Newborn Screening for CF across the Globe—Where Is It Worthwhile?
Abstract
1. Introduction
2. Requirements That Must Be Met for CF NBS to Be Worthwhile
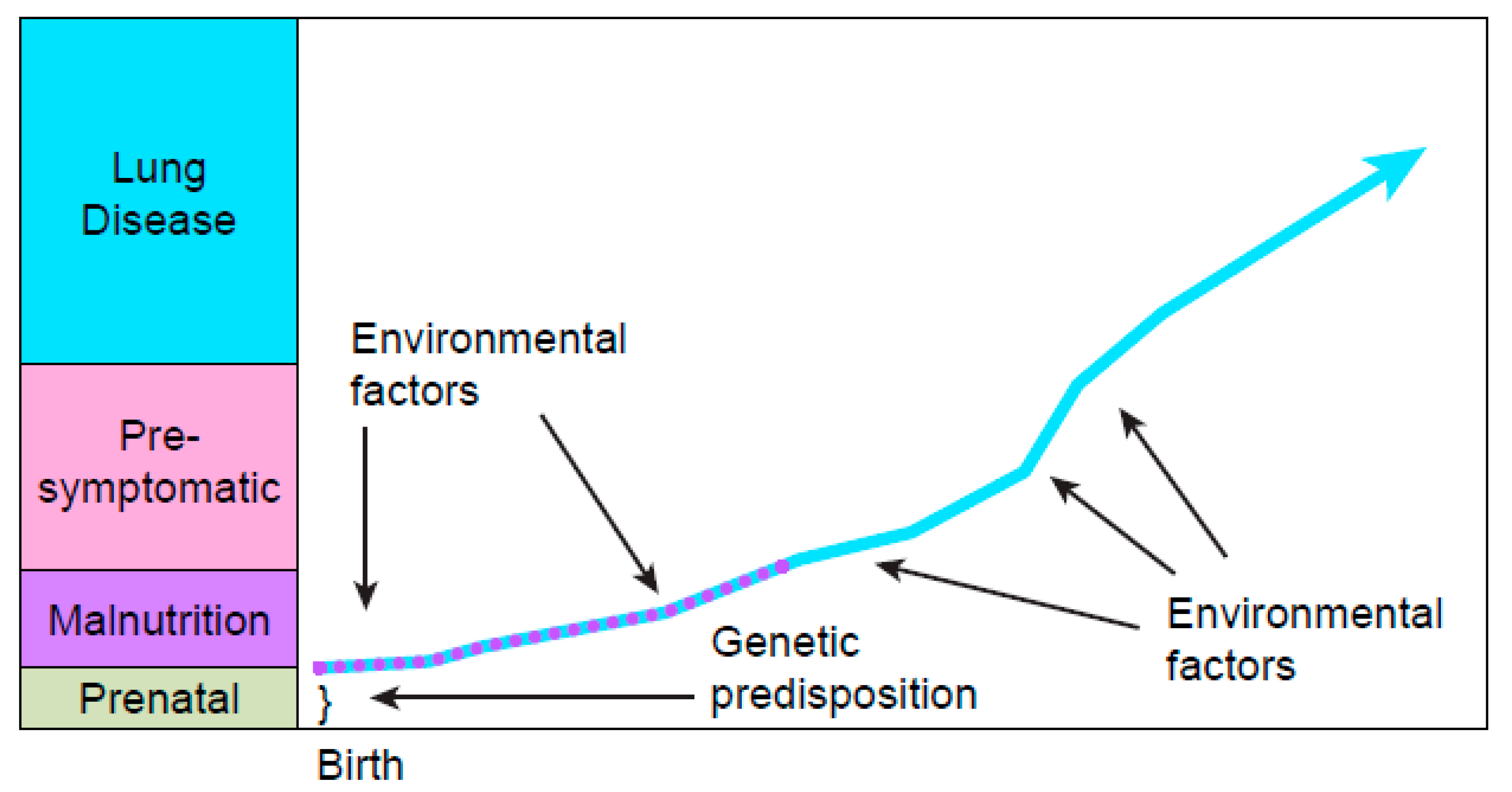
2.1. Feasibility of Screening Newborns for CF
2.2. The Need for an Excellent Screening Test: Limitations of IRT/IRT
2.3. The Value of the IRT/DNA Screening Test When CFTR Mutations Are Known
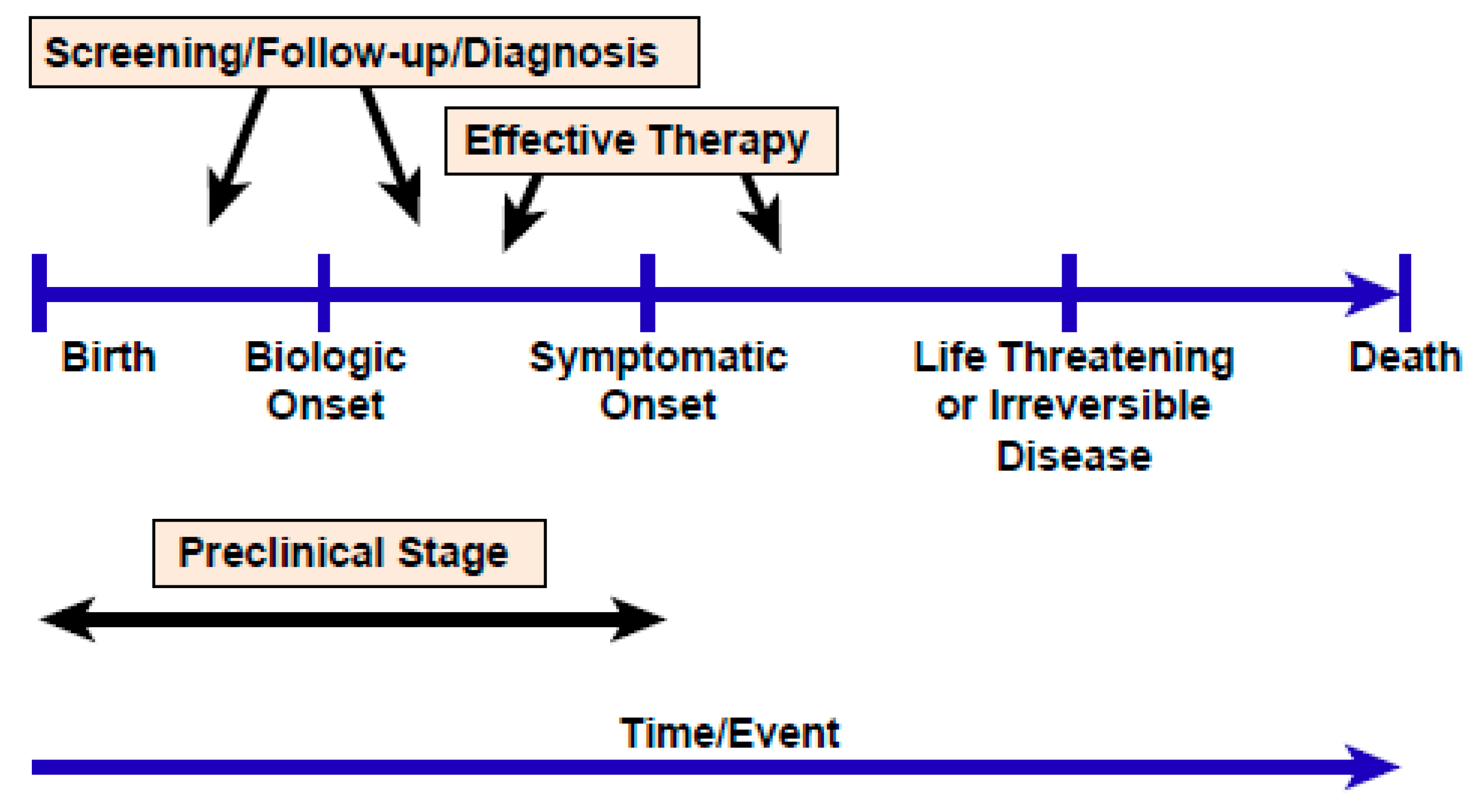
2.4. The Challenge of Evaluating and Achieving Benefits That Outweigh Risks
3. Criteria to Implement Screening
3.1. European CF Society Guidelines
3.2. Clinical and Laboratory Standards Institute, the Association of Public Health Laboratories, the Centers for Disease Control and Prevention, and the Cystic Fibrosis Foundation
- (1)
- Reassessed IRT cutoff value guidelines and discussed the use of a floating rather than fixed cutoff value. The floating cutoff strategy using the 95th or 96th percentile helps overcome the seasonal and kit-related variations in IRT [11]. The recommendations included: “Recent data have shown that the traditional IRT cutoff values in the IRT/IRT algorithm were too high to minimize false-negative screening results and the 95th to 97th percentile (approximately 60 ng/mL) should be used.” As expanded genetic analyses and next-generation sequencing are becoming less expensive, some CF NBS programs are operating with a lower fixed IRT (for example 40 ng/mL), thus allowing more samples for genetic testing to reduce false-negative screening results.
- (2)
- Revised recommendations regarding CFTR variant panels based on the most current information including new biotechnologies such as next-generation sequencing, pointing out that “Guidelines published in 2001 and revised in 2004 include recommendations for screening with a CFTR variant panel of 23 disease-causing variants with a prevalence of at least 0.1% in the CF population. Although this recommended panel provides a high CF detection rate... additional variants may need to be added for improved CF detection in other ethnic groups. Many NBS programs use larger CFTR variant panels...”
- (3)
- Assessed using PAP for detecting babies at risk for CF but did not make a recommendation.
- (4)
- Discussed communications strategies related to the detecting of CF heterozygote babies and providing genetic counseling.
- (5)
- Reviewed emerging issues related to using genetic and genomic sequencing in NBS.
- (6)
- Described the existing CF NBS algorithms, while commenting on the advantages and disadvantages of each protocol.
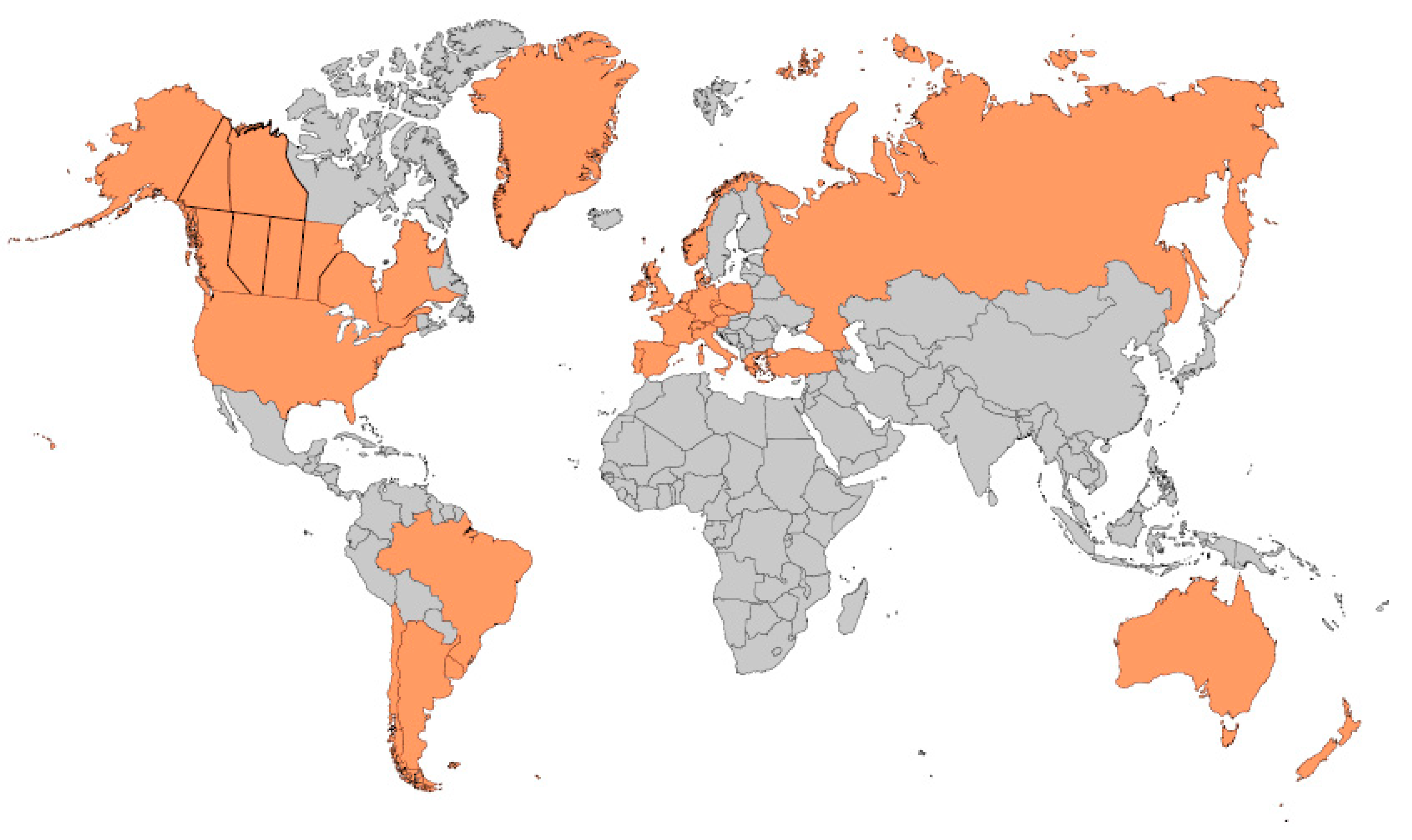
4. Incidence of CF around the World and Screening Protocols Being Employed
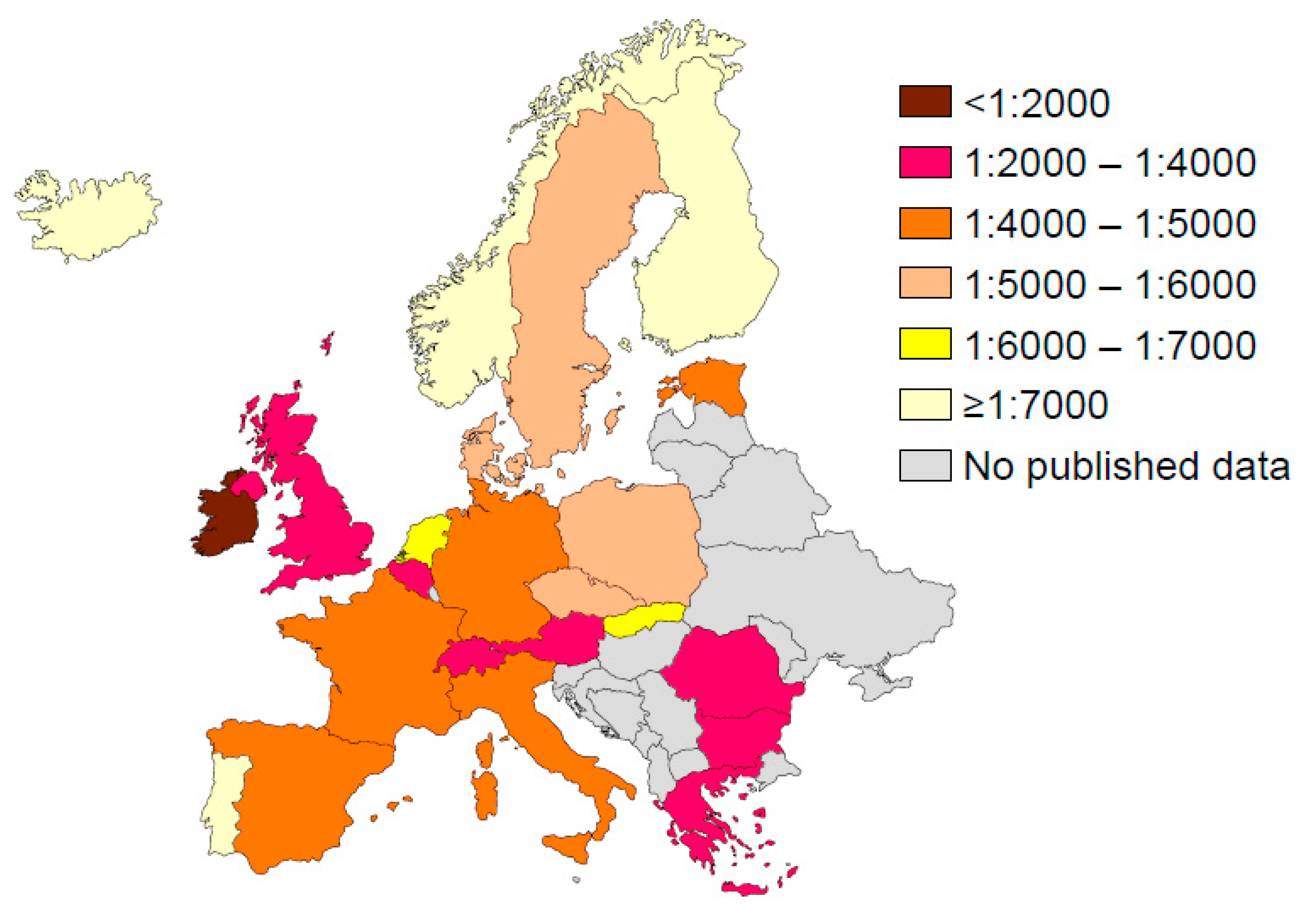
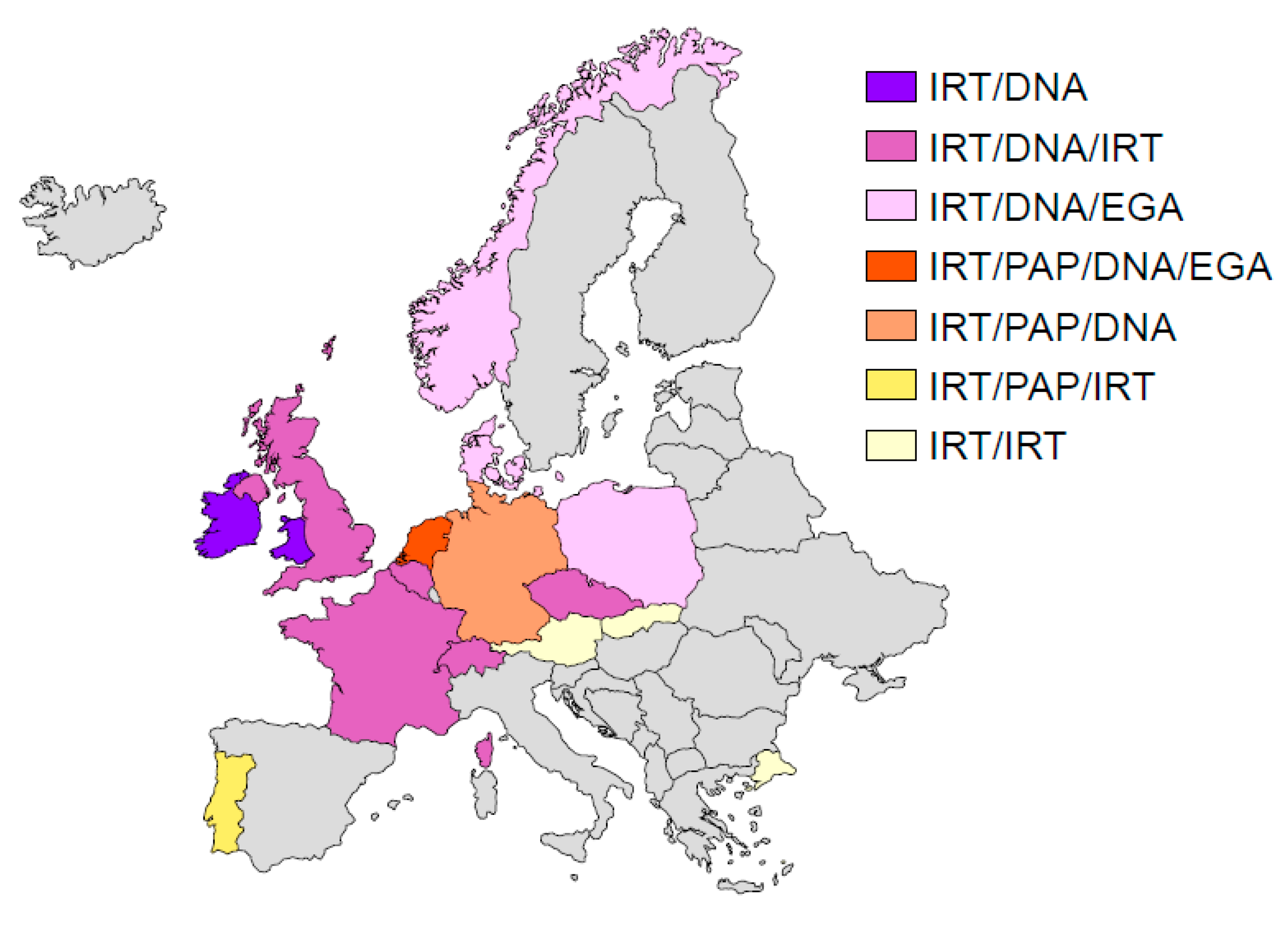
4.1. Europe
4.2. Australasia
4.3. United States of America
4.4. Canada
4.5. Latin America—Mexico (North America), Central, and South America
4.5.1. Argentina
4.5.2. Brazil
4.5.3. Chile
4.5.4. Mexico
4.5.5. Uruguay
4.5.6. Other Latin American Countries
4.5.7. Is CF Neonatal Screening Worthwhile in Latin American Countries?
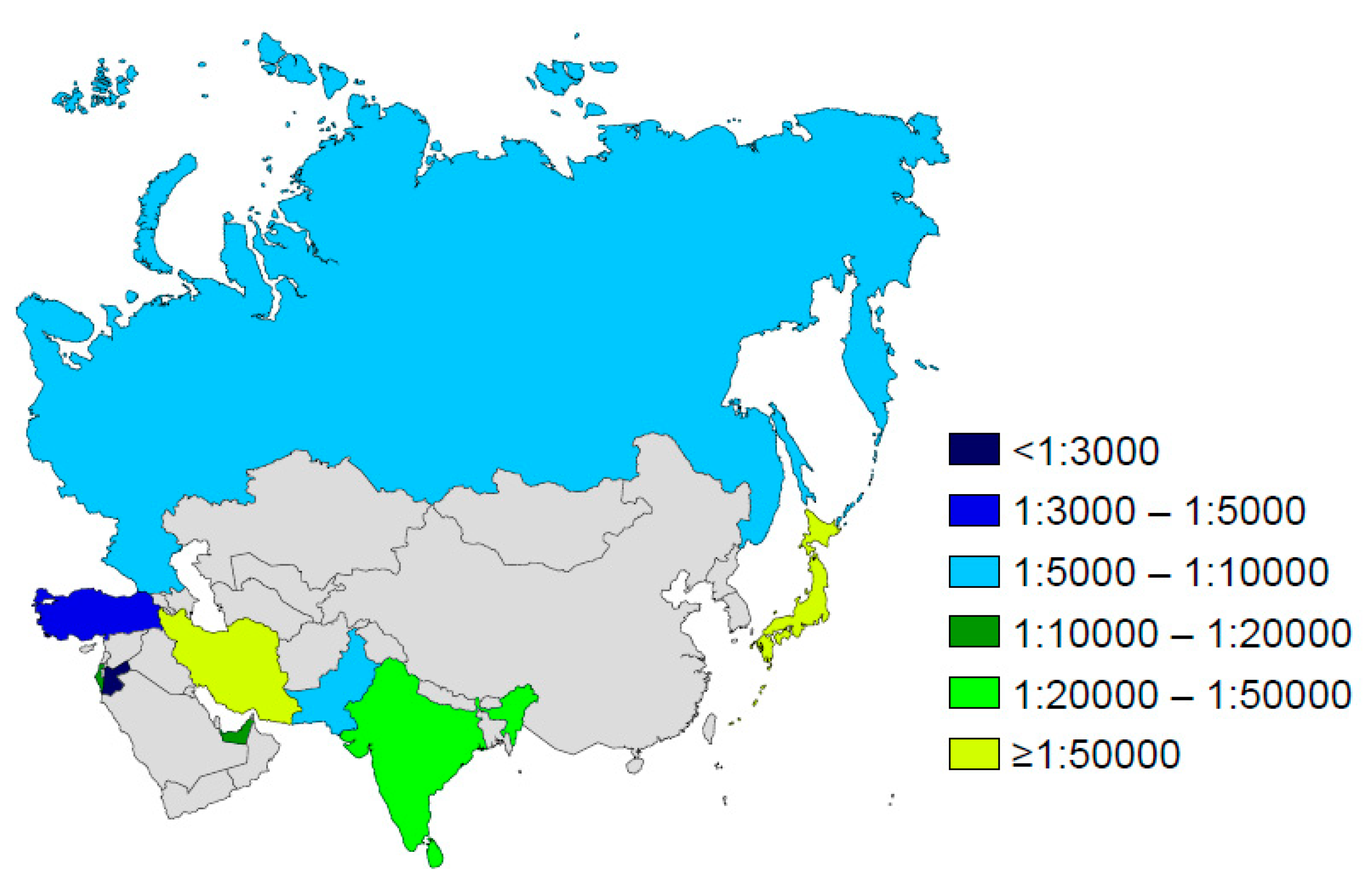
4.6. Asia
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Travert, G.; Heeley, M.; Heeley, A. History of newborn screening for CF—The Early Years. Int. J. Neonatal Screen. 2020, 6, 8. [Google Scholar] [CrossRef]
- Bruns, W.T.; Connell, T.R.; Lacey, J.A.; Whisler, K.E. Test strip meconium screening for cystic fibrosis. Am. J. Dis. Child. 1977, 131, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Crossley, J.R.; Elliott, R.B.; Smith, P.A. Dried-blood spot screening for cystic fibrosis in the newborn. Lancet 1979, 1, 472–474. [Google Scholar] [CrossRef]
- Guthrie, R.; Susi, A. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics 1963, 32, 338–343. [Google Scholar]
- Wilson, J.M.G.; Jungner, Y.G. Principles and Practice of Screening for Disease; WHO: Geneva, Switzerland, 1968; Available online: http://www.who.int/bulletin/volumes/86/4/07-050112BP.pdf (accessed on 1 March 2020).
- Wilcken, B.; Brown, A.R.; Urwin, R.; Brown, D.A. Cystic fibrosis screening by dried blood spot trypsin assay: Results in 75,000 newborn infants. J. Pediatr. 1983, 102, 383–387. [Google Scholar] [CrossRef]
- Reardon, M.C.; Hammond, K.B.; Accurso, F.J.; Fisher, C.D.; McCabe, E.R.; Cotton, E.K.; Bowman, C.M. Nutritional deficits exist before 2 months of age in some infants with cystic fibrosis identified by screening test. J. Pediatr. 1984, 105, 271–274. [Google Scholar] [CrossRef]
- Travert, G.; Duhamel, J.F. Systematic neonatal screening for mucoviscidosis using an immunoreactive trypsin blood assay. Evaluation of 80,000 tests. Arch. Fr. Pediatr. 1983, 40, 295–298. [Google Scholar]
- Cystic Fibrosis Foundation, Ad Hoc Committee Task Force on Neonatal Screening. Neonatal screening for cystic fibrosis: Position paper. Pediatrics 1983, 72, 741–745. [Google Scholar]
- Kerem, B.; Rommens, J.M.; Buchanan, J.A.; Markiewicz, D.; Cox, T.K.; Chakravarti, A.; Buchwald, M.; Tsui, L.C. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef]
- Kloosterboer, M.; Hoffman, G.; Rock, M.; Gershan, W.; Laxova, A.; Li, Z.; Farrell, P.M. Clarification of laboratory and clinical variables that influence cystic fibrosis newborn screening with initial analysis of immunoreactive trypsinogen. Pediatrics 2009, 123, e338–e346. [Google Scholar] [CrossRef]
- Gregg, R.G.; Wilfond, B.S.; Farrell, P.M.; Laxova, A.; Hassemer, D.; Mischler, E.H. Application of DNA analysis in a population-screening program for neonatal diagnosis of cystic fibrosis (CF): Comparison of screening protocols. Am. J. Hum. Genet. 1993, 52, 616–626. [Google Scholar]
- Comeau, A.M.; Parad, R.B.; Dorkin, H.L.; Dovey, M.; Gerstle, R.; Haver, K.; Lapey, A.; O’Sullivan, B.P.; Waltz, D.A.; Zwerdling, R.G.; et al. Population-based newborn screening for genetic disorders when multiple mutation DNA testing is incorporated: A cystic fibrosis newborn screening model demonstrating increased sensitivity but more carrier detections. Pediatrics 2004, 113, 1573–1581. [Google Scholar] [CrossRef]
- Sommerburg, O.; Hammermann, J.; Lindner, M.; Stahl, M.; Muckenthaler, M.; Kohlmueller, D.; Happich, M.; Kulozik, A.E.; Stopsack, M.; Gahr, M.; et al. Five years of experience with biochemical cystic fibrosis newborn screening based on IRT/PAP in Germany. Pediatr. Pulmonol. 2015, 50, 655–664. [Google Scholar] [CrossRef]
- Accurso, F.J.; Sontag, M.K.; Wagener, J.S. Complications associated with symptomatic diagnosis in infants with cystic fibrosis. J. Pediatr. 2005, 147, S37–S41. [Google Scholar] [CrossRef]
- Chatfield, S.; Owen, G.; Ryley, H.C.; Williams, J.; Alfaham, M.; Goodchild, M.C.; Weller, P. Neonatal screening for cystic fibrosis in Wales and the West Midlands: Clinical assessment after five years of screening. Arch. Dis. Child. 1991, 66, 29–33. [Google Scholar] [CrossRef]
- Farrell, P.M.; Kosorok, M.R.; Laxova, A.; Shen, G.; Koscik, R.E.; Bruns, W.T.; Splaingard, M.; Mischler, E.H. Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. N. Engl. J. Med. 1997, 337, 963–969. [Google Scholar] [CrossRef]
- Balfour-Lynn, I.M. Newborn screening for cystic fibrosis: Evidence for benefit. Arch. Dis. Child. 2008, 93, 7–10. [Google Scholar] [CrossRef]
- Farrell, P.M.; Kosorok, M.R.; Rock, M.J.; Laxova, A.; Zeng, L.; Lai, H.C.; Hoffman, G.; Laessig, R.H.; Splaingard, M.L. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Pediatrics 2001, 107, 1–13. [Google Scholar] [CrossRef]
- Farrell, P.M.; Lai, H.J.; Li, Z.; Kosorok, M.R.; Laxova, A.; Green, C.G.; Collins, J.; Hoffman, G.; Laessig, R.; Rock, M.J.; et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: Enough is enough! J. Pediatr. 2005, 147, S30–S36. [Google Scholar] [CrossRef]
- Sanders, D.B.; Zhang, Z.; Farrell, P.M.; Lai, H.J.; Wisconsin, C.F.N.S.G. Early life growth patterns persist for 12 years and impact pulmonary outcomes in cystic fibrosis. J. Cyst. Fibros. 2018, 17, 528–535. [Google Scholar] [CrossRef]
- Kosorok, M.R.; Jalaluddin, M.; Farrell, P.M.; Shen, G.; Colby, C.E.; Laxova, A.; Rock, M.J.; Splaingard, M. Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr. Pulmonol. 1998, 26, 81–88. [Google Scholar] [CrossRef]
- Rosenfeld, M.; Emerson, J.; McNamara, S.; Thompson, V.; Ramsey, B.W.; Morgan, W.; Gibson, R.L.; Group, E.S. Risk factors for age at initial Pseudomonas acquisition in the cystic fibrosis epic observational cohort. J. Cyst. Fibros. 2012, 11, 446–453. [Google Scholar] [CrossRef]
- Baussano, I.; Tardivo, I.; Bellezza-Fontana, R.; Forneris, M.P.; Lezo, A.; Anfossi, L.; Castello, M.; Aleksandar, V.; Bignamini, E. Neonatal screening for cystic fibrosis does not affect time to first infection with Pseudomonas aeruginosa. Pediatrics 2006, 118, 888–895. [Google Scholar] [CrossRef]
- Tluczek, A.; Orland, K.M.; Cavanagh, L. Psychosocial consequences of false-positive newborn screens for cystic fibrosis. Qual. Health Res. 2011, 21, 174–186. [Google Scholar] [CrossRef]
- Johnson, F.; Southern, K.W.; Ulph, F. Psychological impact on parents of an inconclusive diagnosis following newborn bloodspot screening for cystic fibrosis: A qualitative study. Int. J. Neonatal Screen. 2019, 5, 23. [Google Scholar] [CrossRef]
- Tluczek, A.; Clark, R.; McKechnie, A.C.; Brown, R.L. Factors affecting parent-child relationships one year after positive newborn screening for cystic fibrosis or congenital hypothyroidism. J. Dev. Behav. Pediatr. 2015, 36, 24–34. [Google Scholar] [CrossRef]
- Tluczek, A.; Laxova, A.; Grieve, A.; Heun, A.; Brown, R.L.; Rock, M.J.; Gershan, W.M.; Farrell, P.M. Long-term follow-up of cystic fibrosis newborn screening: Psychosocial functioning of adolescents and young adults. J. Cyst. Fibros. 2014, 13, 227–234. [Google Scholar] [CrossRef]
- Ciske, D.J.; Haavisto, A.; Laxova, A.; Rock, L.Z.; Farrell, P.M. Genetic counseling and neonatal screening for cystic fibrosis: An assessment of the communication process. Pediatrics 2001, 107, 699–705. [Google Scholar] [CrossRef]
- Munck, A.; Mayell, S.J.; Winters, V.; Shawcross, A.; Derichs, N.; Parad, R.; Barben, J.; Southern, K.W.; ECFS Neonatal Screening Working Group. Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID): A new designation and management recommendations for infants with an inconclusive diagnosis following newborn screening. J. Cyst. Fibros. 2015, 14, 706–713. [Google Scholar] [CrossRef]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef]
- David, J.; Chrastina, P.; Peskova, K.; Kozich, V.; Friedecky, D.; Adam, T.; Hlidkova, E.; Vinohradska, H.; Novotna, D.; Hedelova, M.; et al. Epidemiology of rare diseases detected by newborn screening in the Czech Republic. Cent. Eur. J. Public Health 2019, 27, 153–159. [Google Scholar] [CrossRef]
- Scotet, V.; Dugueperoux, I.; Saliou, P.; Rault, G.; Roussey, M.; Audrezet, M.P.; Ferec, C. Evidence for decline in the incidence of cystic fibrosis: A 35-year observational study in Brittany, France. Orphanet J. Rare Dis. 2012, 7, 14. [Google Scholar] [CrossRef]
- De Boeck, K. Cystic fibrosis in the year 2020: A disease with a new face. Acta Paediatr. 2020. [Google Scholar] [CrossRef]
- CLSI. Newborn Screening for Cystic Fibrosis, 2nd ed.; CLSI Guideline NBS05; CLSI: Wayne, PA, USA, 2019. [Google Scholar]
- Ren, C.L.; Borowitz, D.S.; Gonska, T.; Howenstine, M.S.; Levy, H.; Massie, J.; Milla, C.; Munck, A.; Southern, K.W. Cystic Fibrosis Transmembrane Conductance Regulator-Related Metabolic Syndrome and Cystic Fibrosis Screen Positive, Inconclusive Diagnosis. J. Pediatr. 2017, 181S, S45–S51. [Google Scholar] [CrossRef]
- Romeo, G.; Devoto, M.; Galietta, L.J. Why is the cystic fibrosis gene so frequent? Hum. Genet. 1989, 84, 1–5. [Google Scholar] [CrossRef]
- Southern, K.W.; Munck, A.; Pollitt, R.; Travert, G.; Zanolla, L.; Dankert-Roelse, J.; Castellani, C.; ECFS CF Neonatal Screening Working Group. A survey of newborn screening for cystic fibrosis in Europe. J. Cyst. Fibros. 2007, 6, 57–65. [Google Scholar] [CrossRef]
- Farrell, P.; Joffe, S.; Foley, L.; Canny, G.J.; Mayne, P.; Rosenberg, M. Diagnosis of cystic fibrosis in the Republic of Ireland: Epidemiology and costs. Ir. Med. J. 2007, 100, 557–560. [Google Scholar]
- Newsletter ECFS Neonatal Screening Working Group. January 2014. Available online: https://www.ecfs.eu/sites/default/files/general-content-files/working-groups/NSWG_newsletter06Jan14. pdf (accessed on 1 March 2020).
- Lucotte, G.; Hazout, S.; De Braekeleer, M. Complete map of cystic fibrosis mutation DF508 frequencies in Western Europe and correlation between mutation frequencies and incidence of disease. Hum. Biol. 1995, 67, 797–803. [Google Scholar]
- Audrezet, M.P.; Munck, A.; Scotet, V.; Claustres, M.; Roussey, M.; Delmas, D.; Ferec, C.; Desgeorges, M. Comprehensive CFTR gene analysis of the French cystic fibrosis screened newborn cohort: Implications for diagnosis, genetic counseling, and mutation-specific therapy. Genet. Med. 2015, 17, 108–116. [Google Scholar] [CrossRef]
- Castellani, C.; Picci, L.; Tridello, G.; Casati, E.; Tamanini, A.; Bartoloni, L.; Scarpa, M.; Assael, B.M.; Veneto, C.F.L.N. Cystic fibrosis carrier screening effects on birth prevalence and newborn screening. Genet. Med. 2016, 18, 145–151. [Google Scholar] [CrossRef]
- Terlizzi, V.; Mergni, G.; Buzzetti, R.; Centrone, C.; Zavataro, L.; Braggion, C. Cystic fibrosis screen positive inconclusive diagnosis (CFSPID): Experience in Tuscany, Italy. J. Cyst. Fibros. 2019, 18, 484–490. [Google Scholar] [CrossRef]
- Bauca, J.M.; Morell-Garcia, D.; Vila, M.; Perez, G.; Heine-Suner, D.; Figuerola, J. Assessing the improvements in the newborn screening strategy for cystic fibrosis in the Balearic Islands. Clin. Biochem. 2015, 48, 419–424. [Google Scholar] [CrossRef]
- Skov, M.; Baekvad-Hansen, M.; Hougaard, D.M.; Skogstrand, K.; Lund, A.M.; Pressler, T.; Olesen, H.V.; Duno, M. Cystic fibrosis newborn screening in Denmark: Experience from the first 2 years. Pediatr. Pulmonol. 2020, 55, 549–555. [Google Scholar] [CrossRef]
- Dankert-Roelse, J.E.; Bouva, M.J.; Jakobs, B.S.; Janssens, H.M.; de Winter-de Groot, K.M.; Schonbeck, Y.; Gille, J.J.P.; Gulmans, V.A.M.; Verschoof-Puite, R.K.; Schielen, P.; et al. Newborn blood spot screening for cystic fibrosis with a four-step screening strategy in the Netherlands. J. Cyst. Fibros. 2019, 18, 54–63. [Google Scholar] [CrossRef]
- Sobczynska-Tomaszewska, A.; Oltarzewski, M.; Czerska, K.; Wertheim-Tysarowska, K.; Sands, D.; Walkowiak, J.; Bal, J.; Mazurczak, T.; NBS CF Working Group. Newborn screening for cystic fibrosis: Polish 4 years’ experience with CFTR sequencing strategy. Eur. J. Hum. Genet. 2013, 21, 391–396. [Google Scholar] [CrossRef]
- Soltysova, A.; Tothova Tarova, E.; Ficek, A.; Baldovic, M.; Polakova, H.; Kayserova, H.; Kadasi, L. Comprehensive genetic study of cystic fibrosis in Slovak patients in 25 years of genetic diagnostics. Clin. Respir. J. 2018, 12, 1197–1206. [Google Scholar] [CrossRef]
- Lannefors, L.; Lindgren, A. Demographic transition of the Swedish cystic fibrosis community–results of modern care. Respir. Med. 2002, 96, 681–685. [Google Scholar] [CrossRef]
- Marcao, A.; Barreto, C.; Pereira, L.; Guedes Vaz, L.; Cavaco, J.; Casimiro, A.; Felix, M.; Reis Silva, T.; Barbosa, T.; Freitas, C.; et al. Cystic fibrosis newborn screening in Portugal: PAP value in populations with stringent rules for genetic studies. Int. J. Neonatal Screen. 2018, 4, 22. [Google Scholar] [CrossRef]
- Lundman, E.; Gaup, H.J.; Bakkeheim, E.; Olafsdottir, E.J.; Rootwelt, T.; Storrosten, O.T.; Pettersen, R.D. Implementation of newborn screening for cystic fibrosis in Norway. Results from the first three years. J. Cyst. Fibros. 2016, 15, 318–324. [Google Scholar] [CrossRef]
- Popa, I.; Pop, L.; Popa, Z.; Schwarz, M.J.; Hambleton, G.; Malone, G.M.; Haworth, A.; Super, M. Cystic fibrosis mutations in Romania. Eur. J. Pediatr. 1997, 156, 212–213. [Google Scholar] [CrossRef]
- Kere, J.; Estivill, X.; Chillon, M.; Morral, N.; Nunes, V.; Norio, R.; Savilahti, E.; de la Chapelle, A. Cystic fibrosis in a low-incidence population: Two major mutations in Finland. Hum. Genet. 1994, 93, 162–166. [Google Scholar] [CrossRef]
- Barben, J.; Castellani, C.; Dankert-Roelse, J.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.; Munck, A.; Sands, D.; Sommerburg, O.; et al. The expansion and performance of national newborn screening programmes for cystic fibrosis in Europe. J. Cyst. Fibros. 2017, 16, 207–213. [Google Scholar] [CrossRef]
- Wilcken, B.; Wiley, V.; Sherry, G.; Bayliss, U. Neonatal screening for cystic fibrosis: A comparison of two strategies for case detection in 1.2 million babies. J. Pediatr. 1995, 127, 965–970. [Google Scholar] [CrossRef]
- Massie, R.J.; Curnow, L.; Glazner, J.; Armstrong, D.S.; Francis, I. Lessons learned from 20 years of newborn screening for cystic fibrosis. Med. J. Aust. 2012, 196, 67–70. [Google Scholar] [CrossRef]
- Wesley, A.W.; Stewart, A.W. Cystic fibrosis in New Zealand: Incidence and mortality. N. Z. Med. J. 1985, 98, 321–323. [Google Scholar]
- Kosorok, M.R.; Wei, W.H.; Farrell, P.M. The incidence of cystic fibrosis. Stat. Med. 1996, 15, 449–462. [Google Scholar] [CrossRef]
- Lilley, M.; Christian, S.; Hume, S.; Scott, P.; Montgomery, M.; Semple, L.; Zuberbuhler, P.; Tabak, J.; Bamforth, F.; Somerville, M.J. Newborn screening for cystic fibrosis in Alberta: Two years of experience. Paediatr. Child Health 2010, 15, 590–594. [Google Scholar] [CrossRef]
- Eyheramendy, S.; Martinez, F.I.; Manevy, F.; Vial, C.; Repetto, G.M. Genetic structure characterization of Chileans reflects historical immigration patterns. Nat. Commun. 2015, 6, 6472. [Google Scholar] [CrossRef]
- Lay-Son, G.; Puga, A.; Astudillo, P.; Repetto, G.M.; Collaborative Group of the Chilean National Cystic Fibrosis Program. Cystic fibrosis in Chilean patients: Analysis of 36 common CFTR gene mutations. J. Cyst. Fibros. 2011, 10, 66–70. [Google Scholar] [CrossRef]
- Silva Filho, L.V.; Castanos, C.; Ruiz, H.H. Cystic fibrosis in Latin America-Improving the awareness. J. Cyst. Fibros. 2016, 15, 791–793. [Google Scholar] [CrossRef]
- Nazer, H.M. Early diagnosis of cystic fibrosis in Jordanian children. J. Trop. Pediatr. 1992, 38, 113–115. [Google Scholar] [CrossRef]
- Al-Mahroos, F. Cystic fibrosis in Bahrain incidence, phenotype, and outcome. J. Trop. Pediatr. 1998, 44, 35–39. [Google Scholar] [CrossRef]
- Frossard, P.M.; Lestringant, G.; Girodon, E.; Goossens, M.; Dawson, K.P. Determination of the prevalence of cystic fibrosis in the United Arab Emirates by genetic carrier screening. Clin. Genet. 1999, 55, 496–497. [Google Scholar] [CrossRef]
- Stafler, P.; Mei-Zahav, M.; Wilschanski, M.; Mussaffi, H.; Efrati, O.; Lavie, M.; Shoseyov, D.; Cohen-Cymberknoh, M.; Gur, M.; Bentur, L.; et al. The impact of a national population carrier screening program on cystic fibrosis birth rate and age at diagnosis: Implications for newborn screening. J. Cyst. Fibros. 2016, 15, 460–466. [Google Scholar] [CrossRef]
- Goodchild, M.C.; Insley, J.; Rushton, D.I.; Gaze, H. Cystic fibrosis in 3 Pakistani children. Arch. Dis. Child. 1974, 49, 739–741. [Google Scholar] [CrossRef]
- Powers, C.A.; Potter, E.M.; Wessel, H.U.; Lloyd-Still, J.D. Cystic fibrosis in Asian Indians. Arch. Pediatr. Adolesc. Med. 1996, 150, 554–555. [Google Scholar] [CrossRef]
- Kapoor, V.; Shastri, S.S.; Kabra, M.; Kabra, S.K.; Ramachandran, V.; Arora, S.; Balakrishnan, P.; Deorari, A.K.; Paul, V.K. Carrier frequency of F508del mutation of cystic fibrosis in Indian population. J. Cyst. Fibros. 2006, 5, 43–46. [Google Scholar] [CrossRef]
- Kabra, S.K.; Kabra, M.; Lodha, R.; Shastri, S. Cystic fibrosis in India. Pediatr. Pulmonol. 2007, 42, 1087–1094. [Google Scholar] [CrossRef]
- Wright, S.W.; Morton, N.E. Genetic studies on cystic fibrosis in Hawaii. Am. J. Hum. Genet. 1968, 20, 157–169. [Google Scholar]
- Yamashiro, Y.; Shimizu, T.; Oguchi, S.; Shioya, T.; Nagata, S.; Ohtsuka, Y. The estimated incidence of cystic fibrosis in Japan. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 544–547. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Tian, X.; Xu, K.F.; Xu, W.; Li, X.; Yue, C.; Zhang, P.; Xiao, Y.; Zhang, X. Characterization of gene mutations and phenotypes of cystic fibrosis in Chinese patients. Respirology 2015, 20, 312–318. [Google Scholar] [CrossRef]
- Singh, M.; Rebordosa, C.; Bernholz, J.; Sharma, N. Epidemiology and genetics of cystic fibrosis in Asia: In preparation for the next-generation treatments. Respirology 2015, 20, 1172–1181. [Google Scholar] [CrossRef]
- Al-Sadeq, D.; Abunada, T.; Dalloul, R.; Fahad, S.; Taleb, S.; Aljassim, K.; Al Hamed, F.A.; Zayed, H. Spectrum of mutations of cystic fibrosis in the 22 Arab countries: A systematic review. Respirology 2019, 24, 127–136. [Google Scholar] [CrossRef]
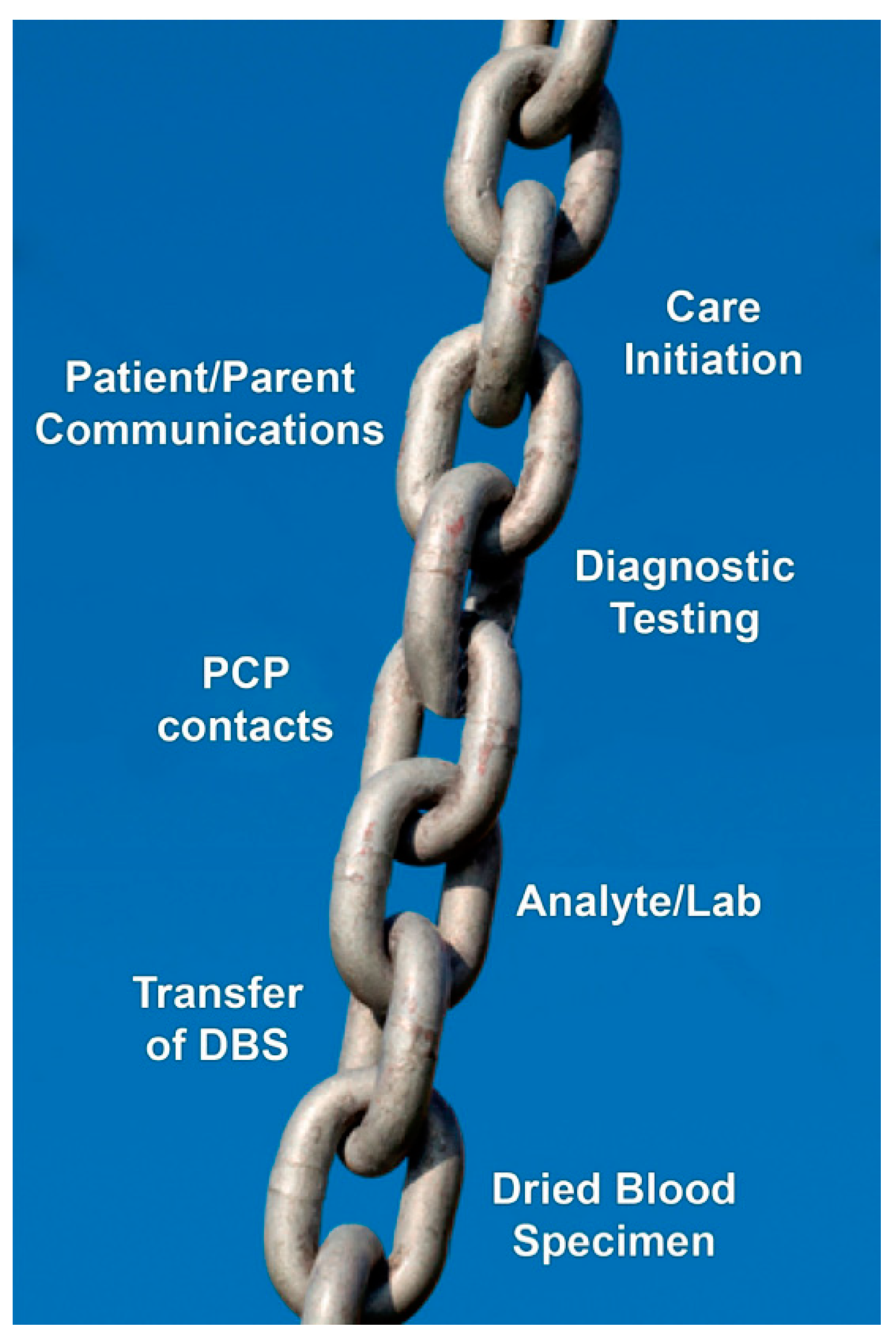
| 1 | A system must be established and functioning well for the universal collection of dried blood spot specimens and their analysis in a central laboratory with quality assurance mechanisms in place and a goal to maximum sensitivity with acceptable specificity. |
| 2 | Collaborative efforts by a team that includes NBS laboratory leadership and CF center follow-up clinicians organized to operate efficiently. |
| 3 | Effective CF NBS analytical tests organized as a sequential protocol (algorithm) to maximize sensitivity and optimize specificity. |
| 4 | Quality improvements in laboratory methods must be planned for and implemented as technologies advance rather than accepting the status quo and resisting change. |
| 5 | Expeditious follow-up care must ensure that not only will high-quality sweat testing be provided promptly to confirm diagnoses but that the nutritional benefits are achieved immediately by a team of dedicated, experienced caregivers with gastrointestinal/nutritional expertise. |
| 6 | A cohort follow-up system must be ensured for patients diagnosed as neonates to segregate them from older patients and avoid exposure to virulent respiratory pathogens. |
| 7 | To ensure a favorable benefit: risk relationship, preventive management of potential psychosocial harms must be given priority by a skilled, dedicated follow-up team. |
| 8 | The incidence of CF must be high enough to warrant CF care centers in the NBS region. |
| 9 | The NBS system must be organized as a highly efficient operation that avoids preventable delays and ensures consistently diagnostic timeliness. |
| 10 | CF NBS guidelines should be known and adhered to throughout the sequence of integrated processes. |
| 1 | Population characteristics that validate screening newborn infants for CF.“Health authorities need to balance the benefit/risk ratio of screening newborns for CF in their population. If the incidence of CF is <1/7000 births, careful evaluation is required as to whether NBS is valid. The protocol must be shown to cause the minimum negative impact possible on the population. Other factors in making the decision on whether to implement screening should include available healthcare resources and the ability to provide a clear pathway to treatment.” |
| 2 | Health and social resources that are minimally acceptable for NBS to be a valid undertaking.“Infants identified with CF through a NBS program should have prompt access to specialist CF care that achieves ECFS standards. A NBS program may be a mechanism to better organize CF services, through the direct referral of infants for specialist CF care. Countries with limited resources should consider a pilot study to assess the validity of NBS and the adequacy of referral services for newly diagnosed infants in their population.” |
| 3 | Acceptable number of repeat tests required for inadequate dried blood samples for every 1000 infants screened.“The number of requests for repeat dried blood samples should be monitored and should be 0.5%. More than 20 repeats for every 1000 infants, is unacceptable (2%).” |
| 4 | Acceptable number of false-positive NBS results (infants referred for clinical assessment and sweat testing).“Programmes should aim for a minimum positive predictive value of 0.3 (PPV is the number of infants with a true positive NBS test divided by the total number of positive NBS tests).” |
| 5 | Acceptable number of false-negative NBS results. These are infants with a negative NBS test that are subsequently diagnosed with CF (a delayed diagnosis).“Programmes should aim for a minimum sensitivity of 95%.” |
| 6 | Maximum acceptable delay between a sweat test being undertaken and the result given to the family. “The sweat test should be analyzed immediately and the result reported to the family on the same day.” |
| 7 | Maximum acceptable age of an infant on the day they are first reviewed by a specialist CF team following a diagnosis of CF after NBS.“The majority of infants with a confirmed diagnosis after NBS should be seen by a specialist CF team by 35 days and no later than 58 days after birth.” |
| 8 | Minimum acceptable information for families of an infant recognized to be a carrier of a CF-causing mutation after NBS.Families should receive a verbal report of the result. They should also receive written information to refer to. Information should also be sent to the family Primary Care Physician. The information should be clear that the infant does not have CF; the baby is a healthy carrier; future pregnancies for this couple are not free of risk of CF and the parents may opt for genetic counseling, and there are implications that could affect reproductive decision making for extended family members and the infant when they are of childbearing age. |
| Incidence of CF: greater than 1:25,000 |
| Aim at minimum sensitivity of 95% |
| IRT/DNA—unless unavailable or not feasible |
| Diagnosis including sweat chloride within 4 weeks of age |
| Assessment program for tests, including plans for monitoring and updating |
| Availability of a complete specialist CF team |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scotet, V.; Gutierrez, H.; Farrell, P.M. Newborn Screening for CF across the Globe—Where Is It Worthwhile? Int. J. Neonatal Screen. 2020, 6, 18. https://doi.org/10.3390/ijns6010018
Scotet V, Gutierrez H, Farrell PM. Newborn Screening for CF across the Globe—Where Is It Worthwhile? International Journal of Neonatal Screening. 2020; 6(1):18. https://doi.org/10.3390/ijns6010018
Chicago/Turabian StyleScotet, Virginie, Hector Gutierrez, and Philip M. Farrell. 2020. "Newborn Screening for CF across the Globe—Where Is It Worthwhile?" International Journal of Neonatal Screening 6, no. 1: 18. https://doi.org/10.3390/ijns6010018
APA StyleScotet, V., Gutierrez, H., & Farrell, P. M. (2020). Newborn Screening for CF across the Globe—Where Is It Worthwhile? International Journal of Neonatal Screening, 6(1), 18. https://doi.org/10.3390/ijns6010018





