Giant Gastric Polyp Mimicking a Duodenal Tumor
Abstract
Introduction
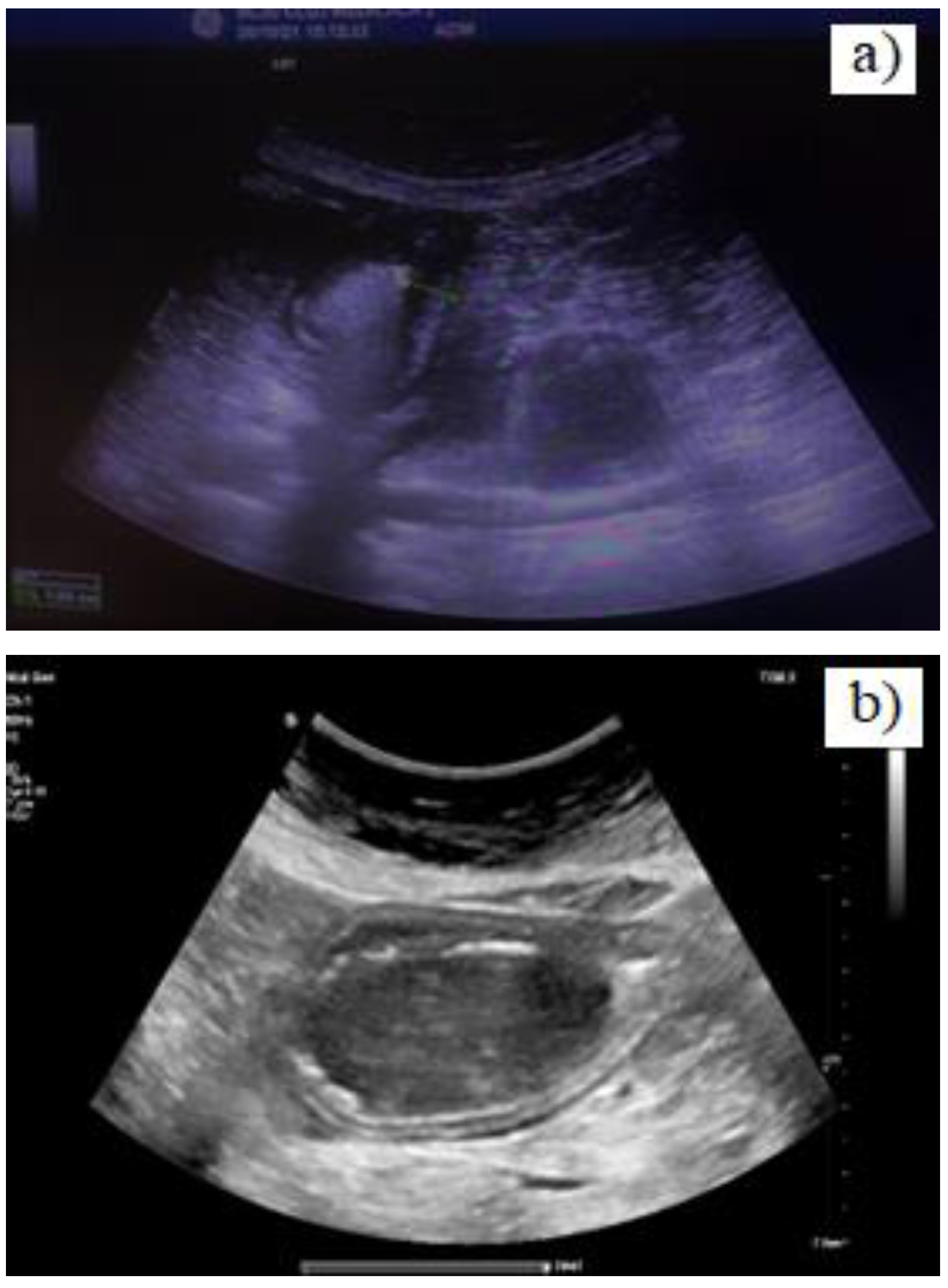
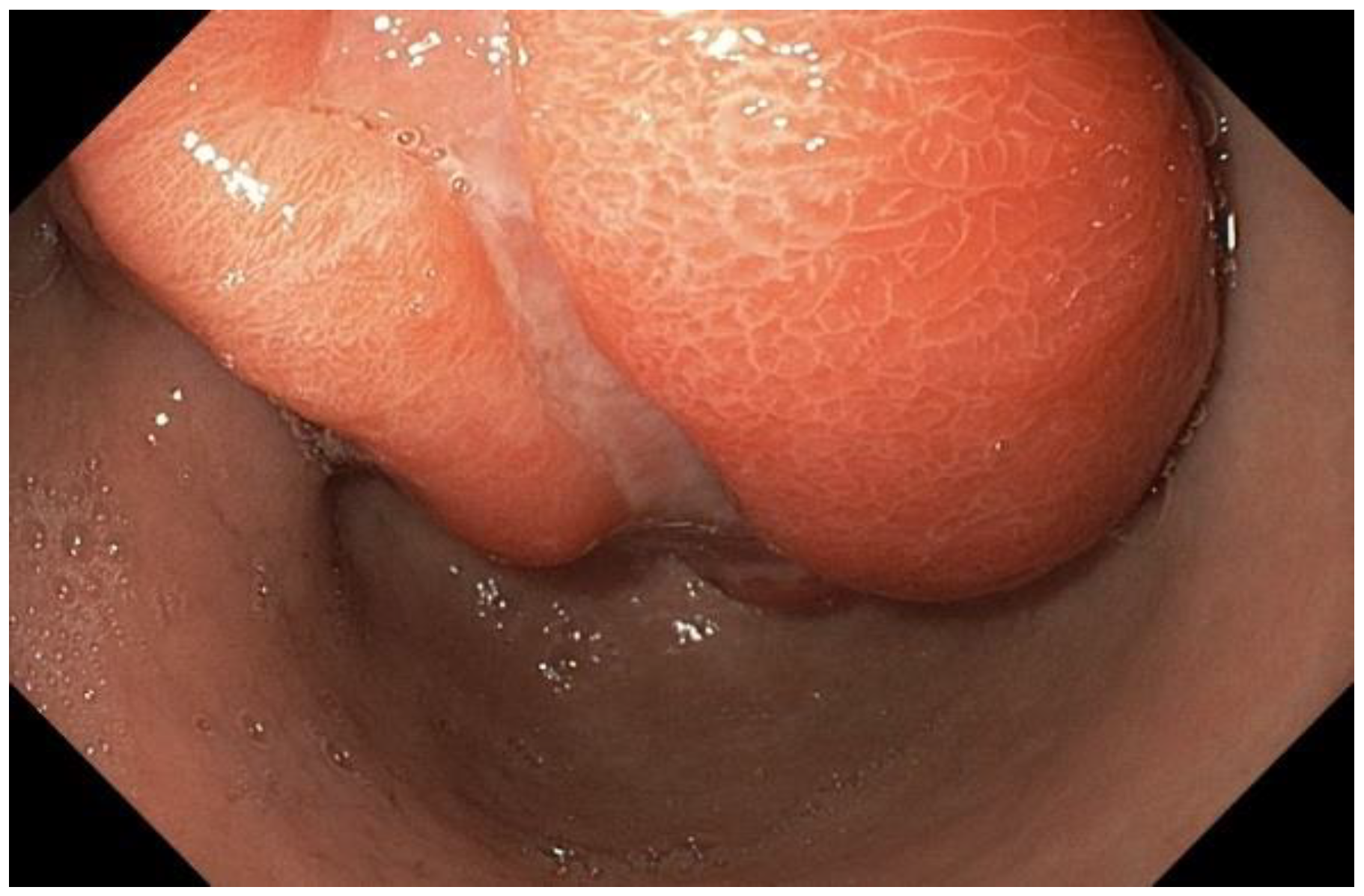
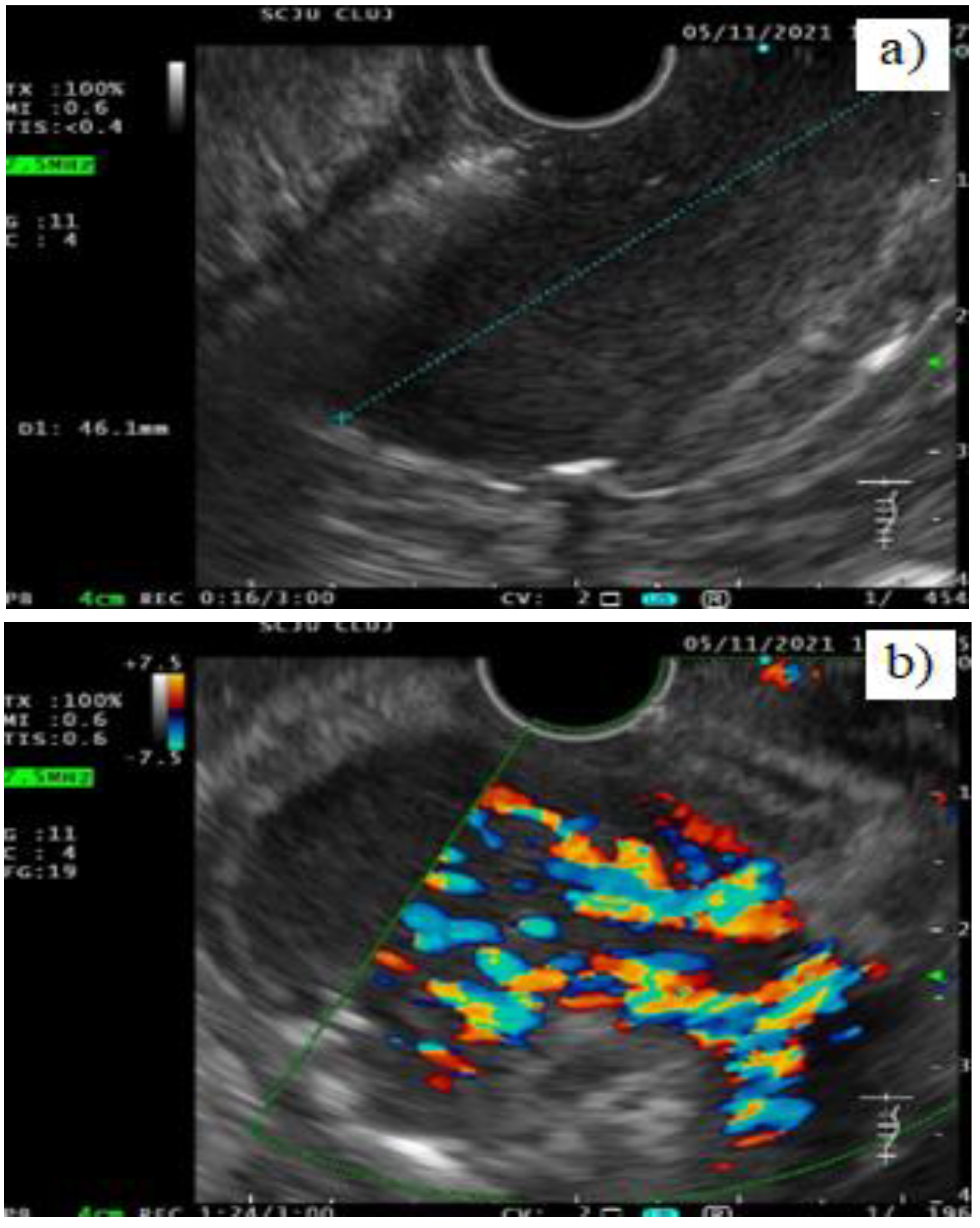
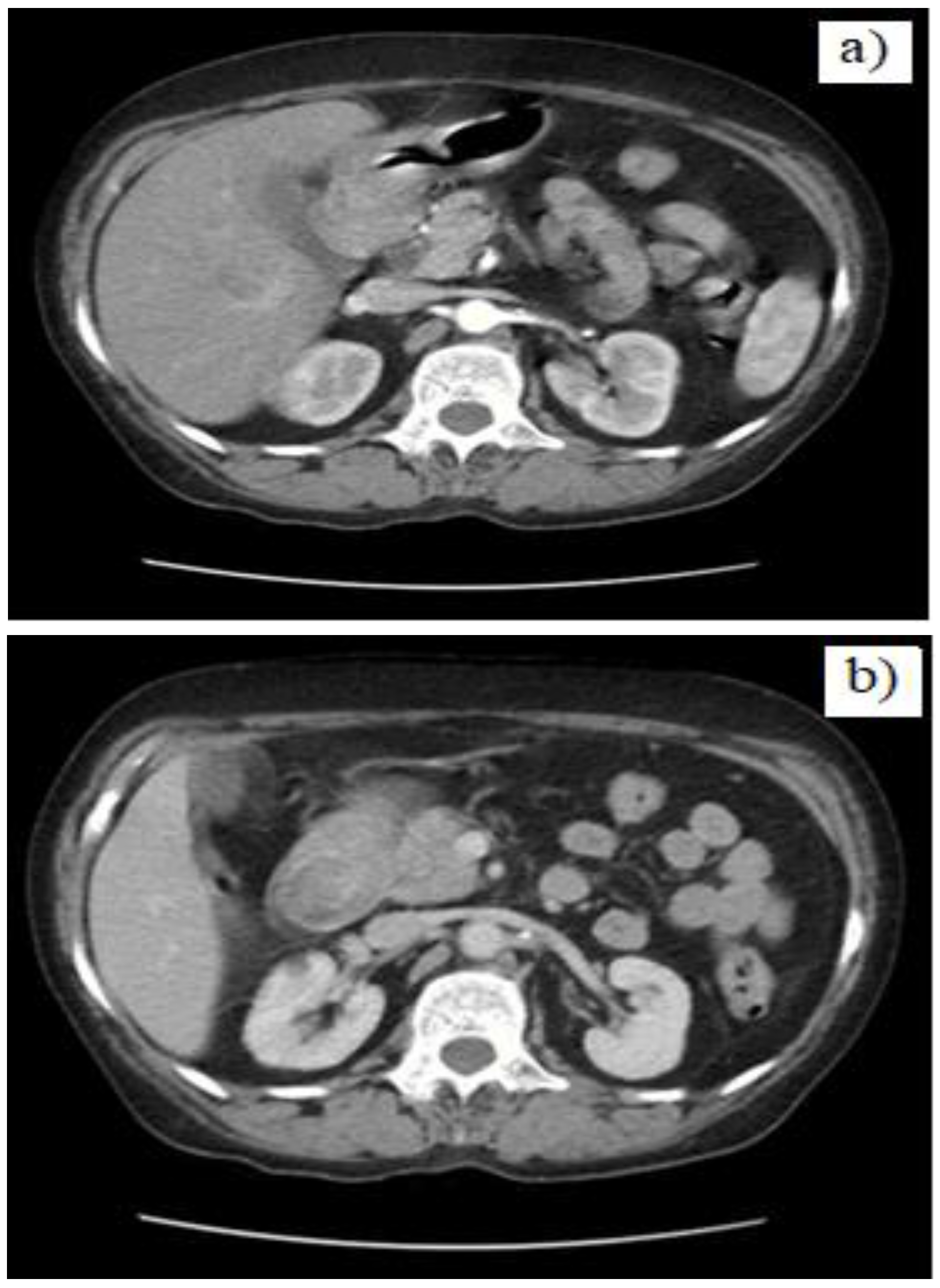
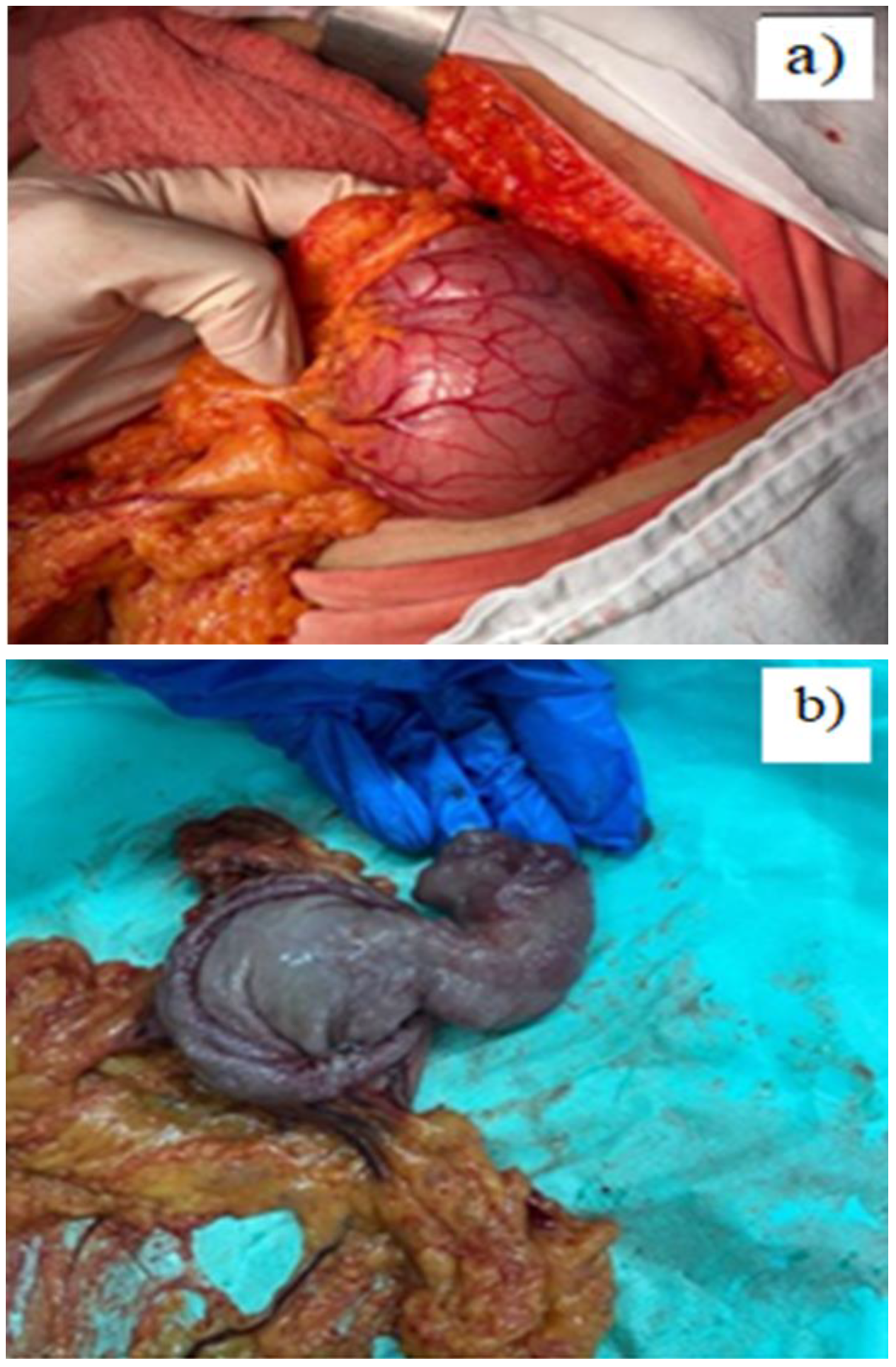
Case presentation
Discussion
Conclusions
Conflict of Interest disclosure
Compliance with ethical standards
References
- Carneiro, F. Familial and hereditary gastric cancer, an overview. Best Pract Res Clin Gastroenterol. 2022, 58, 101800. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.S.; Patel, N.C.; Lam-Himlin, D.; Nguyen, C.C. Gastric polyps: a review of clinical, endoscopic, and histopathologic features and management decisions. Gastroenterol Hepatol (N Y). 2013, 9, 640–651. [Google Scholar]
- Borch, K.; Skarsgård, J.; Franzén, L.; Mårdh, S.; Rehfeld, J.F. Benign gastric polyps: morphological and functional origin. Dig Dis Sci. 2003, 48, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Carmack, S.W.; Genta, R.M.; Schuler, C.M.; Saboorian, M.H. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. 2009, 104, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.M.; Waters, P.S.; Waldron, R.M.; Khan, I.; Orosz, Z.S.; Németh, T.; Barry, K. Recurrent adult jejuno-jejunal intussusception due to inflammatory fibroid polyp - Vanek's tumour: a case report. Diagn Pathol. 2014, 9, 127. [Google Scholar] [CrossRef]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Georgakopoulou, V.E.; Sakellariou, S.; Liakea, A.; Schizas, D.; Diamantis, E.; Farmaki, P.; Voutyritsa, E.; Syllaios, A.; Patsouras, A.; Sypsa, G.; Agorogianni, A.; Stelianidi, A.; Antoniou, E.A.; Kontzoglou, K.; Trakas, N.; Dimitroulis, D. Inflammatory Fibroid Polyp of the Gastrointestinal Tract: A Systematic Review for a Benign Tumor. In Vivo. 2021, 35, 81–93. [Google Scholar] [CrossRef]
- Kimura, N.; Hight, M.; Liang, J.; Willy, R.; Liang, K.; Camp, J. Adult Intussusception Secondary to Inflammatory Fibroid Polyp. West J Emerg Med. 2015, 16, 581–582. [Google Scholar] [CrossRef][Green Version]
- Wysocki, A.P.; Taylor, G.; Windsor, J.A. Inflammatory fibroid polyps of the duodenum: a review of the literature. Dig Surg. 2007, 24, 162–168. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, W.H. Inflammatory fibroid polyps of gastrointestinal tract. Evolution of histologic patterns. Am J Clin Pathol. 1988, 89, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Higgins, J.; Cho, E.Y.; Ko, Y.H.; Oh, Y.L. Expression of CD34, bcl-2, and kit in inflammatory fibroid polyps of the gastrointestinal tract. Appl Immunohistochem Mol Morphol. 2000, 8, 147–153. [Google Scholar] [CrossRef]
- Bae, J.S.; Song, J.S.; Hong, S.M.; Moon, W.S. An unusual presentation of an inflammatory fibroid polyp of the ileum: A case report. Oncol Lett. 2015, 9, 327–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rockey, D.C. Occult and obscure gastrointestinal bleeding: causes and clinical management. Nat Rev Gastroenterol Hepatol. 2010, 7, 265–279. [Google Scholar] [CrossRef]
- Mitsui, Y.; Kagemoto, K.; Itagaki, T.; Inoue, S.; Naruse, K.; Muguruma, N.; Takayama, T. Gastric inflammatory fibroid polyp morphologically changed by Helicobacter pylori eradication. Clin J Gastroenterol. 2015, 8, 77–81. [Google Scholar] [CrossRef]
- Jayasundara, J.; Sellahewa, C.S.; Hall, A.D.; Patel, R.T. A case of gastroduodenal lipomatosis. Ann R Coll Surg Engl. 2016, 98, e203–e205. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, Y.; Nishimura, T.; Komoto, S.; Yuasa, T.; Tamura, R.; Okamoto, T.; Ishido, N. Gastroduodenal intussusception caused by a gastric collision tumor consisting of adenocarcinoma and neuroendocrine carcinoma. Case Rep Gastroenterol. 2014, 8, 89–94. [Google Scholar] [CrossRef]
- Sulu, B.; Günerhan, Y.; Kösemehmetoğlu, K. A rare ileal tumor causing anemia and intussusception: inflammatory fibroid polyp. Turk J Gastroenterol. 2014, 25, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Fleres, F.; Mazzeo, C.; Ieni, A.; Rossitto, M.; Cucinotta, E. Gastric inflammatory fibroid polyp tumor with acute intestinal obstruction-Vanek's tumor can mimick a giant gastrointestinal stromal tumor or a gastric lymphoma. J Vis Surg. 2018, 4, 54. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Hsu, W.H.; Lee, C.C.; Wu, C.C.; Wu, D.C.; Wu, J.Y. Gastroduodenal intussusception caused by gastric gastrointestinal stromal tumor: A case report and review of the literature. World J Clin Cases. 2021, 9, 838–846. [Google Scholar] [CrossRef]
- Silva, M.; Albuquerque, A.; Cardoso, H.; Costa, J.; Macedo, G. Gastric inflammatory fibroid polyp mimicking a gastrointestinal stromal tumour. Rev Esp Enferm Dig. 2016, 108, 497–498. [Google Scholar]
- Ortiz de Solórzapo Aurusa, F.J.; Yarritu Viilanueva, C.; Ruiz Adrados, E.; Obelar Bernal, L.; Acebo García, M.; Alvarez Rabanal, R.; Viguri Díaz, A. Invaginación gastroduodenal y hemorragia digestiva alta secundaria a un lipoma gástrico [Gastroduodenal invagination and upper gastrointestinal hemorrhage secondary to gastric lipoma]. Gastroenterol Hepatol. 1997, 20, 303–305. [Google Scholar]
- Sankaranunni, B.; Ooi, D.S.; Sircar, T.; Smith, R.C.; Barry, J. Gastric lipoma causing gastroduodenal intussusception. Int J Clin Pract. 2001, 55, 731–732. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Hajiro, K.; Okazaki, K.; Takakuwa, H. Gastric inflammatory fibroid polyps: endoscopic ultrasonographic analysis in comparison with the histology. Gastrointest Endosc. 1997, 46, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Uysal, E.; Dokur, M.; Maralcan, G. Abdominal surgery in autoimmune and autoimmune related diseases; A review. J Clin Investig Surg. 2021, 6, 76–87. [Google Scholar] [CrossRef]
- Lin, F.; Setya, V.; Signor, W. Gastroduodenal intussusception secondary to a gastric lipoma: a case report and review of the literature. Am Surg. 1992, 58, 772–774. [Google Scholar]
- Euanorasetr, C.; Suwanthanma, W. Transpyloric prolapse of a pedunculated polypoid gastric carcinoma: a case report and review of the literature. J Med Assoc Thai. 2011, 94, 1008–1012. [Google Scholar]
- Park, Y.M.; Na, K.; Sung, J.Y.; Jang, J.Y.; Kim, Y.W. Adenocarcinoma and Neuroendocrine Collision Tumor in a Giant Gastric Hyperplastic Polyp. J Nippon Med Sch. 2020, 87, 157–161. [Google Scholar] [CrossRef]
- Dumitriu, B.; Valcea, S.; Andrei, G.; Beuran, M. The impact of patient-dependent risk factors on morbidity and mortality following gastric surgery for malignancies. J Mind Med Sci. 2021, 8, 267–272. [Google Scholar] [CrossRef]
- Zinkiewicz, K.; Zgodzinski, W.; Dabrowski, A.; Szumilo, J.; Cwik, G.; Wallner, G. Recurrent inflammatory fibroid polyp of cardia: a case report. World J Gastroenterol. 2004, 10, 767–768. [Google Scholar] [CrossRef]
- Spencer, D. Recurrent familial inflammatory fibroid polyps of the small intestine. J Clin Pathol. 1969, 22, 743. [Google Scholar] [CrossRef]
- Inayat, F.; Ur Rahman, A.; Wahab, A.; Riaz, A.; Zahid, E.; Bejarano, P.; Pimentel, R. Gastric Inflammatory Fibroid Polyp: A Rare Cause of Occult Upper Gastrointestinal Bleeding. J Investig Med High Impact Case Rep. 2020, 8, 2324709620936840. [Google Scholar] [CrossRef]

© 2022 by the authors. 2022 Ioana Duca, Florin Mihaileanu, Alexandra Chira, Romeo Chira, Teodora Surdea Blaga, Diana Popovici, Adriana Albu, Dan Lucian Dumitrascu
Share and Cite
Duca, I.; Mihaileanu, F.; Chira, A.; Chira, R.; Surdea Blaga, T.; Popovici, D.; Albu, A.; Dumitrascu, D.L. Giant Gastric Polyp Mimicking a Duodenal Tumor. J. Mind Med. Sci. 2022, 9, 324-329. https://doi.org/10.22543/2392-7674.1316
Duca I, Mihaileanu F, Chira A, Chira R, Surdea Blaga T, Popovici D, Albu A, Dumitrascu DL. Giant Gastric Polyp Mimicking a Duodenal Tumor. Journal of Mind and Medical Sciences. 2022; 9(2):324-329. https://doi.org/10.22543/2392-7674.1316
Chicago/Turabian StyleDuca, Ioana, Florin Mihaileanu, Alexandra Chira, Romeo Chira, Teodora Surdea Blaga, Diana Popovici, Adriana Albu, and Dan Lucian Dumitrascu. 2022. "Giant Gastric Polyp Mimicking a Duodenal Tumor" Journal of Mind and Medical Sciences 9, no. 2: 324-329. https://doi.org/10.22543/2392-7674.1316
APA StyleDuca, I., Mihaileanu, F., Chira, A., Chira, R., Surdea Blaga, T., Popovici, D., Albu, A., & Dumitrascu, D. L. (2022). Giant Gastric Polyp Mimicking a Duodenal Tumor. Journal of Mind and Medical Sciences, 9(2), 324-329. https://doi.org/10.22543/2392-7674.1316


