Exceptionally High Cystic Fibrosis-Related Morbidity and Mortality in Infants and Young Children in India: The Need for Newborn Screening and CF-Specific Capacity Building
Abstract
1. Introduction—Cystic Fibrosis in India
- If NBS cannot be immediately implemented to identify all babies affected by CF, what can be done now?
- Can we diagnose CF reliably in the absence of NBS?
- Can we create a simple clinical screening tool to identify those who are at risk of dying in the first few months of life and focus our attention on them?
- Can we integrate such clinical tools during routine healthcare for infants?
2. Methods
2.1. Location
2.2. Subjects and Diagnostic Confirmation
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Clinical Profiles of 56 Children Diagnosed with CF in Infancy
3.3. Comparative Analysis Between Infants with CF Who Survived and Those Who Died
3.4. Risk Factor Analysis for Infants Who Died Prior to One-Year of Age
4. Discussion
4.1. Significance of the Quantitative Assessments of Infant Growth in the Study Cohort
4.2. The Deadly Triad
4.3. Severe Anemia—An Ominous Sign
4.4. Dehydration and PseudoBartter Syndrome
4.5. Systematic Disease Recognition Tools
4.6. Misdiagnosis and Missed Diagnosis
4.7. Newborn Screening—An Important Goal
4.8. Limitations
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farrell, P.M.; Paul, G.R.; Varkki, S.D. India: The Last and Best Frontier for Cystic Fibrosis Newborn Screening with Perspectives on Special Challenges. Int. J. Neonatal Screen. 2025, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Kabra, S.K.; Kabra, M.; Lodha, R.; Shastri, S. Cystic fibrosis in India. Pediatr. Pulmonol. 2007, 42, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Kawoosa, M.S.; Bhat, M.A.; Ali, S.W.; Hafeez, I.; Shastri, S. Clinical and mutation profile of children with cystic fibrosis in Jammu and Kashmir. Indian. Pediatr. 2014, 51, 185–189. [Google Scholar] [CrossRef] [PubMed]
- CFF Patient Registry 2024. 2024. Available online: https://www.cff.org/medical-professionals/2024-patient-registry-highlights (accessed on 25 June 2025).
- European CF Society Patient Registry 2023. 2023. Available online: https://www.ecfs.eu/sites/default/files/general-content-files/Annual%20Report_2023_vs1.0_ECFSPR_20250507.pdf (accessed on 25 June 2025).
- Kabra, S.K.; Kabra, M.; Lodha, R.; Shastri, S.; Ghosh, M.; Pandey, R.M.; Kapil, A.; Aggarwal, G.; Kapoor, V. Clinical profile and frequency of delta f508 mutation in Indian children with cystic fibrosis. Indian. Pediatr. 2003, 40, 612–619. [Google Scholar]
- Singh, K.; Bijarnia-Mahay, S.; Ramprasad, V.L.; Puri, R.D.; Nair, S.; Sharda, S.; Saxena, R.; Kohli, S.; Kulshreshtha, S.; Ganguli, I.; et al. NGS-based expanded carrier screening for genetic disorders in North Indian population reveals unexpected results—A pilot study. BMC Med. Genet. 2020, 21, 216. [Google Scholar] [CrossRef]
- Clinical and Functional Translation of CFTR. 2024. Available online: https://cftr2.org/ (accessed on 25 June 2025).
- American College of Medical Genetics. 2023. Available online: https://www.acmg.net/ACMG/Medical-Genetics-Practice-Resources/Practice_Resources/ACMG/Medical-Genetics-Practice-Resources/Medical-Genetics-Practice-Resources.aspx?hkey=d56a0de8-cfb0-4c6e-bf1e-ffb96e5f86aa (accessed on 22 June 2023).
- Saiman, L.; Waters, V.; LiPuma, J.J.; Hoffman, L.R.; Alby, K.; Zhang, S.X.; Yau, Y.C.; Downey, D.G.; Sermet-Gaudelus, I.; Bouchara, J.P.; et al. Practical Guidance for Clinical Microbiology Laboratories: Updated guidance for processing respiratory tract samples from people with cystic fibrosis. Clin. Microbiol. Rev. 2024, 37, e0021521. [Google Scholar] [CrossRef]
- Wald, E.R. Recurrent and nonresolving pneumonia in children. Semin. Respir. Infect. 1993, 8, 46–58. [Google Scholar]
- Kabra, S.K. Challenges in the care of cystic fibrosis in low-to-middle income countries. Pediatr. Pulmonol. 2025, 60 (Suppl. S1), S48–S50. [Google Scholar] [CrossRef]
- Varkki, S.; James, E.J.G. Is Cystic Fibrosis Contributing Significantly to Infant Mortality Rate in India? Indian. Pediatr. 2022, 59, 728. [Google Scholar] [CrossRef]
- Thomas, L.; Kumar, M.; Lionel, B.A.P.; Varkki, S.; Rebekah, G. Pancreatic, hepatobiliary, and gastrointestinal manifestations of children with cystic fibrosis: A 10-year experience from a tertiary care center in southern India. Indian. J. Gastroenterol. 2022, 41, 266–272. [Google Scholar] [CrossRef]
- Thomas, L.; John, S.T.; Lionel, B.A.P.; Rebekah, G.; Kumar, M.; Punnen, A.; Varkki, S. Effect of malnutrition in infants with cystic fibrosis in India: An underestimated danger. J. Family Med. Prim. Care 2021, 10, 1994–1997. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Larsen, B.F. The incidence of anemia, hypoproteinemia, and edema in infants as presenting symptoms of cystic fibrosis: A retrospective survey of the frequency of this symptom complex in 130 patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 1982, 1, 355–359. [Google Scholar] [CrossRef]
- Wilfond, B.S.; Farrell, P.M.; Laxova, A.; Mischler, E. Severe hemolytic anemia associated with vitamin E deficiency in infants with cystic fibrosis. Implications for neonatal screening. Clin. Pediatr. 1994, 33, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Bieri, J.G.; Fratantoni, J.F.; Wood, R.E.; di Sant’Agnese, P.A. The occurrence and effects of human vitamin E deficiency. A study in patients with cystic fibrosis. J. Clin. Investig. 1977, 60, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Mantoo, M.R.; Kabra, M.; Kabra, S.K. Cystic Fibrosis Presenting as Pseudo-Bartter Syndrome: An Important Diagnosis that is Missed! Indian. J. Pediatr. 2020, 87, 726–732. [Google Scholar] [CrossRef] [PubMed]
- da Silva Filho, L.; Zampoli, M.; Cohen-Cymberknoh, M.; Kabra, S.K. Cystic fibrosis in low and middle-income countries (LMIC): A view from four different regions of the world. Paediatr. Respir. Rev. 2021, 38, 37–44. [Google Scholar] [CrossRef]
- Scotet, V.; L’Hostis, C.; Ferec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef]
- Guo, J.; Garratt, A.; Hill, A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J. Cyst. Fibros. 2022, 21, 456–462. [Google Scholar] [CrossRef]
- Dhochak, N.; Lodha, R.; Jat, K.R.; Bhat, J.I.; Kumar, P.; Goyal, J.P.; Varkki, S.; Kabra, S.K.; Sankar, J.; Madhan Kumar, P.; et al. Diagnostic accuracy of ancillary tests in diagnosis of cystic fibrosis and development of cystic fibrosis clinical diagnostic score: A multicentre prospective cohort study. Respir. Med. 2025, 242, 108087. [Google Scholar] [CrossRef]
- Fuhrer, M.; Zampoli, M.; Abriel, H. Diagnosing cystic fibrosis in low- and middle-income countries: Challenges and strategies. Orphanet J. Rare Dis. 2024, 19, 482. [Google Scholar] [CrossRef]
- Panchbudhe, S.A.; Shivkar, R.R.; Banerjee, A.; Deshmukh, P.; Maji, B.K.; Kadam, C.Y. Improving newborn screening in India: Disease gaps and quality control. Clin. Chim. Acta 2024, 557, 117881. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Abadingo, M.E.; Adhikari, S.P.; Aung, T.; Aye, T.T.; Dey, S.K.; Faizi, M.; Ganbaatar, E.; Giang, T.T.H.; et al. Consolitated Newborn Bloodspot Screening Efforts in Developing Countries in the Asia Pacific-2024. Int. J. Neonatal Screen. 2025, 11, 2. [Google Scholar] [CrossRef]
- Kaur, H.; Pandey, S.; Jat, K.R.; Lodha, R.; Kabra, S.K. Predictors of mortality in children with cystic fibrosis in India. Pediatr. Pulmonol. 2022, 57, 648–654. [Google Scholar] [CrossRef]
- Mir, T.A.; Ashraf, M.; Ahmed, K.; Chowdhary, J.; Rehana, B.; Ahmed, J. Clinical profile, diagnostic delay, and genetic make-up of cystic fibrosis in Kashmir, India. Lung India 2011, 28, 97–100. [Google Scholar] [CrossRef] [PubMed]
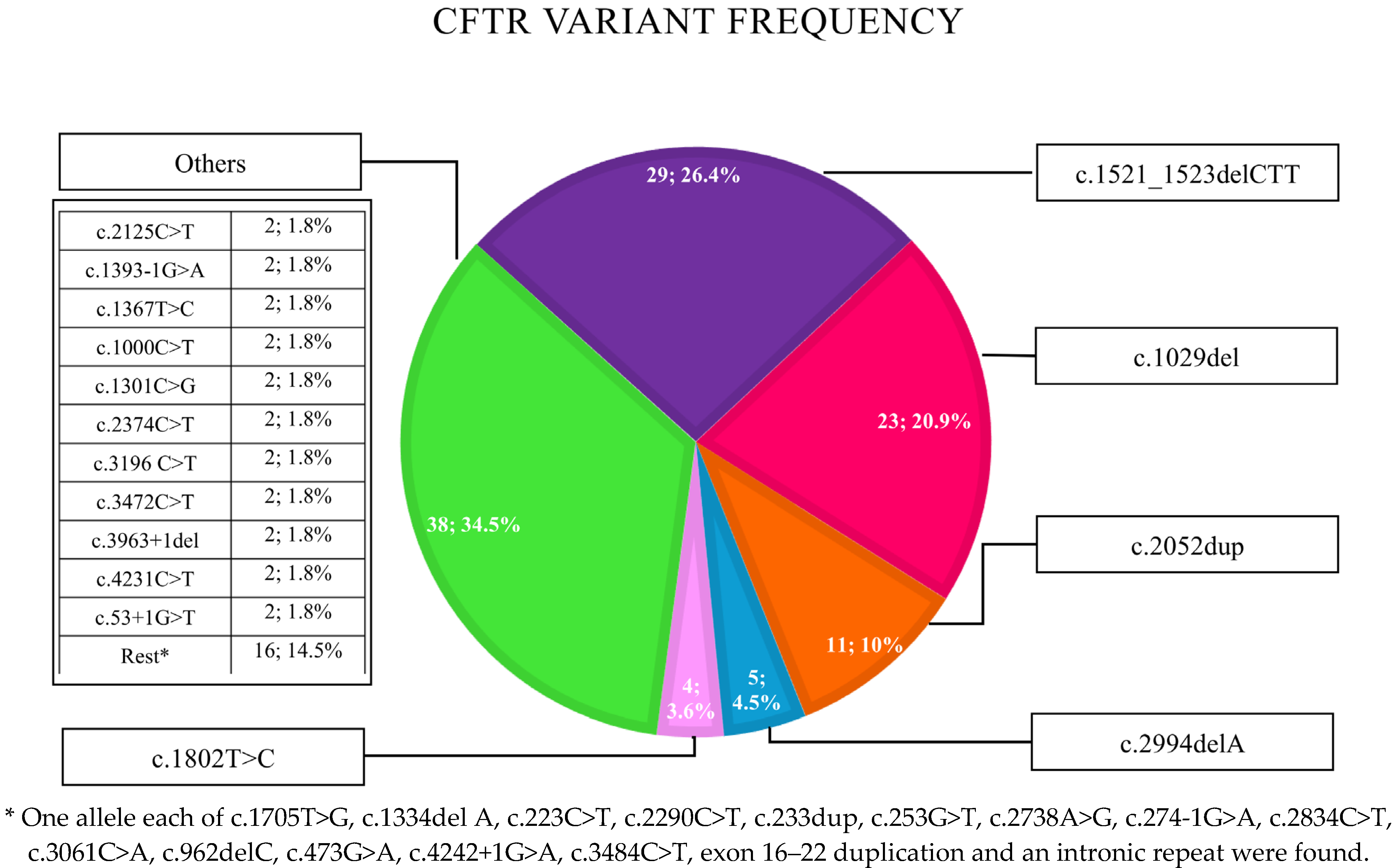
| Demographics | Results |
|---|---|
| Median current age of those alive (months) n = 33 | 52.4 (7–110 months) |
| Age breakdown | |
| - <1 year | 4 |
| - 1–5 years | 15 |
| - >5 year | 14 |
| Median age of death of non-survivors (months) n = 23 | 5 (2–87 months) |
| Age breakdown | |
| - <1 year | 18 |
| - 1–5 years | 4 |
| - >5 years | 1 |
| Median age at Diagnosis (months) n = 56 | 5.4 (1–11) months |
| Male:Female | 2:1 |
| Consanguineous parentage (N = 55 families) | 19 (33.9%) |
| Clinical Features at Diagnosis | Numbers (%) |
| Term Birth (Gestational age ≥ 37 weeks) | 53 (93.6%) |
| Low Birth weight (≤2500 g) | 14 (25.0%) |
| Meconium ileus | 7 (12.5%) |
| Exocrine pancreatic insufficiency * | 49 (87.5%) |
| PseudoBartter presentation | 14 (25.0%) |
| Hypoalbuminemia < 2 gm/dL | 11 (20.7%) |
| Severe malnutrition with edema ** at presentation | 15 (28.6%) |
| Recurrent pneumonia prior to diagnosis # | 42 (75.0%) |
| Severe Anemia needing blood transfusion ## | 24 (42.8%) |
| Investigations | |
| Sweat Chloride ≥60 mmol/L | 30 (81%) |
| 30–59 mmol/L | 4 (10.8%) |
| <30 mmol/L | 3 (8.1%) |
| Not available | 19 |
| Zygosity of Common CFTR Variants | |
| - Patients with at least one F508del variant | 20/55 (36.4%) |
| - Patients homozygous for F508del variant | 9/55 (16.4%) |
| - Patients homozygous for c.1029del variant | 8/55 (14.5%) |
| - Patients with other mutations ^ | 18/55 (32.7%) |
| - No data | 1/56 |
| Mortality Related to CF | |
| No. of families who had already lost a child to confirmed CF/probable CF | 15 (26.8%) |
| Mortality in the cohort | 23/56 (41.0%) |
| Variables | Patients Who Died (n = 23, 41%) | Patients Who Survived (n = 33, 59%) | p Value |
|---|---|---|---|
| Median current age of those alive (months) | N/A | 55 (7–110) months | N/A |
| Median age of death of non-survivors (months) | 5 (2–87 months) | N/A | N/A |
| Mean age at diagnosis (months) | 4.9 (±2.5) | 5.7 (±3.1) | 0.28 |
| Presence of homozygous CFTR variants. (Most common: F508del, c.1029del) | 19 (82.6) | 13 (40.6) | <0.002 |
| Mean weight at birth (gm) | 2590 (±560) | 2770 (±390) | 0.16 |
| Weight z-score at birth | −1.726 | −1.43 | 0.39 |
| Mean weight at 6–8 weeks (gm) | 2700 (±670) | 3500 (±750) | 0.002 |
| Weight for age z-score at 6–8 weeks | −3.908 | −3.136 | 0.22 |
| Decrease in weight z-score from birth to 6–8 weeks | −2.33 | −1.75 | 0.37 |
| Exocrine pancreatic insufficiency * (%) | 23 (100) | 26 (78.8) | <0.034 |
| Hypoalbuminemia (<2 gm/dL) (n, %) | 10 (43.5) | 1 (3) | <0.0001 |
| Severe malnutrition with edema ** (n, %) | 11 (47.8) | 5 (15.2) | <0.008 |
| Severe anemia requiring blood transfusion before 6 months of age # (n, %) | 16 (69.6) | 8 (24.2) | <0.001 |
| Triad of hypoalbuminemia, anemia, and edema at diagnosis (n, %) | 11 (47.8) | 3 (9.1) | <0.001 |
| Infants who had LRTI ^ requiring hospital admission before diagnosis (n, %) | 16 (69.6) | 26 (78.8) | <0.433 |
| PseudoBartter presentation (n, %) | 1 (4.3) | 13 (39.4) | <0.003 |
| Infants who never had an opportunity to receive PERT for >2 weeks | 14 (60.9) | 0 | <0.0001 |
| Sibling death with suspected CF (%) | 10 (43.5) | 5 (15.2) | 0.019 |
| Dead ≤ 12 Months N (%) | Alive > 12 Months N (%) | Odds Ratio | p Value | ||
|---|---|---|---|---|---|
| Albumin < 2 g/dL | Yes | 8 (47.1 *) | 3 (8.3 *) | 9.78 (2.14–44.6) | 0.002 |
| No | 9 (52.9 *) | 33 (91.7 *) | |||
| Severe malnutrition with edema | Yes | 9 (50.0) | 7 (18.4) | 4.43 (1.28–15.23) | 0.018 |
| No | 9 (50.0) | 31 (81.6) | |||
| PseudoBartter syndrome | Yes | 1 (5.6) | 13 (34.2) | 0.11 (0.01–0.95) | 0.044 |
| No | 17 (94.4) | 25 (65.8) | |||
| Blood transfusion for severe anemia | Yes | 11 (61.1) | 13 (34.2) | 3.02 (0.95–9.65) | 0.062 |
| No | 7 (38.9) | 25 (65.8) | |||
| Edema | Yes | 8 (44.4) | 6 (15.8) | 4.26 (1.19–15.3) | 0.026 |
| No | 10 (55.6) | 32 (84.2) | |||
| Triad of anemia, edema, hypoalbuminemia | Yes | 8 (44.4) | 6 (15.8) | 4.27 (1.19–15.3) | 0.026 |
| No | 10 (55.6) | 32 (84.2) | |||
| Homozygous CFTR pathogenic variants | Yes | 15 (83.3) | 17 (45.9) | 5.88 (1.45–23.8) | 0.013 |
| No | 3 (16.7) | 20 (54.1) | |||
| Suspected/confirmed diagnosis of CF in sibling | Yes | 9 (50.0) | 6 (15.8) | 5.33 (1.49–18.9) | 0.01 |
| No | 9 (50.0) | 32 (84.2) | |||
| Meconium Ileus | Yes | 1 (5.6) | 6 (15.8) | 0.31 (0.04–2.82) | 0.314 |
| No | 17 (94.4) | 32 (84.2) | |||
| PERT for at least 2 weeks | Yes | 4 (22.2) | 31 (100.0) | Convincing, but cannot be calculated | Highly significant |
| No | 14 (77.8) | 0 (0.0) | |||
| PI | Yes | 18 (100.0) | 31 (81.6) | Convincing, but cannot be calculated | Highly significant |
| No | 0 (0.0) | 7 (18.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medhi, P.; Paul, G.R.; Kumar, M.; Rebekah, G.; Farrell, P.M.; Chandran, J.; Aaron, R.; Chapla, A.; Varkki, S.D. Exceptionally High Cystic Fibrosis-Related Morbidity and Mortality in Infants and Young Children in India: The Need for Newborn Screening and CF-Specific Capacity Building. Int. J. Neonatal Screen. 2025, 11, 67. https://doi.org/10.3390/ijns11030067
Medhi P, Paul GR, Kumar M, Rebekah G, Farrell PM, Chandran J, Aaron R, Chapla A, Varkki SD. Exceptionally High Cystic Fibrosis-Related Morbidity and Mortality in Infants and Young Children in India: The Need for Newborn Screening and CF-Specific Capacity Building. International Journal of Neonatal Screening. 2025; 11(3):67. https://doi.org/10.3390/ijns11030067
Chicago/Turabian StyleMedhi, Priyanka, Grace R. Paul, Madhan Kumar, Grace Rebekah, Philip M. Farrell, Jolly Chandran, Rekha Aaron, Aaron Chapla, and Sneha D. Varkki. 2025. "Exceptionally High Cystic Fibrosis-Related Morbidity and Mortality in Infants and Young Children in India: The Need for Newborn Screening and CF-Specific Capacity Building" International Journal of Neonatal Screening 11, no. 3: 67. https://doi.org/10.3390/ijns11030067
APA StyleMedhi, P., Paul, G. R., Kumar, M., Rebekah, G., Farrell, P. M., Chandran, J., Aaron, R., Chapla, A., & Varkki, S. D. (2025). Exceptionally High Cystic Fibrosis-Related Morbidity and Mortality in Infants and Young Children in India: The Need for Newborn Screening and CF-Specific Capacity Building. International Journal of Neonatal Screening, 11(3), 67. https://doi.org/10.3390/ijns11030067






