MCT8 Deficiency in Infancy: Opportunities for Early Diagnosis and Screening †
Abstract
1. Introduction
2. Case Description
2.1. Diagnosis and Early Treatment Initiation During Infancy
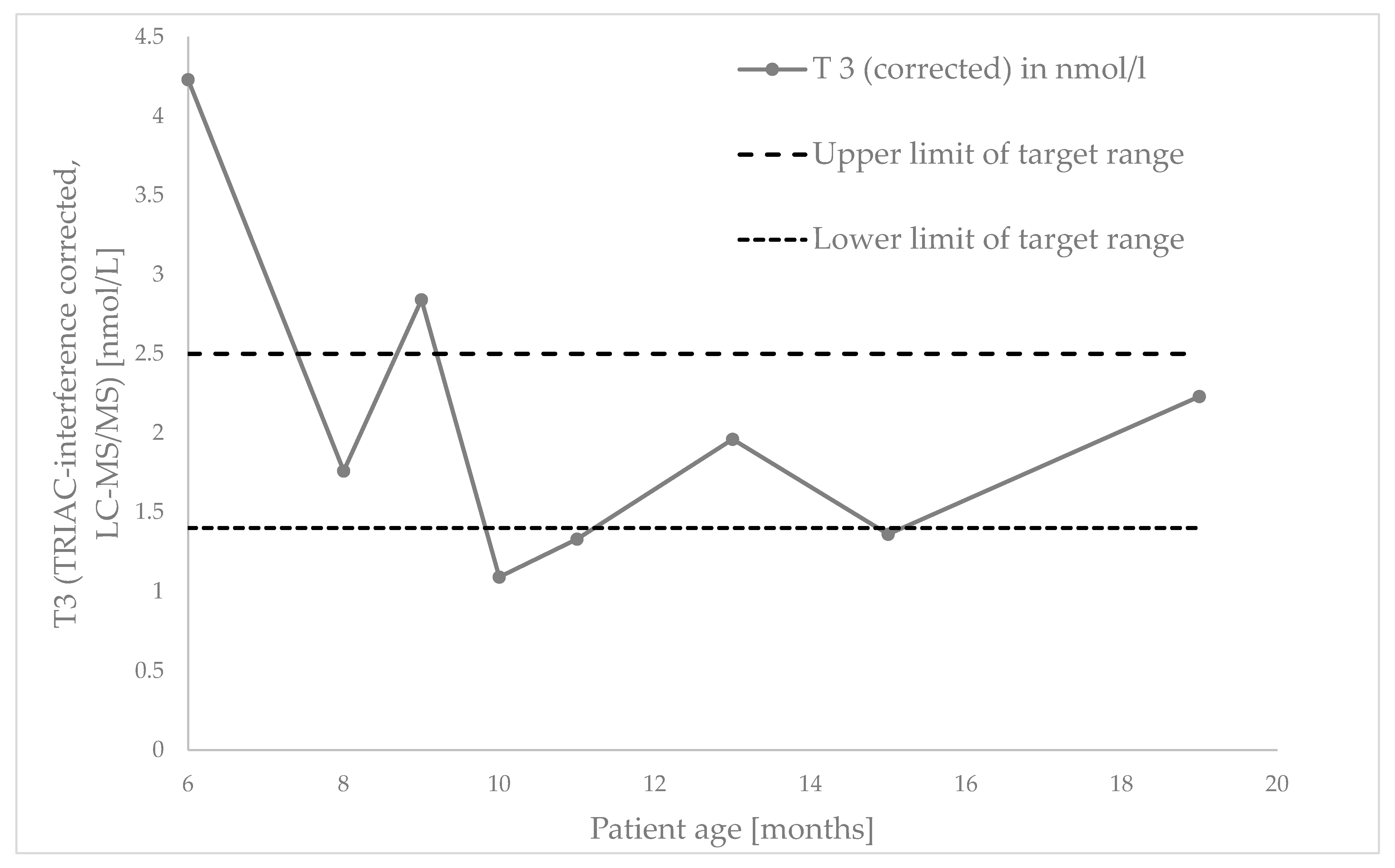
2.2. Follow-Up at Three Years
3. Discussion/Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCT8 | Monocarboxylate transporter 8 |
| AHDS | Allan–Herndon–Dudley syndrome |
| TRIAC | Triiodothyroacetic acid (Trade name: Emcitate®) |
References
- Vatine, G.D.; Shelest, O.; Barriga, B.K.; Ofan, R.; Rabinski, T.; Mattis, V.B.; Heuer, H.; Svendsen, C.N. Oligodendrocyte progenitor cell maturation is dependent on dual function of MCT8 in the transport of thyroid hormone across brain barriers and the plasma membrane. Glia 2021, 69, 2146–2159. [Google Scholar] [CrossRef]
- Bernal, J.; Guadaño-Ferraz, A.; Morte, B. Thyroid hormone transporters--functions and clinical implications. Nat. Rev. Endocrinol. 2015, 11, 406–417, Correction in Nat. Rev. Endocrinol. 2015, 11, 506; Correction in Nat. Rev. Endocrinol. 2015, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Friesema, E.C.; Kuiper, G.G.; Jansen, J.; Visser, T.J.; Kester, M.H. Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol. Endocrinol. 2006, 20, 2761–2772. [Google Scholar] [CrossRef]
- Friesema, E.C.; Ganguly, S.; Abdalla, A.; Manning Fox, J.E.; Halestrap, A.P.; Visser, T.J. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem. 2003, 278, 40128–40135. [Google Scholar] [CrossRef]
- Heuer, H.; Maier, M.K.; Iden, S.; Mittag, J.; Friesema, E.C.H.; Visser, T.J.; Bauer, K. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology 2005, 146, 1701–1706. [Google Scholar] [CrossRef]
- Nishimura, M.; Naito, S. Tissue-specific mRNA expression profiles of human solute carrier transporter superfamilies. Drug Metab. Pharmacokinet. 2008, 23, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, S.; van Geest, F.S.; Abacı, A.; Alcantud, A.; Ambegaonkar, G.P.; Armour, C.M.; Bakhtiani, P.; Barca, D.; Bertini, P.E.S.; van Beynum, I.M.; et al. Disease characteristics of MCT8 deficiency: An international, retrospective, multicentre cohort study. Lancet Diabetes Endocrinol. 2020, 8, 594–605, Correction in Lancet Diabetes Endocrinol. 2022, 10, e7. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.M.; Liao, X.H.; Best, T.B.; Brockmann, K.; Refetoff, S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am. J. Hum. Genet. 2004, 74, 168–175, Correction in Am. J. Hum. Genet. 2004, 74, 598. [Google Scholar] [CrossRef]
- Visser, W.E.; Vrijmoeth, P.; Visser, F.E.; Arts, W.F.; van Toor, H.; Visser, T.J. Identification, functional analysis, prevalence and treatment of monocarboxylate transporter 8 (MCT8) mutations in a cohort of adult patients with mental retardation. Clin. Endocrinol. 2013, 78, 310–315. [Google Scholar] [CrossRef]
- van Geest, F.S.; Groeneweg, S.; Visser, W.E. Monocarboxylate transporter 8 deficiency: Update on clinical characteristics and treatment. Endocrine 2021, 71, 689–695. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Groeneweg, S.; Peeters, R.P.; Moran, C.; Stoupa, A.; Auriol, F.; Tonduti, D.; Dica, A.; Paone, L.; Rozenkova, K.; Malikova, J.; et al. Effectiveness and safety of the tri-iodothyronine analogue Triac in children and adults with MCT8 deficiency: An international, single-arm, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019, 7, 695–706. [Google Scholar] [CrossRef]
- van Geest, F.S.; Groeneweg, S.; van den Akker, E.L.T.; Bacos, I.; Barca, D.; van den Berg, S.A.A.; Bertini, E.; Brunner, D.; Brunetti-Pierri, N.; Cappa, M.; et al. Long-Term Efficacy of T3 Analogue Triac in Children and Adults With MCT8 Deficiency: A Real-Life Retrospective Cohort Study. J. Clin. Endocrinol. Metab. 2022, 107, e1136–e1147. [Google Scholar] [CrossRef]
- Reinwald, J.R.; Weber-Fahr, W.; Cosa-Linan, A.; Becker, R.; Sack, M.; Falfan-Melgoza, C.; Gass, N.; Braun, U.; Clemm von Hohenberg, C.; Chen, J.; et al. TRIAC Treatment Improves Impaired Brain Network Function and White Matter Loss in Thyroid Hormone Transporter Mct8/Oatp1c1 Deficient Mice. Int. J. Mol. Sci. 2022, 23, 15547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.; Salveridou, E.; Liebmann, L.; Sundaram, S.M.; Doycheva, D.; Markova, B.; Hübner, C.A.; Boelen, A.; Visser, W.E.; Heuer, H.; et al. Triac Treatment Prevents Neurodevelopmental and Locomotor Impairments in Thyroid Hormone Transporter Mct8/Oatp1c1 Deficient Mice. Int. J. Mol. Sci. 2023, 24, 3452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freund, M.E.T.; van der Most, F.; Visser, W.E. Diagnosis and Therapy in MCT8 Deficiency: Ongoing Challenges. J. Clin. Res. Pediatr. Endocrinol. 2024, 16, 1–3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Geest, F.S.; Groeneweg, S.; Popa, V.M.; Stals, M.A.M.; Visser, W.E. Parent Perspectives on Complex Needs in Patients With MCT8 Deficiency: An International, Prospective, Registry Study. J. Clin. Endocrinol. Metab. 2023, 109, e330–e335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Groeneweg, S.; van Geest, F.S.; van der Most, F.; Abela, L.; Alfieri, P.; Bauer, A.J.; Bertini, E.; Cappa, M.; Çelik, N.; de Coo, I.F.M.; et al. MCT8 deficiency in females. J. Clin. Endocrinol. Metab. 2025, dgaf311. [Google Scholar] [CrossRef] [PubMed]
- Iwayama, H.; Kakita, H.; Iwasa, M.; Adachi, S.; Takano, K.; Kikuchi, M.; Fujisawa, Y.; Osaka, H.; Yamada, Y.; Okumura, A.; et al. Measurement of Reverse Triiodothyronine Level and the Triiodothyronine to Reverse Triiodothyronine Ratio in Dried Blood Spot Samples at Birth May Facilitate Early Detection of Monocarboxylate Transporter 8 Deficiency. Thyroid 2021, 31, 1316–1321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yiu, R.S.; Ling, T.K.; Ko, C.H.; Poon, S.W.; Poon, G.W.; Wong, F.C.; Law, C.Y.; Iwayama, H.; Lam, C.W. Allan-Herndon-Dudley syndrome in Hong Kong: Implication for newborn screening. Clin. Chim. Acta. 2023, 551, 117621. [Google Scholar] [CrossRef] [PubMed]
- Wilpert, N.M.; Thamm, R.; Thamm, M.; Kratzsch, J.; Seelow, D.; Vogel, M.; Krude, H.; Schuelke, M. Normal Values for the fT3/fT4 Ratio: Centile Charts (0–29 Years) and Their Application for the Differential Diagnosis of Children with Developmental Delay. Int. J. Mol. Sci. 2024, 25, 8585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, S.L.; Refetoff, S.; Babic, N.; Jin, M.; Garg, U.; Yeo, K.J. Triiodothyroacetic Acid Cross-Reacts With Measurement of Triiodothyronine (T3) on Various Immunoassay Platforms. Am. J. Clin. Pathol. 2022, 157, 156–158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Islam, M.S.; Namba, N.; Ohata, Y.; Fujiwara, M.; Nakano, C.; Takeyari, S.; Miyata, K.; Nakano, Y.; Yamamoto, K.; Nakayama, H.; et al. Functional analysis of monocarboxylate transporter 8 mutations in Japanese Allan-Herndon-Dudley syndrome patients. Endocr. J. 2019, 66, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Remerand, G.; Boespflug-Tanguy, O.; Tonduti, D.; Touraine, R.; Rodriguez, D.; Curie, A.; Perreton, N.; Des Portes, V.; Sarret, C.; RMLX/AHDS Study Group. Expanding the phenotypic spectrum of Allan-Herndon-Dudley syndrome in patients with SLC16A2 mutations. Dev. Med. Child Neurol. 2019, 61, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Markova, B.; Mayerl, S.; Heuer, H. Toward a treatment for thyroid hormone transporter MCT8 deficiency—Achievements and challenges. Eur. Thyroid. J. 2024, 13, e240286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Category | Typical Phenotype |
|---|---|
| General signs | Severe underweight (71.1%) Feeding problems (71.4%) Mild-to-moderate intellectual disability (100%) Severe delay in motor development (100%) Truncal hypotonia (100%) Lack of head control (75.3%) |
| Neurological signs | Seizures (with EEG signs) (23.1%) Neurodevelopmental delay (100%) Sleep problems (39.2%) Apneusis (21.9%) Peripheral hypertonia (90.5%) Dystonia (82.6%)/Spasticity (80.3%) Persistent primitive reflexes (91.1%) Delayed myelination (100%) Abnormal cerebral white matter volume (100%) Periventricular WML/Prominent ventricles (100%) Scoliosis (88.2%) Hip luxation (66.7%) |
| Cardiovascular signs | Elevated systolic blood pressure (53.2%) Premature atrial complexes (75.6%) Tachycardia in rest (31.3%) Sudden death (18.8%) Pulmonary infection (69.1%) |
| Thyroid function tests | Chronic thyrotoxicosis Serum T3 ↑↑↑ (95.1%) Serum free T4 ↓/⇔ (88.7%) Serum T3/T4 ratio ↑↑↑ (90.5%) Serum TSH ⇔/slightly ↑(88.6%) |
| Other lab results | Serum sex hormone binding globulin (SHBG) ↑ (88.5%) Serum creatinine ↓/⇔ (27.8%) Serum creatine kinase (CK) ↓/⇔ (96.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the International Society for Neonatal Screening. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubinski, I.; Debor, B.; Petrova, S.; Schiergens, K.A.; Weigand, H.; Schmidt, H. MCT8 Deficiency in Infancy: Opportunities for Early Diagnosis and Screening. Int. J. Neonatal Screen. 2025, 11, 66. https://doi.org/10.3390/ijns11030066
Dubinski I, Debor B, Petrova S, Schiergens KA, Weigand H, Schmidt H. MCT8 Deficiency in Infancy: Opportunities for Early Diagnosis and Screening. International Journal of Neonatal Screening. 2025; 11(3):66. https://doi.org/10.3390/ijns11030066
Chicago/Turabian StyleDubinski, Ilja, Belana Debor, Sofia Petrova, Katharina A. Schiergens, Heike Weigand, and Heinrich Schmidt. 2025. "MCT8 Deficiency in Infancy: Opportunities for Early Diagnosis and Screening" International Journal of Neonatal Screening 11, no. 3: 66. https://doi.org/10.3390/ijns11030066
APA StyleDubinski, I., Debor, B., Petrova, S., Schiergens, K. A., Weigand, H., & Schmidt, H. (2025). MCT8 Deficiency in Infancy: Opportunities for Early Diagnosis and Screening. International Journal of Neonatal Screening, 11(3), 66. https://doi.org/10.3390/ijns11030066






