Overview of the Surgical Management of Liver Oligometastatic Disease in Colorectal Cancer
Abstract
1. Introduction
2. Methods: Literature Search
3. Results and Discussion
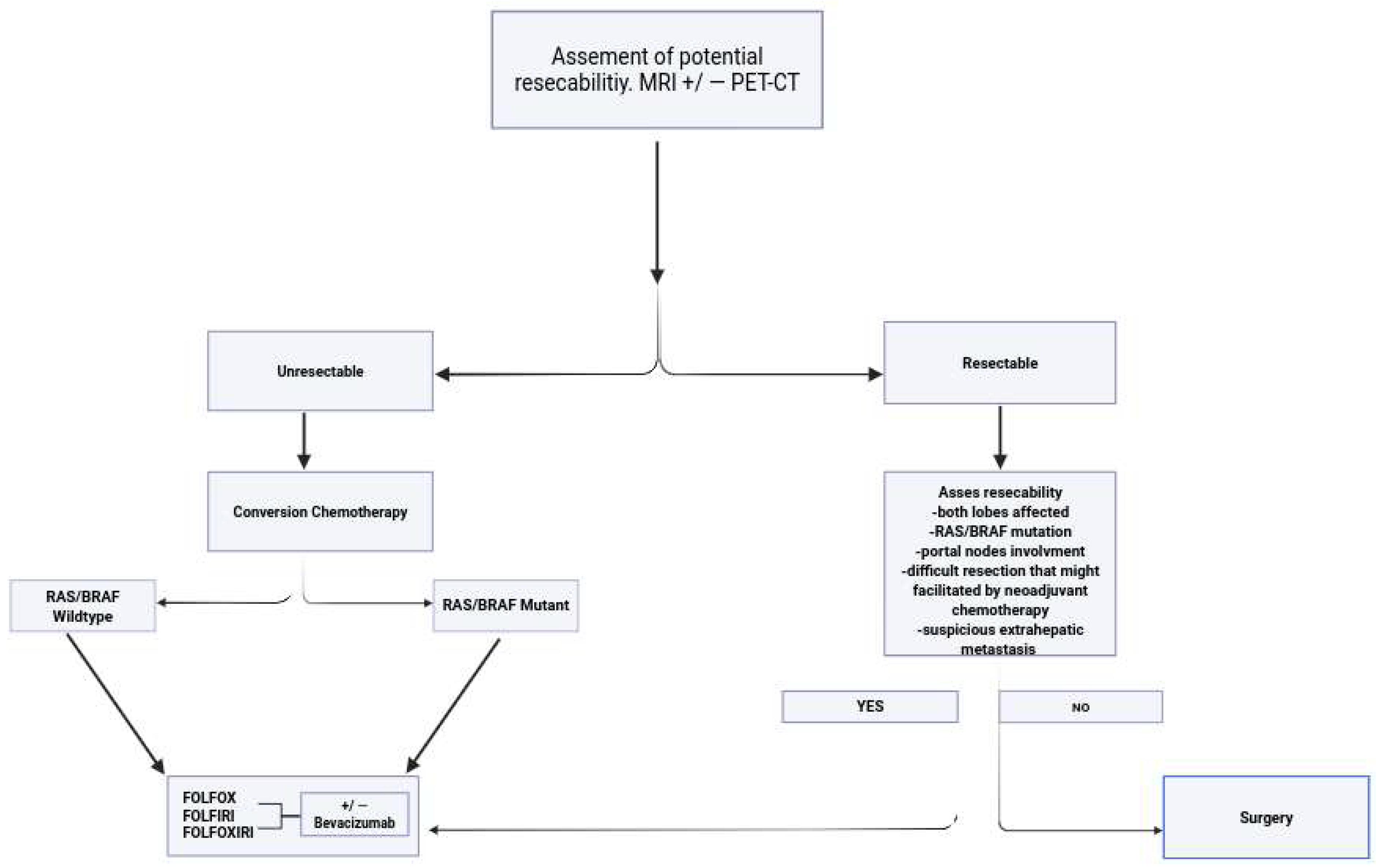
3.1. Presurgery Evaluation
3.2. Simultaneous Versus Staged Resection
3.3. Hepatic Metastases Defined as Unresectable
3.4. RAS/RAF Mutated Tumors
3.5. RAS/RAF Wild-Type Tumors
3.6. Hepatic Metastases Are Defined as Resectable
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berri, R.N.; Abdalla, E.K. Curable metastatic colorectal cancer: Recommended paradigms. Curr. Oncol. Rep. 2009, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Folprecht, G.; Grothey, A.; Alberts, S.; Raab, H.-R.; Köhne, C.-H. Neoadjuvant treatment of unresectable colorectal liver metastases: Correlation between tumour response and resection rates. Ann. Oncol. 2005, 16, 1311. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Hedrick, E.E.; Mass, R.D.; Sarkar, S.; Suzuki, S.; Ramanathan, R.K.; Hurwitz, H.I.; Goldberg, R.M.; Sargent, D.J. Response-independent survival benefit in metastatic colorectal cancer: A comparative analysis of N9741 and AVF2107. J. Clin. Oncol. 2008, 26, 183. [Google Scholar] [CrossRef]
- Izmailov, T.; Ryzhkin, S.; Borshchev, G.; Boichuk, S. Oligometastatic Disease (OMD): The Classification and Practical Review of Prospective Trials. Cancers 2023, 15, 5234. [Google Scholar] [CrossRef]
- Bipat, S.; van Leeuwen, M.S.; Comans, E.F.; Pijl, M.E.; Bossuyt, P.M.; Zwinderman, A.H.; Stoker, J. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis—Meta-analysis. Radiology 2005, 237, 123. [Google Scholar] [CrossRef]
- Kamel, I.R.; Choti, M.A.; Horton, K.M.; Braga, H.J.V.; Birnbaum, B.A.; Fishman, E.K.; Thompson, R.E.; Bluemke, D.A. Surgically staged focal liver lesions: Accuracy and reproducibility of dual-phase helical CT for detection and characterization. Radiology 2003, 227, 752. [Google Scholar] [CrossRef]
- Floriani, I.; Torri, V.; Rulli, E.; Garavaglia, D.; Compagnoni, A.; Salvolini, L.; Giovagnoni, A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: A systematic review and meta-analysis. J. Magn. Reson. Imaging 2010, 31, 19. [Google Scholar] [CrossRef]
- Kulemann, V.; Schima, W.; Tamandl, D.; Kaczirek, K.; Gruenberger, T.; Wrba, F.; Ba-Ssalamah, A. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur. J. Radiol. 2011, 79, e1. [Google Scholar] [CrossRef]
- Scharitzer, M.; Ba-Ssalamah, A.; Ringl, H.; Kölblinger, C.; Grünberger, T.; Weber, M.; Schima, W. Preoperative evaluation of colorectal liver metastases: Comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur. Radiol. 2013, 23, 2187. [Google Scholar] [CrossRef]
- Sahani, D.V.; Bajwa, M.A.; Andrabi, Y.; Bajpai, S.; Cusack, J.C. Current status of imaging and emerging techniques to evaluate liver metastases from colorectal carcinoma. Ann. Surg. 2014, 259, 861. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Colon Cancer. Version 3. 2024. Available online: https://jnccn.org/view/journals/jnccn/22/2D/article-e240029.xml (accessed on 29 December 2024).
- Ruers, T.J.; Wiering, B.; van der Sijp, J.R.; Roumen, R.M.; de Jong, K.P.; Comans, E.F.; Pruim, J.; Dekker, H.M.; Krabbe, P.F.; Oyen, W.J. Improved selection of patients for hepatic surgery of colorectal liver metastases with 18F-FDG pet: A randomized study. J. Nucl. Med. 2009, 50, 1036. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, T.; Gönen, M.; Baser, R.E.; Schwartz, L.H.; Tuorto, S.; Brody, L.A.; Covey, A.; Brown, K.T.; Larson, S.M.; Fong, Y. Prospective evaluation of 18F-FDG positron emission tomography in the preoperative staging of patients with hepatic colorectal metastases. HepatoBiliary Surg. Nutr. 2022, 11, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.T.M.; Fulham, M.; Stephen, M.S.; Chu, K.-M.; Thompson, J.F.; Sheldon, D.M.; Storey, D.W. The role of whole-body positron emission tomography with [18F]Fluorodeoxyglucose in identifying operable colorectal cancer metastases to the liver. Arch. Surg. 1996, 131, 703. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, D.; Vitola, J.V.; Sandler, M.P.; Arildsen, R.C.; Powers, T.A.; Wright, J.K.; Chapman, W.C.; Pinson, C.W. Staging recurrent metastatic colorectal carcinoma with PET. J. Nucl. Med. 1997, 38, 1196–1201. [Google Scholar]
- Mekenkamp, L.J.M.; Koopman, M.; Teerenstra, S.; van Krieken, J.H.J.M.; Mol, L.; Nagtegaal, I.D.; A Punt, C.J. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br. J. Cancer 2010, 103, 159. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Su, Y.-H.; Ho, M.-C.; Liang, J.-T.; Chen, T.-P.; Lai, H.-S.; Lee, P.-H. Clinicopathological features and prognosis in resectable synchronous and metachronous colorectal liver metastasis. Ann. Surg. Oncol. 2007, 14, 786–794. [Google Scholar] [CrossRef]
- Driedger, M.R.; Yamashita, T.S.; Starlinger, P.; Mathis, K.L.; Smoot, R.L.; Cleary, S.P.; Nagorney, D.M. Synchronous resection of colorectal cancer primary and liver metastases: An outcomes analysis. HPB 2021, 23, 1277. [Google Scholar] [CrossRef]
- McCahill, L.E.; Yothers, G.; Sharif, S.; Petrelli, N.J.; Lai, L.L.; Bechar, N.; Giguere, J.K.; Dakhil, S.R.; Fehrenbacher, L.; Lopa, S.H.; et al. Primary mFOLFOX6 plus Bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: Definitive analysis of NSABP trial C-10. J. Clin. Oncol. 2012, 30, 3223. [Google Scholar] [CrossRef]
- Shimada, H.; Tanaka, K.; Masui, H.; Nagano, Y.; Matsuo, K.; Kijima, M.; Ichikawa, Y.; Ike, H.; Ooki, S.; Togo, S. Results of surgical treatment for multiple (> or =5 nodules) bi-lobar hepatic metastases from colorectal cancer. Langenbeck’s Arch. Surg. 2004, 389, 114–121. [Google Scholar] [CrossRef]
- Gruenberger, T.; Morris, D.L. Staged treatment of bilobar hepatic metastases from colorectal cancer. Eur. J. Surg. 2002, 168, 516–518. [Google Scholar] [CrossRef]
- Birrer, D.L.; Tschuor, C.; Reiner, C.S.; Fritsch, R.; Pfammatter, T.; Schüler, H.G.; Pavic, M.; De Oliveira, M.; Petrowsky, H.; Dutkowski, P.; et al. Multimodal treatment strategies for colorectal liver metastases. Swiss Med. Wkly. 2021, 5, 151. [Google Scholar] [CrossRef]
- Boudiaf, Z.; Bouzid, C.; Ait-Arab, M.R.; Cherchar, K.; Kheloufi, M.; Chibane, A.; Boutekedjiret, I.H.; Hattou, Z.; Gouaref, F.; Bentabak, K. La chirurgie laparoscopique de downstaging pour cancer colorectal avec métastases hépatiques synchrones: Quel intérêt dans les hépatectomies en deux temps? à propos d’une série de 6 cas [Laparoscopic downstaging surgery for colorectal cancer with synchronous liver metastases: What value in two-stage hepatectomies?]. Pan. Afr. Med. J. 2023, 27, 38. [Google Scholar]
- Ono, Y.; Inoue, Y.; Kobayashi, K.; Sato, S.; Kitano, Y.; Oba, A.; Sato, T.; Ito, H.; Takahashi, Y. Laparoscopic Portal Vein Ligation and Embolization During First-Stage Hepatectomy for Initially Unresectable Colorectal Liver Metastases. Ann. Surg. Oncol. 2024, 31, 3069–3070. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Wicherts, D.A.; De Haas, R.J.; Aloia, T.; Lévi, F.; Paule, B.; Castaing, D. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: Myth or reality? J. Clin. Oncol. 2008, 26, 1635. [Google Scholar] [CrossRef] [PubMed]
- Blazer, D.G., 3rd; Kishi, Y.; Maru, D.M.; Kopetz, S.; Chun, Y.S.; Overman, M.J.; Vauthey, J.N. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J. Clin. Oncol. 2008, 26, 5344. [Google Scholar] [CrossRef]
- Benoist, S.; Brouquet, A.; Penna, C.; Julié, C.; El Hajjam, M.; Chagnon, S.; Nordlinger, B. Complete response of colorectal liver metastases after chemotherapy: Does it mean cure? J. Clin. Oncol. 2006, 24, 3939. [Google Scholar] [CrossRef]
- Song, K.D.; Kim, Y.K.; Kim, H.C.; Huh, J.W.; Park, Y.S.; Park, J.O.; Kim, S.T. Disappearing or residual tiny (≤5 mm) colorectal liver metastases after chemotherapy on gadoxetic acid-enhanced liver MRI and diffusion-weighted imaging: Is local treatment required? Eur. Radiol. 2017, 27, 3088–3096. [Google Scholar]
- Kepenekian, V.; Muller, A.; Valette, P.J.; Rousset, P.; Chauvenet, M.; Phelip, G.; Walter, T.; Adham, M.; Glehen, O.; Passot, G. Evaluation of a strategy using pretherapeutic fiducial marker placement to avoid missing liver metastases. BJS Open 2019, 22, 344–353. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Paredes, A.Z.; Moris, D.; Gavriatopoulou, M.; Cloyd, J.M.; Pawlik, T.M. Disappearing liver metastases: A systematic review of the current evidence. Surg. Oncol. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Alberts, S.R.; Horvath, W.L.; Sternfeld, W.C.; Goldberg, R.M.; Mahoney, M.R.; Dakhil, S.R.; Donohue, J.H. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group phase II study. J. Clin. Oncol. 2005, 23, 9243. [Google Scholar] [CrossRef]
- Hayashi, H.; Miyamoto, Y.; Higashi, T.; Hiyoshi, Y.; Yamao, T.; Uemura, N.; Matsumura, K.; Imai, K.; Yamashita, Y.I.; Baba, H. CD44 expression enhances chemoresistance and implies occult micrometastases after conversion hepatectomy for initially unresectable colorectal liver metastases. Am. J. Transl. Res. 2020, 15, 5955–5966. [Google Scholar]
- Bridgewater, J.; Pugh, S.A.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; O Griffiths, G.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, S.; Modest, D.P.; Rossius, L.; Lerch, M.M.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016, 17, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.A.; Meade, A.M.; Seymour, M.T.; Wilson, R.H.; Madi, A.; Fisher, D.; Kenny, S.L.; Kay, E.; Hodgkinson, E.; Pope, M.; et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011, 12, 642–653. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Lang, I.; Folprecht, G.; Nowacki, M.; Barone, C.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Celik, I.; Kohne, C. Cetuximab plus FOLFIRI: Final data from the CRYSTAL study on the association of KRAS and BRAF biomarker status with treatment outcome. J. Clin. Oncol. 2010, 28, 3570. [Google Scholar] [CrossRef]
- Tournigand, C.; Cervantes, A.; Figer, A.; Lledo, G.; Flesch, M.; Buyse, M.; Mineur, L.; Carola, E.; Etienne, P.-L.; Rivera, F.; et al. OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—A Gercor study. J. Clin. Oncol. 2006, 24, 394–400. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.-L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A randomized, multicenter Phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 Metastatic colorectal Cancer. J. Clin. Oncol. 2014, 20, 2240–2247. [Google Scholar]
- Mise, Y.; Hasegawa, K.; Saiura, A.; Oba, M.; Yamamoto, J.; Nomura, Y.; Kokudo, N. A Multicenter Phase 2 Trial to Evaluate the Efficacy of mFOLFOX6 + Cetuximab as Induction Chemotherapy to Achieve R0 Surgical Resection for Advanced Colorectal Liver Metastases (NEXTO Trial). Ann. Surg. Oncol. 2020, 27, 4188. [Google Scholar] [CrossRef]
- Masi, G.; Loupakis, F.; Pollina, L.; Vasile, E.; Cupini, S.; Ricci, S.; Brunetti, I.M.; Ferraldeschi, R.; Naso, G.; Filipponi, F.; et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann. Surg. 2009, 249, 420. [Google Scholar] [CrossRef]
- Ychou, M.; Viret, F.; Kramar, A.; Desseigne, F.; Mitry, E.; Guimbaud, R.; Delpero, J.R.; Rivoire, M.; Quénet, F.; Portier, G.; et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): A phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother. Pharmacol. 2008, 62, 195. [Google Scholar] [CrossRef]
- Chrabaszcz, S.; Rajeev, R.M.; Witmer, H.D.M.; Dhiman, A.M.; Klooster, B.; Gamblin, T.C.M.; Banerjee, A.; Johnston, F.M.M.; Turaga, K.K. A Systematic Review of Conversion to Resectability in Unresectable Metastatic Colorectal Cancer Chemotherapy Trials. Am. J. Clin. Oncol. 2022, 45, 366. [Google Scholar] [CrossRef] [PubMed]
- Karaoğlan, B.B.; Öz, D.K.; Araz, M.S.; Akyol, C.; Utkan, G. Advancements in the Management of Synchronous Colorectal Liver Metastases: A Comprehensive Review of Surgical, Systemic, and Local Treatment Modalities. Curr. Oncol. Rep. 2024, 26, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Wagman, L.D. Importance of response to neoadjuvant therapy in patients with liver-limited MCRC when the intent is resection and/or ablation. Clin. Color. Cancer 2013, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Damato, A.; Ghidini, M.; Dottorini, L.; Tomasello, G.; Iaculli, A.; Ghidini, A.; Luciani, A.; Petrelli, F. Chemotherapy Duration for Various Indications in Colorectal Cancer: A Review. Curr. Oncol. Rep. 2023, 25, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.J.G.; Bolhuis, K.; Loosveld, O.J.L.; de Groot, J.W.B.; Droogendijk, H.; Helgason, H.H.; Hendriks, M.P.; Klaase, J.M.; Kazemier, G.; Liem, M.S.L.; et al. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): An open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. 2023, 24, 757–771. [Google Scholar] [CrossRef]
- D’angelica, M.; Kornprat, P.; Gonen, M.; Chung, K.-Y.; Jarnagin, W.R.; DeMatteo, R.P.; Fong, Y.; Kemeny, N.; Blumgart, L.H.; Saltz, L.B. Lack of evidence for increased operative morbidity after hepatectomy with perioperative use of bevacizumab: A matched case-control study. Ann. Surg. Oncol. 2007, 14, 759. [Google Scholar] [CrossRef]
- Reddy, S.K.; Morse, M.A.; Hurwitz, H.I.; Bendell, J.C.; Gan, T.J.; Hill, S.E.; Clary, B.M. Addition of bevacizumab to irinotecan- and oxaliplatin-based preoperative chemotherapy regimens does not increase morbidity after resection of colorectal liver metastases. J. Am. Coll. Surg. 2008, 206, 96. [Google Scholar] [CrossRef]
- Okines, A.; del Puerto, O.; Cunningham, D.; Chau, I.; Van Cutsem, E.; Saltz, L.; Cassidy, J. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br. J. Cancer 2009, 101, 1033. [Google Scholar] [CrossRef]
- Kesmodel, S.B.; Ellis, L.M.; Lin, E.; Chang, G.J.; Abdalla, E.K.; Kopetz, S.; Vauthey, J.-N.; Rodriguez-Bigas, M.A.; Curley, S.A.; Feig, B.W. Preoperative bevacizumab does not significantly increase postoperative complication rates in patients undergoing hepatic surgery for colorectal cancer liver metastases. J. Clin. Oncol. 2008, 26, 5254. [Google Scholar] [CrossRef]
- Tamandl, D.; Gruenberger, B.; Klinger, M.; Herberger, B.; Kaczirek, K.; Fleischmann, E.; Gruenberger, T. Liver resection remains a safe procedure after neoadjuvant chemotherapy including bevacizumab: A case-controlled study. Ann. Surg. 2010, 252, 124. [Google Scholar] [CrossRef]
- Wicherts, D.A.; de Haas, R.J.; Sebagh, M.; Corrales, E.S.; Gorden, D.L.; Lévi, F.; Paule, B.; Azoulay, D.; Castaing, D.; Adam, R. Impact of bevacizumab on functional recovery and histology of the liver after resection of colorectal metastases. Br. J. Surg. 2011, 98, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pool, A.E.; Marsman, H.A.; Verheij, J.; Ten Kate, F.J.; Eggermont, A.M.; Ijzermans, J.N.; Verhoef, C. Effect of bevacizumab added preoperatively to oxaliplatin on liver injury and complications after resection of colorectal liver metastases. J. Surg. Oncol. 2012, 106, 892. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, D.A.; de Haas, R.J.; Andreani, P.; Sotirov, D.; Salloum, C.; Castaing, D.; Adam, R.; Azoulay, D. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br. J. Surg. 2010, 97, 240. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, D.; Chun, Y.S.; Madoff, D.C.; Abdalla, E.K.; Vauthey, J.-N. Chemotherapy with bevacizumab does not affect liver regeneration after portal vein embolization in the treatment of colorectal liver metastases. Ann. Surg. Oncol. 2008, 15, 2765. [Google Scholar] [CrossRef]
- O’connell, R.M.; Hoti, E. Challenges and Opportunities for Precision Surgery for Colorectal Liver Metastases. Cancers 2024, 28, 2379. [Google Scholar] [CrossRef]
- Ychou, M.; Rivoire, M.; Thezenas, S.; Guimbaud, R.; Ghiringhelli, F.; Mercier-Blas, A.; Mineur, L.; Francois, E.; Khemissa, F.; Chauvenet, M.; et al. Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br. J. Cancer 2022, 126, 1264. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004, 22, 229. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nagase, M.; Tamagawa, H.; Ueda, S.; Tamura, T.; Murata, K.; Nakajima, T.E.; Baba, E.; Tsuda, M.; Moriwaki, T.; et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann. Oncol. 2016, 27, 1539. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Van Cutsem, E.; Rougier, P.; Ciardiello, F.; Heeger, S.; Schlichting, M.; Celik, I.; Köhne, C.-H. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer 2012, 48, 1466–1475. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Makhson, A.; Hartmann, J.T.; Aparicio, J.; de Braud, F.; Koralewski, P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic col orectal cancer. J. Clin. Oncol. 2009, 27, 663. [Google Scholar] [CrossRef]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol. 2014, 15, 601. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B.; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Mizusawa, J.; Inaba, Y.; Hamaguchi, T.; Shida, D.; Ohue, M.; Komori, K.; Shiomi, A.; Shiozawa, M.; Watanabe, J.; et al. Hepatectomy Followed by mFOLFOX6 Versus Hepatectomy Alone for Liver-Only Metastatic Colorectal Cancer (JCOG0603): A Phase II or III Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 3789. [Google Scholar] [CrossRef]
| Trial Name | Authors | Publication Year | Key Findings/Important Data |
|---|---|---|---|
| New EPOC | Bridgewater JA et al. [33] | 2020 | The multicenter, open-label, randomized study found a detrimental effect on overall survival in patients with metastatic colorectal cancer, RAS wild type with resectable or suboptimal resectable liver metastases who received perioperative cetuximab. |
| FIRE-3 | Stintzing S et al. [34] | 2015 | Comparison of FOLFIRI plus cetuximab vs. FOLFIRI plus bevacizumab in mCRC. The study highlighted that cetuximab significantly increased the rate of conversion to resectability compared to bevacizumab, especially in patients with RAS wild-type tumors. |
| COIN (Chemotherapy versus Chemotherapy plus Cetuximab) | Seymour MT et al. [35] | 2007 | This trial assessed the addition of cetuximab to standard chemotherapy (FOLFOX) in mCRC patients. It demonstrated an improvement in progression-free survival (PFS) but did not show a major increase in resection rates for liver metastases. It highlighted the challenge of using cetuximab for conversion. |
| CRISTAL | Van Cutsem et al. [36] | 2010 | Studied FOLFIRI plus cetuximab in RAS wild-type mCRC patients. It found that cetuximab improved overall survival and response rates and supported its role in conversion strategies for patients with limited metastatic spread. |
| OPTIMOX | Tournigand C et al. [37] | 2006 | Investigated the use of oxaliplatin-based chemotherapy in mCRC, showing that alternating chemotherapy (OXALIPLATIN/FOLFOX) regimens led to significant tumor reduction, aiding in conversion to resectability in some patients. |
| PEAK | Schwartzberg LS et al. [38] | 2014 | Focused on the combination of FOLFOX and bevacizumab in patients with liver-limited mCRC. It demonstrated that a high response rate with this regimen increased the likelihood of achieving resectability, especially in patients with isolated liver metastases. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macovei, A.M.O.; Venter, D.P.; Dumitriu, B.; Oprescu, C.; Venter, M.D.; Andrei, G.-N.; Precup, M.S.V.; Socea, B.; Stefan, M. Overview of the Surgical Management of Liver Oligometastatic Disease in Colorectal Cancer. J. Mind Med. Sci. 2025, 12, 31. https://doi.org/10.3390/jmms12010031
Macovei AMO, Venter DP, Dumitriu B, Oprescu C, Venter MD, Andrei G-N, Precup MSV, Socea B, Stefan M. Overview of the Surgical Management of Liver Oligometastatic Disease in Colorectal Cancer. Journal of Mind and Medical Sciences. 2025; 12(1):31. https://doi.org/10.3390/jmms12010031
Chicago/Turabian StyleMacovei, Anca Monica Oprescu, Dana Paula Venter, Bogdan Dumitriu, Constantin Oprescu, Mircea Dan Venter, Gabriel-Nicolae Andrei, Mures Sebastian Valcea Precup, Bogdan Socea, and Mihai Stefan. 2025. "Overview of the Surgical Management of Liver Oligometastatic Disease in Colorectal Cancer" Journal of Mind and Medical Sciences 12, no. 1: 31. https://doi.org/10.3390/jmms12010031
APA StyleMacovei, A. M. O., Venter, D. P., Dumitriu, B., Oprescu, C., Venter, M. D., Andrei, G.-N., Precup, M. S. V., Socea, B., & Stefan, M. (2025). Overview of the Surgical Management of Liver Oligometastatic Disease in Colorectal Cancer. Journal of Mind and Medical Sciences, 12(1), 31. https://doi.org/10.3390/jmms12010031






