97.5 | ||||
| 37 | 60 | 157.6 ± 39.9 | 81.09 | 232.95 | nmol/L |
| 12.1 ± 3.1 | 6.30 | 18.10 | µg/dL | ||
| 38 | 155 | 162.1 ± 36.5 | 101.94 | 237.86 | nmol/L |
| 12.5 ± 2.8 | 7.92 | 18.48 | µg/dL | ||
| 39 | 223 | 168.5 ± 44.2 | 95.80 | 269.42 | nmol/L |
| 13.0 ± 3.4 | 7.44 | 20.93 | µg/dL | ||
| 40 | 260 | 156.9 ± 44.1 | 95.32 | 266.66 | nmol/L |
| 12.8 ± 3.4 | 7.40 | 20.72 | µg/dL | ||
| 41 | 147 | 170.3 ± 39.3 | 94.94 | 242.85 | nmol/L |
| 13.1 ± 3.0 | 7.37 | 18.87 | µg/dL | ||
| 42 | 21 | 182.7 ± 37.6 | (120.50–250.90) | nmol/L | |
| 14.1 ± 2.9 | (9.36–19.49) | µg/dL | |||
| 43 | 2 | 148.9 ± 37.5 | (122.40–175.40) | nmol/L | |
| 11.5 ± 2.9 | (9.51–13.63) | µg/dL | |||
| All | 868 | 166.4 ± 41.7 | 95.91 | 256.00 | nmol/L |
| 12.8 ± 3.2 | 7.45 | 19.90 | µg/dL | ||
| Gender | Number Studied (n) | Mean | Standard Deviation |
|---|---|---|---|
| Male | 749 | 124.4 | 52.75 |
| Female | 619 | 123.7 | 55.10 |
| Unknown | 19 | 137.8 | 46.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijman, A.-I.; Konrad, D.; Fingerhut, R. Determining Reference Ranges for Total T4 in Dried Blood Samples for Newborn Screening. Int. J. Neonatal Screen. 2020, 6, 17. https://doi.org/10.3390/ijns6010017
Hijman A-I, Konrad D, Fingerhut R. Determining Reference Ranges for Total T4 in Dried Blood Samples for Newborn Screening. International Journal of Neonatal Screening. 2020; 6(1):17. https://doi.org/10.3390/ijns6010017
Chicago/Turabian StyleHijman, Anna-Isabella, Daniel Konrad, and Ralph Fingerhut. 2020. "Determining Reference Ranges for Total T4 in Dried Blood Samples for Newborn Screening" International Journal of Neonatal Screening 6, no. 1: 17. https://doi.org/10.3390/ijns6010017
APA StyleHijman, A.-I., Konrad, D., & Fingerhut, R. (2020). Determining Reference Ranges for Total T4 in Dried Blood Samples for Newborn Screening. International Journal of Neonatal Screening, 6(1), 17. https://doi.org/10.3390/ijns6010017






