Abstract
Objective. To identify those who develop pulmonary embolism with Ddimer levels by evaluating pulmonary CT angiographies of patients who are followed up with suspicion of coronavirus disease 2019 (COVID-19). Methods. Patients who were followed up in a community hospital with suspicion of COVID-19 and underwent Pulmonary CT angiography examination were evaluated. Clinical and demographic parameters and DDimer values for patients with and without pulmonary embolism were evaluated in the pulmonary CT angiogram. Results. During the COVID-19 pandemic, Thorax CT examination was performed in our center for suspicion or follow-up of COVID-19 infection in 3396 patients. Pulmonary CT angiography was applied to 312 (9.2%) of these cases. Of these 312 patients, 141 were identified as COVID-19 patients. Acute pulmonary embolism was detected in 33 (23.4%) of 141 patients with COVID-19 and pulmonary CT angiogram. D-dimer levels (5964.97 ± 4036.8 μg/L) of patients with COVID-19 infection and pulmonary embolism were significantly higher than D-dimer levels (972.4 ± 1766.8 μg/L) of patients without pulmonary embolism. In patients with COVID-19 infection, a Ddimer value higher than 1013 μg/L was determined as a cut-off value with 100% sensitivity for the presence of pulmonary embolism. Conclusions. For those struggling with the COVID-19 pandemic, pulmonary embolism should be kept in mind if D-dimer values increase more than expected in the presence of respiratory distress that Thorax CT findings cannot explain.
Introduction
In January 2020, a new coronavirus SARS-CoV-2 was identified as the cause of the viral pneumonia epidemic in Wuhan, China. This disease was later called coronavirus disease 2019 (COVID-19), as it spread rapidly and globally. The progression of COVID-19 includes primarily serious respiratory complications [1]. Patients may have fever, cough, abdominal pain and diarrhea. After the COVID-19 outbreak, acute pulmonary embolism reports related to COVID-19 have tended to increase. Several studies have reported clinical cases with isolated coagulopathy and pulmonary embolism with COVID-19 pneumonia [2,3,4]. Also, elevated D-dimer levels have been reported in COVID-19 patients [5,6]. In addition, research provides evidence for a relationship between the severity of the disease and D-dimer levels [7]. The purpose of our study was to identify those who develop pulmonary embolism and detect if there is a correlation with D-dimer levels and pulmonary CT angiography findings in patients who are followed up due to suspected COVID-19.
Materials and Methods
Patient group
This retrospective study was approved by Scientific Research Committee in the structure of Ministry of Health for COVID-19 research. The study was also approved (permit no: 135-24.09.20) by the institutional ethics committee for human clinical investigations which oversees the protocols in accordance with the Declaration of Helsinki. Patients who were followed-up with suspicion of COVID-19, and underwent pulmonary CT angiography between March 13 and May 20 at the Training and Research Public Hospital, were evaluated. Clinical and demographic parameters and D-Dimer values for patients with and without pulmonary embolism were evaluated in the pulmonary CT angiogram.
Pulmonary CT Angiography
In our center, 128-multidetector tomography device (Optima CT660, General Electric Healthcare Systems, Milwaukee, USA) was used for examinations. Our standard display parameters: 120 kVp, 150-300 mAs, section thickness 1 mm and 0.6-1.4 pitch is determined depending on body size. “SmartPrep” protocol (GE Healthcare) was used as the monitoring system for the injection of contrast medium. SmartPrep is a feature that provides real-time tracking of contrast in part of its intended area. Follow-up images are taken as a low dose until the contrast medium reaches the preferred point and scanning is initiated by the operator. In our examinations, 50-60 mL of iodinated contrast medium was injected through the antecubital vein at a rate of 4.0 mL/s with an 18-to-20-gauge cannula. Images were reconstructed in the mediastinal and parenchymal windows with a slice thickness of 1 mm. The location of the pulmonary embolism by a single radiologist classified the main pulmonary arteries, lobar, segmental according to the most proximal location of the filling defect.
Computed Tomography without contrast-media was performed in patients who were not suspected of pulmonary embolism and who requested CT for diagnosis or follow-up in terms of COVID-19 pneumonia. Non enhanced chest CT is preferably performed by using a low radiation-dose protocol to minimize radiation burden.
Laboratory analysis
D-dimer levels were recorded for all patients with pulmonary CT angiography. The last D-dimer measurements of the patients before the CTPA examination were evaluated. All patients with pulmonary CT angiography were evaluated with the Reverse Transcription Polymerase Chain Reaction (RT-PCR) test results for SARS-CoV-2. Necessary samples were obtained by nasopharyngeal swab. If necessary, second or third sampling was done using sputum or bronchoalveolar lavage. Any positive results were classified as confirmed COVID-19 infection. In a limited group of patients, in the early period of the pandemic, non-contrast thorax CT examination was used for diagnosis due to the suspicion of false negativity for PCR tests and late test results. In addition to PCR positive patients, Thorax CT images of RT-PCR negative patients were reviewed for comparison by a radiologist with 15 years of experience (AV), and patients were evaluated as COVID-19 in the presence of typical lung parenchymal lesions (bilateral and peripheral ground-glass opacities and / or alveolar consolidation) for COVID-19 [7,8].
Statistical Analysis
Student t test was used for comparing the parameters between the two groups (Mean, Standard deviation, frequency). ROC curve analysis was used to determine the specificity and sensitivity values of D-dimer values and cut-off values. Significance was evaluated at the level of p<0.05. For all analyses, Statistical Package for the Social Science 22 (version 22 for Windows; SPSS, Turkey) program was used.
Results
The flow chart of all patients who applied to the emergency department between 13 March and 20 May 2020 in our public hospital, who underwent CT scan, is shown in Figure 1.
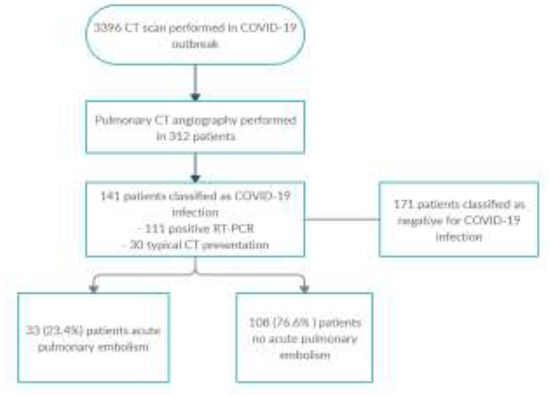
Figure 1.
Flowchart of the study.
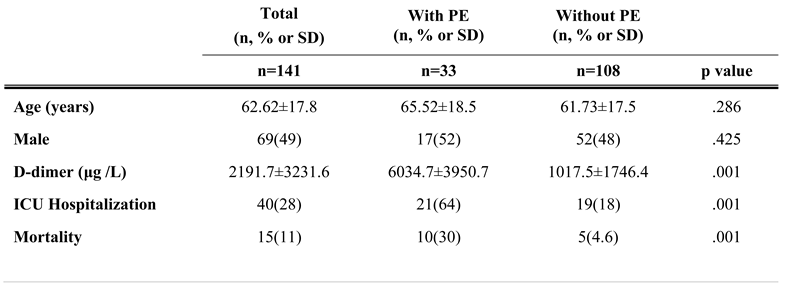
During this time, Thorax CT examination was performed in 3396 patients for suspicion or follow-up of COVID-19 infection. Pulmonary CT angiography was applied to 312/3396 (9.2%) of these cases. Of these 312 patients, 141 patients were classified as COVID-19 infections (111 patients were positive RT-PCR and 30 patients were positive Thorax CT). The reason for requesting CT angiography in these patients was high D-Dimer values in 88/141 (62%) patients, and respiratory distress in 53/141 (38%) patients that could not be explained by Thorax CT findings. 83 of our patients were male (59%) and 58 were female (41%). The age range of our patients was 23-95, and the mean age was calculated as 64.88±18.6 for male patients, 61.04±17.1 for female patients and 62.62±17.8 for all patients. Acute pulmonary embolism was detected in 33 (23.4%) of 141 patients with COVID-19 and pulmonary CT angiogram (Figure 2 and Figure 3).
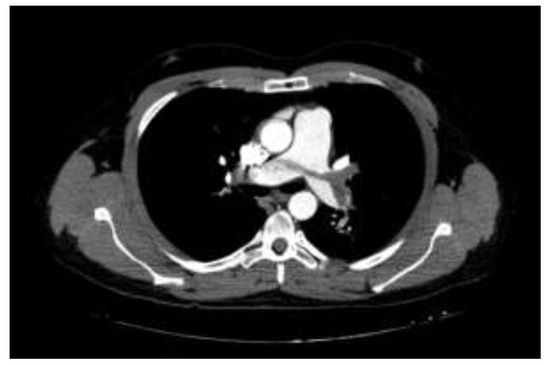
Figure 2.
Pulmonary computed tomographic angiography (PCTA) demonstrating saddle pulmonary embolism (PE) as well as emboli partially obstructing both main pulmonary arteries. A 33-year-old male patient has been followed for 4 days with the diagnosis of COVID-19, and PCTA was performed due to the increased dyspnea with a rapid increase in D-dimer values.
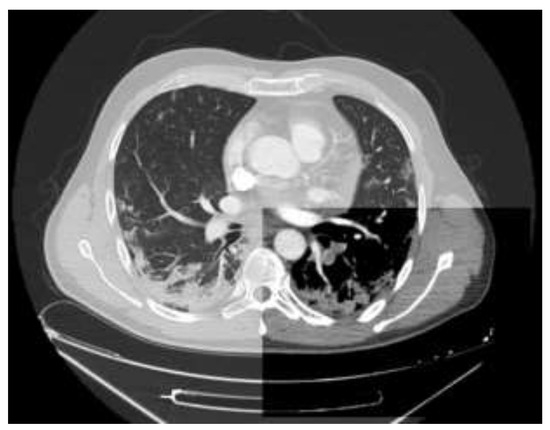
Figure 3.
Axial CT angiography scan of the chest shows filling defects in the left lower lobe pulmonary arteries representing clots (mediastinal window) and findings compatible with COVID-19 pneumonia (lung window).
There were no signs of pulmonary embolism in 108 patients (76.6%). Relevant clinical and tomography data are summarized in Table 1 and Table 2.

Table 1.
Patient Demographics. The values are given as average/median PE: Pulmonary embolism.

Table 2.
Location of embolus on Chest CT.
D-dimer levels (5964.97±4036.8 μg/L) of patients with COVID-19 infection and pulmonary embolism were significantly higher than D-dimer levels (972.4±1766.8 μg/L) of patients without pulmonary embolism (p<0.05). In follow-up of 141 patients, 21/33 (64%) of patients with pulmonary embolism and 19/108 (18%) of patients without pulmonary embolism needed intensive care treatment (p<0.05). Follow-up resulted in death in 10/33 (30%) of patients with pulmonary embolism and in 5/108 (4.6%) of patients without pulmonary embolism (p<0.05) (Table 1). In patients with COVID-19 infection, a D-dimer value greater than 1013 μg/L had 33/33 (100%, 95% CI) sensitivity and 78/108 (72%, 95% CI) specificity for the presence of pulmonary embolism.
The D-dimer levels of 171 patients whose PCR tests for COVID-19 were negative but were applied CTPA due to increased D-dimer levels were found to be 711 ± 416.5 μg/ L. Pulmonary embolism was detected in 13 (7.6%) of 171 patients with negative COVID-19 tests.
Discussions
Despite the need for improvement in infectious involvement in the lung parenchyma in COVID-19 patients, high oxygen requirement continues and it may even increase due to the development of thromboembolic disease [9]. Myocardial damage has been reported to be associated with a fatal course in COVID-19 patients, with mortality rates reaching 37% in patients without prior cardiovascular disease but with high troponin levels [10]. Previous studies have shown poor prognosis in COVID-19 cases, with low comorbidity in patients with severe comorbidity, with low platelet levels, increased d-dimer levels, and increased prothrombin levels [11]. D-dimer levels higher than 1 μg/mL in COVID-19 patients have been identified as an important risk factor for mortality [12]. Autopsy reports of COVID-19 patients showed microtrombus in the lungs and other organs with bleeding focus [13,14]. These findings show that cytokine storm and associated hypoxaemia lead to severe endothelial dysfunction, causing intravascular coagulation and thromboembolic complications. Although some underlying conditions have affected the coagulation process in these patients, the hypothesis that hypercoagulability is due to endothelial dysfunction makes sense. Case studies support observations that show that anticoagulation reduces mortality rates for COVID-19 patients [15]. Follow-up of diffuse intravascular coagulation and measurement of platelet counts, D-dimer and fibrinogen levels, assessment of the International Society on Thrombosis and Haemostasis scores may be useful for early diagnosis of PE in patients with COVID-19 [9]. A normal level of D-dimer may enable the exclusion of pulmonary embolism in patients who are clinically unlikely to have PE. However, the increase in D-dimer level hinders the use of thrombosis as a precise marker due to lack of specificity. The European Society of Radiology and the European Society of Thoracic Imaging recommend performing contrast CT to exclude PE if additional oxygen is needed in patients with COVID-19 pneumonia with limited disease spread [16]. Similarly, the European Society of Cardiology recommends CTPA before the patient leaves the radiology department if the findings obtained with non-contrast CT are not advanced enough to explain severe respiratory failure [17].
It has been observed that pulmonary embolism and deep vein thrombosis (DVT) occur in other viral pneumonias, but not as often as in COVID-19 patients. It was reported that seven (5.9%) of 119 people who applied to the hospital with influenza A H1N1 virus infection developed thrombotic vascular events, one of which was PE and three were DVT [18].
In our center, which is one of the sites designated as a pandemic hospital, we evaluated patients admitted with the suspicion of COVID-19. The use of the D-dimer test is not recommended due to false positive results during clinical evaluation. Although there is no evidence of pneumonia in the COVID-19 patients to explain the respiratory distress in the non-contrast Thoracic CT examination, pulmonary CT angiography is recommended with high risk in case of severe respiratory distress and increased oxygen support requirement. It is recommended to perform D-dimer tests in patients with low and moderate risk of pulmonary embolism, and CT angiography if D-dimer results are positive. Because D-dimer values increase with age [19], it is recommended to accept age-adjusted limit values (such as, age x 10 μg/L) for the D-dimer instead of a fixed cut-off value. Although there is no pulmonary embolism in COVID-19 patients, increased D-dimer levels have been reported compared to normal [20]. In our center, D-dimer levels above 500 μg/L in COVID-19 patients are considered sufficiently high in order to request pulmonary CT angiography. All of the patients included in the study were positive for the RT-PCR test or with typical signs of COVID-19 pneumonia on Thoracic CT examination. Acute pulmonary embolism was present in 33/141 (23.6%) of the patients. This rate of pulmonary embolism is significantly higher than the rates encountered in critically ill patients (1.3%) [21] or emergency service patients (3 to 10%) without COVID-19 infection [22].
In our patient population, a D-dimer threshold of 1013 μg/L detected all patients with pulmonary embolism on chest CT. This 1013 μg/L threshold is a value between 2660 [9], 2400 [23] and 900 [5] previously reported. These cut-off values are normally higher than the cut-off values used to exclude pulmonary embolism, as COVID-19 patients have increased D-dimer levels without pulmonary embolism, which may be associated with a systemic inflammatory response [24].
Hydroxychloroquine and favipiravir treatment was administered to patients who were diagnosed with COVID-19 in the early period of the pandemic in which patients admitted to the study were followed, but anticoagulants were not used for routine prophylaxis. At the time of writing the article, 2x60 mg of enoxaparin sodium was added to the routine treatment protocol of patients with COVID-19 pneumonia. Although a more detailed assessment is required, after anticoagulant prophylaxis was initiated, there was a decrease in high D-dimer levels and the need for CTPA.
Our study has several limitations. First, it is not possible to accurately determine the incidence of PE in COVID-19 patients since CTPA examination is not performed in all patients. Since our study was conducted in a retrospective manner, it was not possible to determine exactly why PE was suspected in terms of the examination decision in all patients. For similar reasons, the comorbidities of patients in terms of thromboembolism and past venous thrombosis histories could not be evaluated. In addition, patients were not evaluated for deep vein thrombosis.
Highlights
- ✓
- Increased D-dimer levels are often seen in patients with COVID-19 pneumonia.
- ✓
- There appears to be a strong association between high D-dimer levels and thrombotic complications in COVID-19 patients.
- ✓
- Prophylaxis with anticoagulant agents may improve prognosis in patients with COVID-19 pneumonia.
Conclusions
Although performed at a single center and with a limited number of patients, we our study may provide useful information for those fighting the COVID-19 pandemic. In the presence of respiratory distress that thorax CT findings cannot explain, pulmonary embolism should be kept in mind if D-dimer values increase more than expected. Our findings may provide guidance in making a decision for pulmonary CT angiography. In addition, studies with more patients are required to establish consensus regarding the use of anticoagulants as prophylactic in COVID-19 patients whose clinical situation has deteriorated.
Conflict of interest disclosure
There are no known conflicts of interest in the publication of this article. The manuscript was read and approved by all authors.
Compliance with ethical standards
Any aspect of the work covered in this manuscript has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. This study was approved by the institutional ethics committee (permit no: 135-24.09.20).
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Danzi, G.B.; Loffi, M.; Galeazzi, G.; Gherbesi, E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020, 41, 1858. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, X.; Yang, P.; Zhang, S. COVID-19 Complicated by Acute Pulmonary Embolism. Radiol Cardiothorac Imaging. 2020, 2, e200067. [Google Scholar] [CrossRef]
- Goeijenbier, M.; van Wissen, M.; van de Weg, C.; Jong, E.; Gerdes, V.E.; Meijers, J.C.; Brandjes, D.P.; van Gorp, E.C. Review: Viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012, 84, 1680–1696. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; Xia, J.; Yu, T.; Zhang, X.; Zhang, L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Griffin, D.O.; Jensen, A.; Khan, M.; Chin, J.; Chin, K.; Saad, J.; Parnell, R.; Awwad, C.; Patel, D. Pulmonary Embolism and Increased Levels of d-Dimer in Patients with Coronavirus Disease. Emerg Infect Dis. 2020, 26, 1941–1943. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; Guan, L.; Wei, Y.; Li, H.; Wu, X.; Xu, J.; Tu, S.; Zhang, Y.; Chen, H.; Cao, B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Danzi, G.B.; Loffi, M.; Galeazzi, G.; Gherbesi, E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020, 41, 1858. [Google Scholar] [CrossRef] [PubMed]
- Zuckier, L.S.; Moadel, R.M.; Haramati, L.B.; Freeman, L.M. Diagnostic Evaluation of Pulmonary Embolism During the COVID-19 Pandemic. J Nucl Med. 2020, 61, 630–631. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.P.; Parkar, A.P.; Prosch, H.; Silva, M.; Sverzellati, N.; Gleeson, F.; Brady, A. European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol. 2020, 30, 4903–4909. [Google Scholar] [CrossRef]
- ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic [Internet]. Available online: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance (accessed on 26 May 2020).
- Bunce, P.E.; High, S.M.; Nadjafi, M.; Stanley, K.; Liles, W.C.; Christian, M.D. Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis. 2011, 52, e14–e17. [Google Scholar] [CrossRef]
- Schouten, H.J.; Geersing, G.J.; Koek, H.L.; Zuithoff, N.P.; Janssen, K.J.; Douma, R.A.; van Delden, J.J.; Moons, K.G.; Reitsma, J.B. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013, 346, f2492. [Google Scholar] [CrossRef]
- Léonard-Lorant, I.; Delabranche, X.; Séverac, F.; Helms, J.; Pauzet, C.; Collange, O.; Schneider, F.; Labani, A.; Bilbault, P.; Molière, S.; Leyendecker, P.; Roy, C.; Ohana, M. Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels. Radiology. 2020, 296, E189–E191. [Google Scholar] [CrossRef]
- Lim, W.; Meade, M.; Lauzier, F.; et al. Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients*. Crit Care Med. 2015, 43, 401–410. [Google Scholar] [CrossRef]
- Corrigan, D.; Prucnal, C.; Kabrhel, C. Pulmonary embolism: the diagnosis, risk-stratification, treatment and disposition of emergency department patients. Clin Exp Emerg Med. 2016, 3, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Douma, R.A.; Tan, M.; Schutgens, R.E.; Bates, S.M.; Perrier, A.; Legnani, C.; Biesma, D.H.; Ginsberg, J.S.; Bounameaux, H.; Palareti, G.; Carrier, M.; Mol, G.C.; Le Gal, G.; Kamphuisen, P.W.; Righini, M. Using an age-dependent D-dimer cut-off value increases the number of older patients in whom deep vein thrombosis can be safely excluded. Haematologica. 2012, 97, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
© 2021 by the author. 2021 Vural Ahmet, Kahraman Ahmet Nedim