The Crosstalk Between Insulin Resistance, Systemic Inflammation, Redox Imbalance and the Thyroid in Subjects with Obesity
Abstract
Introduction
Materials and Methods
Clinical evaluation
Laboratory investigations
Ultrasound evaluation
Statistical analysis
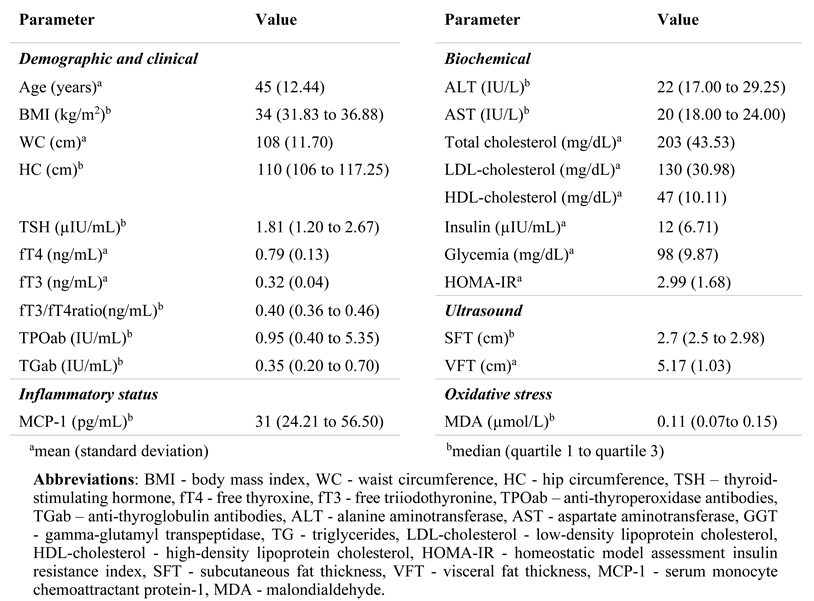
Results
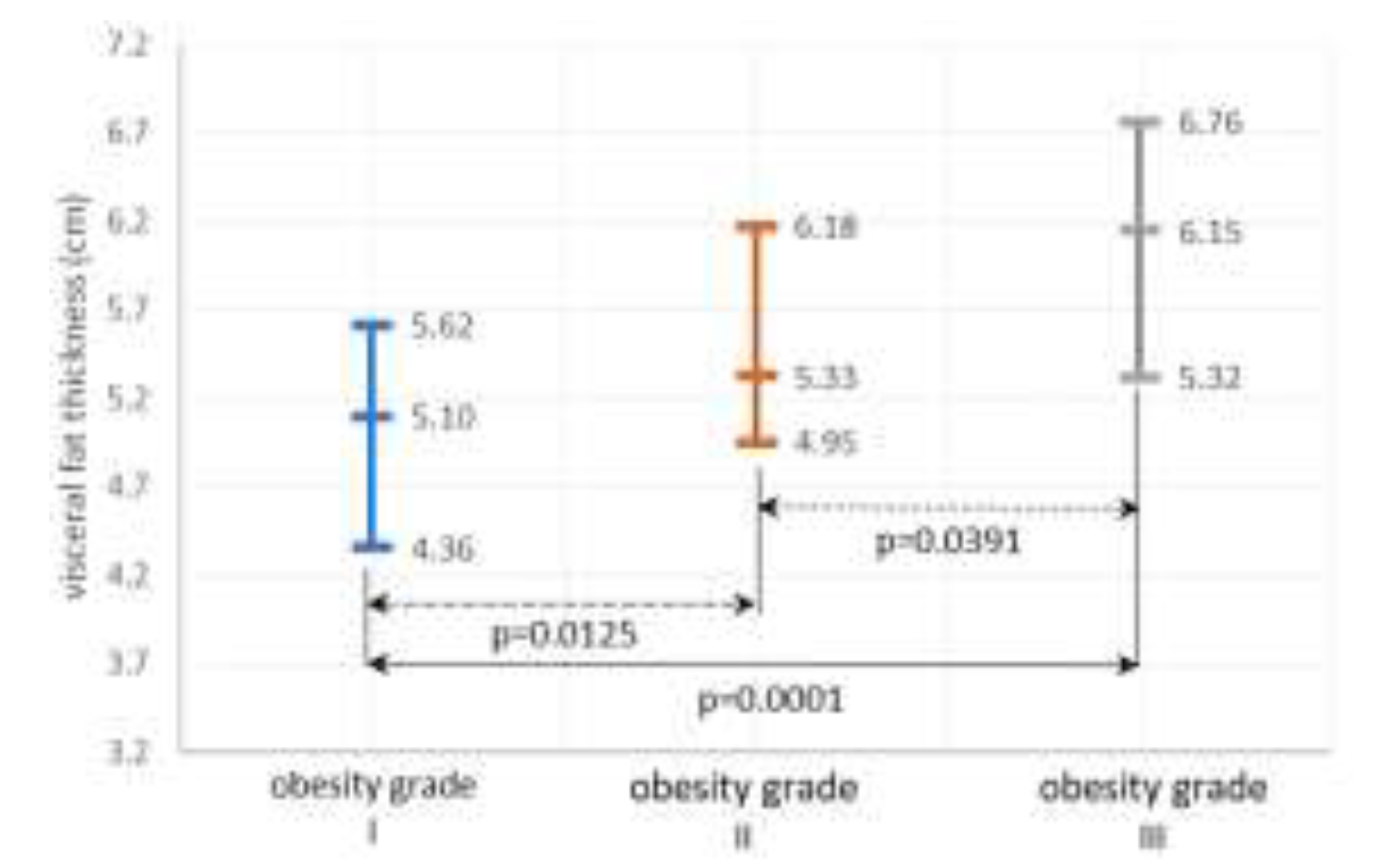
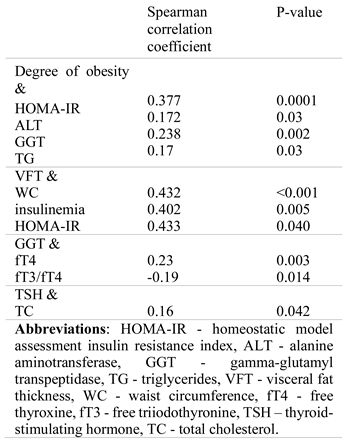
Metabolic and thyroid parameters
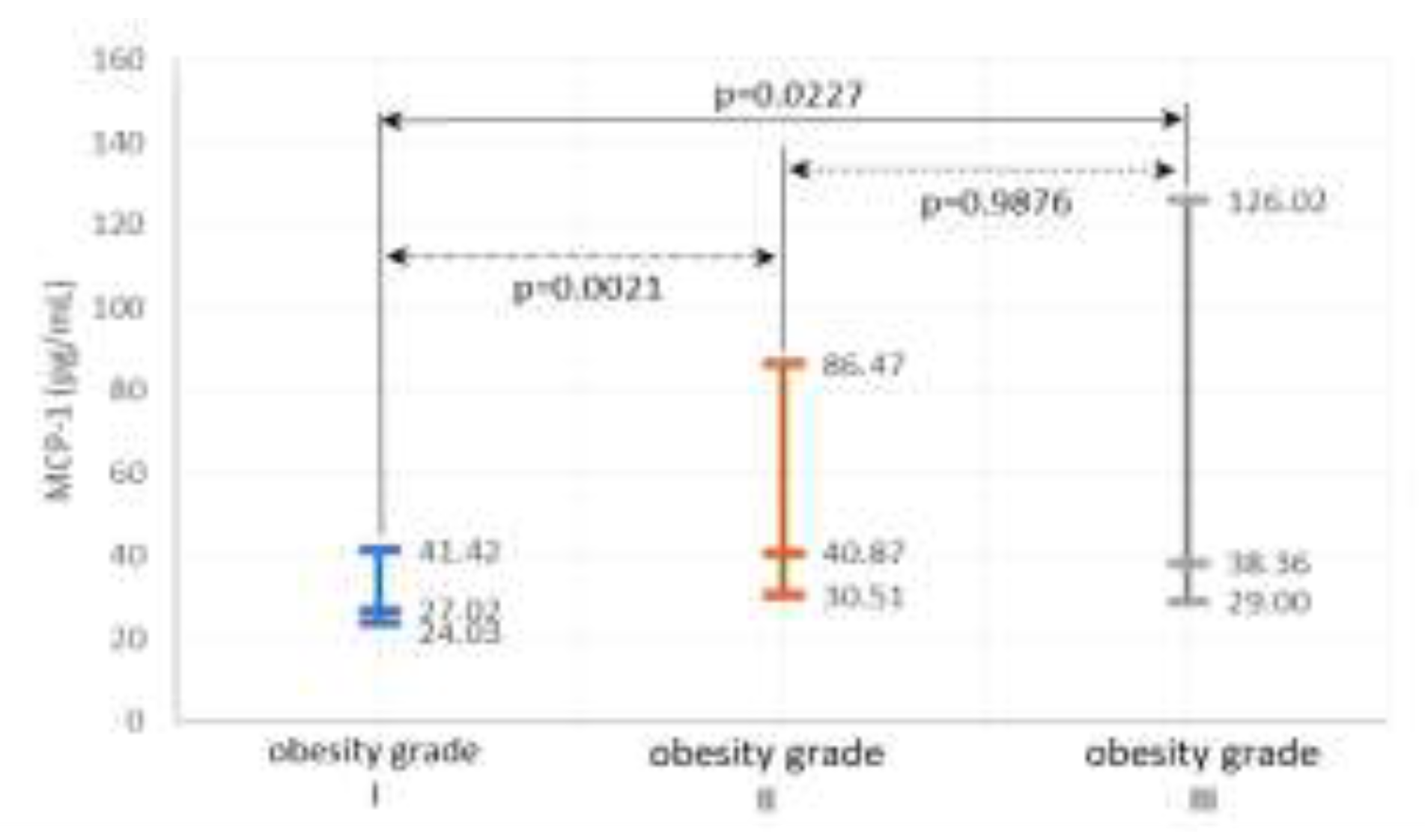
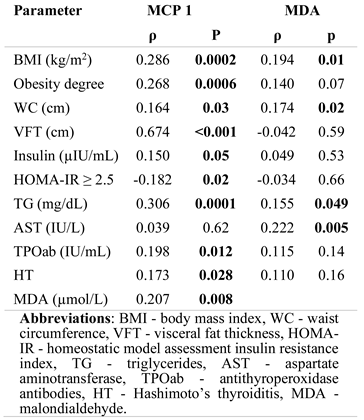
Systemic inflammation and OS
Discussions
Study limitations
Conclusions
Conflict of interest disclosure
Compliance with ethical standards
Acknowledgments
References
- Di Domenico, M.; Pinto, F.; Quagliuolo, L.; Contaldo, M.; Settembre, G.; Romano, A.; Coppola, M.; Ferati, K.; Bexheti-Ferati, A.; Sciarra, A.; Nicoletti, G.F.; Ferraro, G.A.; Boccellino, M. The Role of Oxidative Stress and Hormones in Controlling Obesity. Front Endocrinol (Lausanne). 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Cozma, A.; Sitar-Taut, A.; Urian, L.; Fodor, A.; Suharoschi, R.; Muresan, C.; Negrean, V.; Sampelean, D.; Zdrenghea, D.; Pop, D.; Leucuta, D.; Orasan, O.H. Unhealthy lifestyle and the risk of metabolic syndrome - the Romanian experience. J Mind Med Sci. 2018, 5, 218–229. [Google Scholar] [CrossRef]
- Cozma, A.; Sitar-Taut, A.; Orasan, O.; et al. The Relationship Between eNOS (G894T) Gene Polymorphism and Arterial Stiffness in Patients with Metabolic Syndrome. REV. CHIM. (Bucharest). 2018, 69, 2351–2356. [Google Scholar] [CrossRef]
- Layegh, P.; Asadi, A.; Jangjoo, A.; Layegh, P.; Nematy, M.; Salehi, M.; Shamsian, A.; Ranjbar, G. "Comparison of thyroid volume, TSH, free t4 and the prevalence of thyroid nodules in obese and non-obese subjects and correlation of these parameters with insulin resistance status". Caspian J Intern Med. 2020, 11, 278–282. [Google Scholar] [CrossRef]
- Anil, C.; Akkurt, A.; Ayturk, S.; Kut, A.; Gursoy, A. Impaired glucose metabolism is a risk factor for increased thyroid volume and nodule prevalence in a mild-to-moderate iodine deficient area. Metabolism. 2013, 62, 970–975. [Google Scholar] [CrossRef]
- Rezzonico, J.; Rezzonico, M.; Pusiol, E.; Pitoia, F.; Niepomniszcze, H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid. 2008, 18, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.K. Cytokine actions on the thyroid gland. Dan Med Bull. 2000, 47, 94–114. [Google Scholar] [PubMed]
- Malaguarnera, R.; Vella, V.; Nicolosi, M.L.; Belfiore, A. Insulin Resistance: Any Role in the Changing Epidemiology of Thyroid Cancer? Front Endocrinol (Lausanne). 2017, 8, 314. [Google Scholar] [CrossRef]
- Juiz-Valiña, P.; Cordido, M.; Outeiriño-Blanco, E.; Pértega, S.; Varela-Rodríguez, B.M.; García-Brao, M.J.; Mena, E.; Pena-Bello, L.; Sangiao-Alvarellos, S.; Cordido, F. Central Resistance to Thyroid Hormones in Morbidly Obese Subjects Is Reversed after Bariatric Surgery-Induced Weight Loss. J Clin Med. 2020, 9, 359. [Google Scholar] [CrossRef]
- Muraca, E.; Oltolini, A.; Pizzi, M.; Villa, M.; Manzoni, G.; Perra, S.; Zerbini, F.; Bianconi, E.; Cannistraci, R.; Ciardullo, S.; Pizzi, P.; Lattuada, G.; Perseghin, G. Baseline TSH levels and short-term weight loss after different procedures of bariatric surgery. Int J Obes (Lond). 2021, 45, 326–330. [Google Scholar] [CrossRef]
- Reaven, G.M. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995, 75, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; Kasuga, M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Kokkotou, E.; Marafelia, P.; Mantzos, E.I.; Tritos, N.A. Serum monocyte chemoattractant protein-1 is increased in chronic autoimmune thyroiditis. Metabolism. 2002, 51, 1489–1493. [Google Scholar] [CrossRef]
- Peixoto de Miranda, É.J.; Bittencourt, M.S.; Santos, I.S.; Lotufo, P.A.; Benseñor, I.M. Thyroid Function and High-Sensitivity C-Reactive Protein in Cross-Sectional Results from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil): Effect of Adiposity and Insulin Resistance. Eur Thyroid J. 2016, 5, 240–246. [Google Scholar] [CrossRef][Green Version]
- Al-Rubae'i, S.H.N.; Al-Musawi, A.K. An evaluation of antioxidants and oxidative stress in Iraqi patients with thyroid gland dysfunction. African J Biochem Res. 2011, 5, 188–196. [Google Scholar]
- Leclercq, I.A. Antioxidant defence mechanisms: new players in the pathogenesis of non-alcoholic steatohepatitis? Clin Sci (Lond). 2004, 106, 235–237. [Google Scholar] [CrossRef]
- Kent Holtorf. Thyroid Hormone Transport into Cellular Tissue. J Restor Med. 2014, 3, 53–68. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Navarro-Ruiz, A.; Fernández-Ferri, M.; Arilla-Codoñer, A.; Ballester-Asensio, E.; Valls-Bellés, V. A matter of fat: insulin resistance and oxidative stress. Pediatr Diabetes. 2012, 13, 392–399. [Google Scholar] [CrossRef]
- Yesilova, Z.; Yaman, H.; Oktenli, C.; Ozcan, A.; Uygun, A.; Cakir, E.; Sanisoglu, S.Y.; Erdil, A.; Ates, Y.; Aslan, M.; Musabak, U.; Erbil, M.K.; Karaeren, N.; Dagalp, K. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2005, 100, 850–855. [Google Scholar] [CrossRef]
- Bianco, A.C.; Kim, B.W. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.A.; Mulcahey, M.A.; Crescenzi, A.; Chung, M.; Kim, B.W.; Barnes, C.; Kuijt, W.; Turano, H.; Harney, J.; Larsen, P.R. Transforming growth factor-beta promotes inactivation of extracellular thyroid hormones via transcriptional stimulation of type 3 iodothyronine deiodinase. Mol Endocrinol. 2005, 19, 3126–3136. [Google Scholar] [CrossRef][Green Version]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000, 894, i.
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Arkadievich, O.D. Metabolic markers of myocardium insulin resistance in dogs with heart failure. Open Vet J. 2021, 10, 363–370. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.J.; Hur, K.Y.; Choi, S.H.; Ahn, C.W.; Lim, S.K.; Kim, K.R.; Lee, H.C.; Huh, K.B.; Cha, B.S. Visceral fat thickness measured by ultrasonography can estimate not only visceral obesity but also risks of cardiovascular and metabolic diseases. Am J Clin Nutr. 2004, 79, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Jäntschi, L.; Bolboacã, S.D. Exact probabilities and confidence limits for binomial samples: applied to the difference between two proportions. ScientificWorldJournal. 2010, 10, 865–878. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; Chen, H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003, 100, 7265–7270. [Google Scholar] [CrossRef]
- Molnár, I. Interactions among thyroid hormone (FT4), chemokine (MCP-1) and neurotrophin (NGF-β) levels studied in Hungarian postmenopausal and obese women. Cytokine. 2020, 127, 154948. [Google Scholar] [CrossRef] [PubMed]
- Torres-Leal, F.L.; Fonseca-Alaniz, M.H.; De Oliveira, A.C.; Alonso-Vale, M.I.C. Adipose Tissue Inflammation and Insulin Resistance. Intech. 2012, 6, 137–156. [Google Scholar]
- García-López, M.A.; Sancho, D.; Sánchez-Madrid, F.; Marazuela, M. Thyrocytes from autoimmune thyroid disorders produce the chemokines IP-10 and Mig and attract CXCR3+ lymphocytes. J Clin Endocrinol Metab. 2001, 86, 5008–5016. [Google Scholar] [CrossRef] [PubMed]
- Marina, C.N.; Danciu, R.; Raducu, L.; Scaunasu, R.V.; Jecan, C.R.; Florescu, P.I. The surgical treatment of diabetic foot ulcers. J Clin Invest Surg. 2019, 4, 96–100. [Google Scholar] [CrossRef]
- Duntas, L.H.; Biondi, B. The interconnections between obesity, thyroid function, and autoimmunity: the multifold role of leptin. Thyroid. 2013, 23, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, P.; Minocci, A.; Tagliaferri, M.A.; Guzzaloni, G.; Di Blasio, A.; De Medici, C.; Aimaretti, G.; Liuzzi, A. Investigations of thyroid hormones and antibodies in obesity: leptin levels are associated with thyroid autoimmunity independent of bioanthropometric, hormonal, and weight-related determinants. J Clin Endocrinol Metab. 2010, 95, 3965–3972. [Google Scholar] [CrossRef]
- Ohye, H.; Sugawara, M. Dual oxidase, hydrogen peroxide and thyroid diseases. Exp Biol Med (Maywood). 2010, 235, 424–433. [Google Scholar] [CrossRef]
- Girard, J.; Lafontan, M. Impact of visceral adipose tissue on liver metabolism and insulin resistance. Part II: Visceral adipose tissue production and liver metabolism. Diabetes Metab. 2008, 34, 439–445. [Google Scholar] [CrossRef]
- Pacifico, L.; Bonci, E.; Ferraro, F.; Andreoli, G.; Bascetta, S.; Chiesa, C. Hepatic steatosis and thyroid function tests in overweight and obese children. Int J Endocrinol. 2013, 2013, 381014. [Google Scholar] [CrossRef]
- Farasat, T.; Cheema, A.M.; Khan, M.N. Hyperinsulinemia and insulin resistance is associated with low T₃/T₄ ratio in pre diabetic euthyroid Pakistani subjects. J Diabetes Complications. 2012, 26, 522–525. [Google Scholar] [CrossRef]
- Lee, D.H.; Gross, M.D.; Jacobs, D.R., Jr. Cardiovascular Risk Development in Young Adults Study. Association of serum carotenoids and tocopherols with gamma-glutamyltransferase: the Cardiovascular Risk Development in Young Adults (CARDIA) Study. Clin Chem. 2004, 50, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Albu, A.; Moldovan, A.; Petra, C.; Para, I. Serum Gamma-Glutamyl Transferase is associated with epicardial fat thickness in middle aged woman. Revista de Chimie. 2020, 71, 430–435. [Google Scholar] [CrossRef]
- Rotondi, M.; Leporati, P.; La Manna, A.; Pirali, B.; Mondello, T.; Fonte, R.; Magri, F.; Chiovato, L. Raised serum TSH levels in patients with morbid obesity: is it enough to diagnose subclinical hypothyroidism? Eur J Endocrinol. 2009, 160, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Lathia, T. Rising prevalence of thyroid disorders. J Mahatma Gandhi Inst Med Sci. 2015, 20, 125–127. [Google Scholar] [CrossRef]
- Chubb, S.A.; Davis, W.A.; Davis, T.M. Interactions among thyroid function, insulin sensitivity, and serum lipid concentrations: the Fremantle diabetes study. J Clin Endocrinol Metab. 2005, 90, 5317–5320. [Google Scholar] [CrossRef]
- Farishta, F.; Farishta, S. Insulin resistance and thyroid hypofunction in obese women – A cross sectional study. Integr Obesity Diabetes. 2015, 1. [Google Scholar] [CrossRef]
 |
 |
 |
 |
 |
© 2021 by the author. 2021 Nicoleta Răcătăianu, Nicoleta Valentina Leach, Sorana D. Bolboacă, Maria Loredana Soran, Mirela Flonta, Ana Valea, Andrada-Luciana Lazăr, Cristina Ghervan
Share and Cite
Răcătăianu, N.; Leach, N.V.; Bolboacă, S.D.; Soran, M.L.; Flonta, M.; Valea, A.; Lazăr, A.-L.; Ghervan, C. The Crosstalk Between Insulin Resistance, Systemic Inflammation, Redox Imbalance and the Thyroid in Subjects with Obesity. J. Mind Med. Sci. 2021, 8, 139-148. https://doi.org/10.22543/7674.81.P139148
Răcătăianu N, Leach NV, Bolboacă SD, Soran ML, Flonta M, Valea A, Lazăr A-L, Ghervan C. The Crosstalk Between Insulin Resistance, Systemic Inflammation, Redox Imbalance and the Thyroid in Subjects with Obesity. Journal of Mind and Medical Sciences. 2021; 8(1):139-148. https://doi.org/10.22543/7674.81.P139148
Chicago/Turabian StyleRăcătăianu, Nicoleta, Nicoleta Valentina Leach, Sorana D. Bolboacă, Maria Loredana Soran, Mirela Flonta, Ana Valea, Andrada-Luciana Lazăr, and Cristina Ghervan. 2021. "The Crosstalk Between Insulin Resistance, Systemic Inflammation, Redox Imbalance and the Thyroid in Subjects with Obesity" Journal of Mind and Medical Sciences 8, no. 1: 139-148. https://doi.org/10.22543/7674.81.P139148
APA StyleRăcătăianu, N., Leach, N. V., Bolboacă, S. D., Soran, M. L., Flonta, M., Valea, A., Lazăr, A.-L., & Ghervan, C. (2021). The Crosstalk Between Insulin Resistance, Systemic Inflammation, Redox Imbalance and the Thyroid in Subjects with Obesity. Journal of Mind and Medical Sciences, 8(1), 139-148. https://doi.org/10.22543/7674.81.P139148



