Abstract
Oligometastatic colorectal cancer (CRC) refers to a state in which distant metastatic spread is limited to a few sites, offering the potential for curative treatment with aggressive local therapies. The surgical management of oligometastatic CRC has gained increasing attention due to its potential to improve survival. This review explores the evolving role of surgery in the treatment of oligometastatic disease, focusing on the criteria for selecting patients, surgical techniques, and outcomes. While systemic therapy remains essential, surgery can offer long-term survival benefits for appropriately selected patients with limited metastatic disease, particularly those with metastases confined to the liver. Advances in imaging technologies, minimally invasive surgical techniques, and perioperative care have enhanced the safety and efficacy of these procedures. The integration of multimodal therapies, such as chemotherapy, targeted therapy, and immunotherapy, in conjunction with surgery, is also discussed, with a focus on optimizing outcomes. To conclude, surgical resection of liver metastases improves survival compared to systemic therapy alone; thus, resection should be taken into consideration whenever possible. For initially unresectable diseases, personalized conversion therapy is indicated. This review aims to clarify how and when liver resection can first be chosen; when preoperative systemic treatment is needed; and if this is chosen, what is the best approach.
1. Introduction
The liver is the most common anatomic site where metastases occur in colorectal cancer, and one-third of metastatic patients have lesions located only at this level [1]. Some patients with liver-only metastases may be candidates for surgical intervention, but their initial approach is essential for a good long-term outcome. The option of liver resection is the standard treatment for patients with liver-only disease. This approach improves survival considerably (5-year survival of 40%). When it is feasible, it is performed with intent to cure [1]. Chemotherapy is also essential in patients with resectable metastases and is usually administered postoperatively. However, it is possible to opt for preoperative systemic treatment to check the tumor biology in a patient in whom such a complex intervention may not be curative or in patients with metastases initially considered unresectable due to their size [2,3].
In the literature, more types of oligometastatic disease are listed; depending on the time of appearance of the metastases, these types have different survival and therapeutic implications. It is generally accepted that oligometastatic patients are those with up to 5 metastases in total. Liver, bone, lung, and brain metastases are of particular interest. According to the OligoCare classification, there are 10 major and 7 minor criteria for their classification, which influence the prognosis of patients [4].
This review aims to clarify how and when liver resection per primam is chosen when preoperative systemic treatment is needed and, if this is chosen, what is the best approach.
2. Methods: Literature Search
The present narrative review aims to describe the surgical and systemic treatment options for patients with liver metastases originating from colon cancer. The analyzed literature was selected according to exclusion and inclusion criteria. Peer-reviewed articles and clinical trials published over a period of 20 years (from 2004 to 2024) were taken into account, and the ones published after 2015 were preferred. For this, two databases, the Cochrane Library and the PubMed database, were used, both accessed on 27th of December 2024. The keywords were colorectal cancer, mCRC, oligometastatic, hepatic metastases, resectable, borderline resectable. Only articles in English were included. The initial search resulted in 2367 publications, whose titles and abstracts were evaluated one at a time by the authors, and 68 of them were finally selected, considered relevant due to the period of publication and the quality of the information. The information was structured into six paragraphs.
3. Results and Discussion
3.1. Presurgery Evaluation
Preoperative imaging evaluation is essential in the decision on surgical treatment. This not only informs on the number, anatomical distribution, and resectability of liver metastases but also on the possible extrahepatic extension. Usually, the first proposed imaging investigation is computed tomography with contrast. This has a specificity of 96 percent for the detection of liver metastases. Still, it must be taken into account that the ability to identify those under 1 cm is reduced and that the false negative rate, in this case, is 10 percent. However, this investigation is useful in estimating the liver volume before resection and determining if portal embolization is necessary in advance [5,6,7].
Magnetic resonance imaging is preferable to computed tomography, especially to detect infracentrimetric liver metastases. This is of choice in the case of patients with hepatic steatosis, having a specificity and sensitivity of up to 97 percent when used with contrast enhancement. It is also more efficient than computed tomography in the detection of metastases at the peritoneal level or at the level of the porta hepatis [8,9,10].
The NCCN (National Comprehensive Cancer Center) guideline recommends that any patient with metastatic colorectal cancer with potentially curable disease should be evaluated with positron emission tomography [11].
The data related to the usefulness of PET CT in this clinical situation come from a clinical trial that demonstrated the reduction in the number of futile laparotomies, preventing unnecessary surgery in 1 out of 6 cases [12,13]. However, it is ideal for the patient to have both PET and contrast-enhanced CT acquisitions simultaneously for the best specificity, and false positive results due to inflammatory changes have to be kept in mind [14,15].
3.2. Simultaneous Versus Staged Resection
The decision on the type of surgical intervention is fundamentally influenced by the time of appearance of liver metastases. Those that appear up to 12 months after the identification of the primary tumor are called synchronous metastases, and those that appear after this interval are metachronous [16,17].
The biggest challenge is dealing with synchronous metastases, most of the time requiring complex surgical interventions. Other important aspects to consider in the choice of surgical intervention are severe symptoms and tumor burden. If the symptoms deriving from the primary tumor are disabling (obstruction, perforation, or bleeding), the primary tumor should be operated on first [18,19]. If the patient is asymptomatic, simultaneous hepatic and colonic resection is ideal, depending on how extensive the disease is in the liver.
The algorithm for the management of patients with hepatic metastases originating from colon cancer is illustrated in Figure 1.
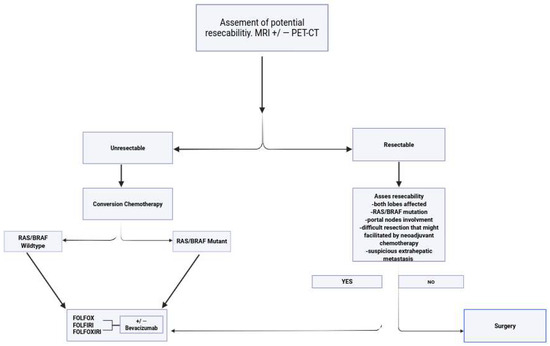
Figure 1.
Algorithm for management of hepatic metastases originating from colorectal cancer (MRI—magnetic resonance imaging, PET-CTpositron emission tomography–computed tomography, RAS—rat sarcoma virus).
Patients with a favorable location of the primary tumor (right colon) and a limited number of liver metastases are ideal candidates for simultaneous surgical intervention.
In patients with extensive bilobar disease, the treatment decision is more controversial from several points of view. First of all, technically, only a two-time resection is feasible. This involves the initial maximum resection first with multiple partial hepatectomies and postponing the anatomical liver resection for the second surgical step. In some cases, portal embolization may also be necessary, which targets the hypertrophy of the remaining liver [20,21].
However, although extensive liver resection at first has the advantage of the absence of changes at the parenchymal level that chemotherapy gives, it also has the disadvantage of a long postoperative recovery time, in which the patient is deprived of systemic treatment. In this interval, the disease can evolve, and these aspects must be carefully weighed by the multidisciplinary team [22]. Liver resections can be performed both open and through minimally invasive techniques. It is proven that the minimally invasive ones have better short-term results and at least comparable, even superior, results for oncological outcomes (survival). Minimally invasive resections are suitable for small (under 5 cm) and peripheral liver tumors (segments II and VI), and their success is dependent on the number of previous hepatectomies the operator has performed. [23,24].
Even in the desired situation in which the patient has a complete clinical response, resection of the respective liver segment is necessary because only 4 to 9 percent have a complete pathological response after neoadjuvant chemotherapy [25,26]. There is evidence that up to 83 percent of lesions with complete clinical response still have viable tumors present [27,28]. These are the reasons why lesions with the highest probability of imaging disappearance (those below 2 cm) and which, due to their location, are easily accessible (subcapsular lesions) must be marked with a fiducial marker before the initiation of treatment [29,30].
3.3. Hepatic Metastases Defined as Unresectable
The decision on treatment in patients with initially unresectable liver metastases depends on the RAS-RAF status, the side tumor, and the general condition of the patient (ability to tolerate aggressive treatment or not). The treatment used in this situation is called conversion therapy. It can be used in patients who are expected to have a sufficient objective response to allow R0 rejection of liver lesions [31,32].
The most important trials that made significant contributions to understanding and improving the efficacy of perioperative chemotherapy in patients with colorectal cancer and hepatic metastases have been summarized in Table 1. Some of these trials also included patients with upfront resectable hepatic disease.

Table 1.
The most important trials that made significant contributions to understanding and improving the efficacy of perioperative chemotherapy in patients with colorectal cancer and hepatic metastases.
The most significant conversion rates reported were with triplet chemotherapy or the combination of chemotherapy with epidermal growth factor receptor inhibitors [39,40,41].
Patients who become resectable and undergo surgery have a substantial benefit in overall survival (improvement from 20 percent to 30–35%) [42,43].
The maximum duration of preoperative treatment until resection is 4 months; after this interval, changes in the liver due to chemotherapy make resection very difficult. Also, if the patient becomes resectable during the 4 months, he will be offered adjuvant treatment for a duration that completes the administration of up to 6 months.
3.4. RAS/RAF Mutated Tumors
In patients with RAS-RAF mutations, the most effective possible option is FOLFOXIRI (irinotecan 165 mg-m2, oxaliplatin 85 mg/m2, fluorouracil 2400 mg–3400 mg/m2 continuous infusion of 46 h, leucovorin 200 mg/m2) with or without bevacizumab. This regimen was compared in the CAIRO5 study, a phase III study that enrolled 294 patients with RAS/BRAF V600E mutations or primary location in the right colon. They were randomized to FOLFOXIRI+ bevacizumab or FOLFOX/FOLFIRI+ bevacizumab. They were re-evaluated by imaging at 8–9 weeks for resectability, and if this was feasible, they received up to 12 postoperative treatments. If resection was not possible, the patients received maintenance with 5-fluorouracil and bevacizumab after 6 months of initial treatment. A total of 51 percent of the FOLFIRINOX arm underwent complete local treatment (R0 liver resection or R1/combined with ablative treatment) versus 33 in the doublet arm. This result translated into a survival benefit (progression-free survival 10.6 versus 9 months, HR 0.76, 95%CI 0.6–0.98) and a higher response rate (objective response rate 54 versus 33 percent). Toxicity was, as expected, greater in the triplet arm, with important rates of neutropenia (12.9/38.2%, p < 0.001) and diarrhea (3.4/19.4%, p < 0.001) [44].
The addition of bevacizumab to chemotherapy in patients with induction treatment is controversial because of its toxicity profile (impaired wound healing) and because of the need for a 6–8 week break from the last administration to the intervention, which can postpone surgery [45,46]. However, retrospective data suggest that the rate of bleeding and functional recovery after bevacizumab is comparable to chemotherapy regimens without bevacizumab [47,48,49,50,51,52]. There is, however, the hypothesis that the use of bevacizumab after portal embolization would be detrimental because of liver regeneration impairment [53,54].
3.5. RAS/RAF Wild-Type Tumors
In RAS/RAF wild-type tumors, the regimens with the highest response rate are preferred, as there is an important correlation between this, the resection rate, and overall survival [55,56].
The triplet (FOLFOXIRI regimen) is, thus, preferred for all patients who can tolerate this more aggressive approach irrespective of RAS status [2,57]. Doublets with irinotecan or oxaliplatin and 5-fluorouracil are options for patients in whom triple treatment is not an option. They are equally effective, and the choice between them should only be based on the different toxicity profiles [52,58,59].
In patients in whom liver resection is considered, the regimen based on oxaliplatin (FOLFOX- oxaliplatin 85 mg/m2, 5-FU bolus administration 400 mg/m2, 5-FU 2400 mg/m2 46 h continuous administration, every 2 weeks) is preferred, irinotecan being associated with more important liver changes. However, if metachronous liver metastases and the patient has received oxaliplatin in the last 12 months, FOLFIRI (irinotecan 180 mg/m2, 5-FU bolus administration 400 mg/m2, 5-FU 2400 mg/m2 46 h continuous administration, every 2 weeks) is the best option [11].
Targeting EGFR (epidermal growth factor receptor) in patients receiving conversion therapy is an option with modest benefits. In the CRISTAL and OPUS trials, the resection rate improved from 3.7% to 7% and from 2.4 to 4.7 when chemotherapy doublet (either with oxaliplatin or irinotecan) was compared with doublet plus cetuximab or panitumumab [36,60,61].
However, the experts do not recommend the combination of the oxaliplatin-based regimen with anti-EGFR, considering the inferior outcomes demonstrated in the Ne EPOC trial. The trial studies the addition of cetuximab to FOLFOX perioperatively in patients with potentially resectable liver metastases. They received FOLFOX with or without cetuximab 12 weeks pre- and 12 weeks postoperatively, those with anti-EGFR treatment had significantly lower survival (14.5 versus 24.5 months). A possible explanation of this result would be that the oxaliplatin—cetuximab combination is detrimental in the first line even in patients with RAS-RAF wild-type left colon tumors [32,62,63].
Therefore, the administration of FOLFIRI and cetuximab remains an option to improve the resection rate, but at the price of possibly more difficult hepatic resections after irinotecan.
3.6. Hepatic Metastases Are Defined as Resectable
Several trials demonstrated that the use of perioperative chemotherapy in patients with primary resectable metastases does not improve survival and that it has the disadvantage of a more difficult rejection (due to changes after chemotherapy, especially after irinotecan) and a greater possibility of postoperative complications.
The EORTC 40983 trial enrolled 364 patients with up to four resectable liver metastases who were randomized to receive or not perioperative treatment with FOLFOX (12 weeks pre- and 12 weeks postoperative). The resection rate was similar in the group with primary surgery versus the one with perioperative chemotherapy (84 versus 83 percent). However, the use of preoperative chemotherapy reduced the rate of failed laparotomy (11 percent in the primary rejection group versus 5 percent in the study group). A higher rate of postoperative complications was reported in the chemotherapy group (25 versus 16 percent, OR 1.58 percent, 95% CI 1.02–2.45), but postoperative mortality was the same. Among the present complications were biliary fistulas, intra-abdominal infections, and reversible hepatocellular insufficiency [33,64].
The study also demonstrated, after a follow-up of 8.5 years, a trend of favorable survival in the group that received chemotherapy (38 versus 30 percent, OR = 0.71), which was statistically significant only when stratified for truly resectable patients [33].
These data led to the indication from the guidelines for surgery first in all patients who do not have more than four liver metastases and whose metastases are limited to one liver lobe, who do not have radiological suspicion of portal node involvement, with no suspicion of non-hepatic metastases and no BRAF or RAS mutation [33].
In patients treated with surgery first, the guidelines recommend 6 months of adjuvant oxaliplatin-based chemotherapy, except for those with metachronous metastases who received oxaliplatin in the last 12 months. This recommendation is extrapolated from the indications in non-metastatic patients. However, the data studying this particular clinical situation are contradictory [11].
A study that enrolled patients with initially resectable disease who received six preoperative and six postoperative chemotherapy administrations did not demonstrate a benefit for overall survival (survival at 5 years 52 in the chemotherapy group versus 48 percent HR 0.88, 95% CI 0.68–1.14), nor for colorectal-cancer-related deaths. However, it should be taken into account that survival was not the main endpoint of this trial [33,65].
Another trial that included the Japanese population randomized patients to observation versus 6 months of FOLFOX type chemotherapy after resection with R0 hepatectomy. It demonstrated a substantial benefit in progression-free survival (5-year DFS 50 versus 39 percent, HR0.67, 95% CI 0.5–0.92) but failed to demonstrate a benefit in overall survival. It can be speculated that the use of regimens based on oxaliplatin would have a greater effect on selecting resistant cells in this particular clinical situation [66].
4. Conclusions
In patients with liver metastases and colorectal cancer, liver resection, when feasible, significantly improves survival. The ideal way to determine resectability preoperatively is an abdominal MRI. Even for patients in whom this investigation reveals unresectable disease, conversion treatment can be considered, which consists of double or triple chemotherapy. Adding targeted treatment increases the response rate but also the rate of postoperative complications, and there is evidence that EGFR treatment combined with oxaliplatin-based chemotherapy is detrimental. Conversion chemotherapy can last a maximum of 4 months, and response assessment must be performed at a maximum of 2 months. In patients with primary resectable liver metastases, surgery is preferred, followed by postoperative chemotherapy. Therapeutic decisions, both in resectable and unresectable situations, are complex and require a multidisciplinary team with experience in this clinical situation. This review includes both the point of view of medical and surgical oncologists and tries to bring the information to a common denominator, this aspect making it unique compared to the literature published on the same subject.
Author Contributions
Conceptualization, A.M.O.M.; methodology, D.P.V.; software, B.D.; validation, C.O.; formal analysis, M.D.V.; investigation, G.-N.A. and M.S.V.P.; resources, B.S.; data curation M.S.; writing—original draft preparation, A.M.O.M.; writing—B.S.; visualization, C.O.; supervision, M.S.; project administration, B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Patient consent was waived as the study is retrospective. All of the patients at admission signed an informed consent regarding future studies as this is a clinical and research hospital.
Data Availability Statement
Data are available upon requirement.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Berri, R.N.; Abdalla, E.K. Curable metastatic colorectal cancer: Recommended paradigms. Curr. Oncol. Rep. 2009, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Folprecht, G.; Grothey, A.; Alberts, S.; Raab, H.-R.; Köhne, C.-H. Neoadjuvant treatment of unresectable colorectal liver metastases: Correlation between tumour response and resection rates. Ann. Oncol. 2005, 16, 1311. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Hedrick, E.E.; Mass, R.D.; Sarkar, S.; Suzuki, S.; Ramanathan, R.K.; Hurwitz, H.I.; Goldberg, R.M.; Sargent, D.J. Response-independent survival benefit in metastatic colorectal cancer: A comparative analysis of N9741 and AVF2107. J. Clin. Oncol. 2008, 26, 183. [Google Scholar] [CrossRef]
- Izmailov, T.; Ryzhkin, S.; Borshchev, G.; Boichuk, S. Oligometastatic Disease (OMD): The Classification and Practical Review of Prospective Trials. Cancers 2023, 15, 5234. [Google Scholar] [CrossRef]
- Bipat, S.; van Leeuwen, M.S.; Comans, E.F.; Pijl, M.E.; Bossuyt, P.M.; Zwinderman, A.H.; Stoker, J. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis—Meta-analysis. Radiology 2005, 237, 123. [Google Scholar] [CrossRef]
- Kamel, I.R.; Choti, M.A.; Horton, K.M.; Braga, H.J.V.; Birnbaum, B.A.; Fishman, E.K.; Thompson, R.E.; Bluemke, D.A. Surgically staged focal liver lesions: Accuracy and reproducibility of dual-phase helical CT for detection and characterization. Radiology 2003, 227, 752. [Google Scholar] [CrossRef]
- Floriani, I.; Torri, V.; Rulli, E.; Garavaglia, D.; Compagnoni, A.; Salvolini, L.; Giovagnoni, A. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: A systematic review and meta-analysis. J. Magn. Reson. Imaging 2010, 31, 19. [Google Scholar] [CrossRef]
- Kulemann, V.; Schima, W.; Tamandl, D.; Kaczirek, K.; Gruenberger, T.; Wrba, F.; Ba-Ssalamah, A. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur. J. Radiol. 2011, 79, e1. [Google Scholar] [CrossRef]
- Scharitzer, M.; Ba-Ssalamah, A.; Ringl, H.; Kölblinger, C.; Grünberger, T.; Weber, M.; Schima, W. Preoperative evaluation of colorectal liver metastases: Comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur. Radiol. 2013, 23, 2187. [Google Scholar] [CrossRef]
- Sahani, D.V.; Bajwa, M.A.; Andrabi, Y.; Bajpai, S.; Cusack, J.C. Current status of imaging and emerging techniques to evaluate liver metastases from colorectal carcinoma. Ann. Surg. 2014, 259, 861. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Colon Cancer. Version 3. 2024. Available online: https://jnccn.org/view/journals/jnccn/22/2D/article-e240029.xml (accessed on 29 December 2024).
- Ruers, T.J.; Wiering, B.; van der Sijp, J.R.; Roumen, R.M.; de Jong, K.P.; Comans, E.F.; Pruim, J.; Dekker, H.M.; Krabbe, P.F.; Oyen, W.J. Improved selection of patients for hepatic surgery of colorectal liver metastases with 18F-FDG pet: A randomized study. J. Nucl. Med. 2009, 50, 1036. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, T.; Gönen, M.; Baser, R.E.; Schwartz, L.H.; Tuorto, S.; Brody, L.A.; Covey, A.; Brown, K.T.; Larson, S.M.; Fong, Y. Prospective evaluation of 18F-FDG positron emission tomography in the preoperative staging of patients with hepatic colorectal metastases. HepatoBiliary Surg. Nutr. 2022, 11, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.T.M.; Fulham, M.; Stephen, M.S.; Chu, K.-M.; Thompson, J.F.; Sheldon, D.M.; Storey, D.W. The role of whole-body positron emission tomography with [18F]Fluorodeoxyglucose in identifying operable colorectal cancer metastases to the liver. Arch. Surg. 1996, 131, 703. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, D.; Vitola, J.V.; Sandler, M.P.; Arildsen, R.C.; Powers, T.A.; Wright, J.K.; Chapman, W.C.; Pinson, C.W. Staging recurrent metastatic colorectal carcinoma with PET. J. Nucl. Med. 1997, 38, 1196–1201. [Google Scholar]
- Mekenkamp, L.J.M.; Koopman, M.; Teerenstra, S.; van Krieken, J.H.J.M.; Mol, L.; Nagtegaal, I.D.; A Punt, C.J. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br. J. Cancer 2010, 103, 159. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Su, Y.-H.; Ho, M.-C.; Liang, J.-T.; Chen, T.-P.; Lai, H.-S.; Lee, P.-H. Clinicopathological features and prognosis in resectable synchronous and metachronous colorectal liver metastasis. Ann. Surg. Oncol. 2007, 14, 786–794. [Google Scholar] [CrossRef]
- Driedger, M.R.; Yamashita, T.S.; Starlinger, P.; Mathis, K.L.; Smoot, R.L.; Cleary, S.P.; Nagorney, D.M. Synchronous resection of colorectal cancer primary and liver metastases: An outcomes analysis. HPB 2021, 23, 1277. [Google Scholar] [CrossRef]
- McCahill, L.E.; Yothers, G.; Sharif, S.; Petrelli, N.J.; Lai, L.L.; Bechar, N.; Giguere, J.K.; Dakhil, S.R.; Fehrenbacher, L.; Lopa, S.H.; et al. Primary mFOLFOX6 plus Bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: Definitive analysis of NSABP trial C-10. J. Clin. Oncol. 2012, 30, 3223. [Google Scholar] [CrossRef]
- Shimada, H.; Tanaka, K.; Masui, H.; Nagano, Y.; Matsuo, K.; Kijima, M.; Ichikawa, Y.; Ike, H.; Ooki, S.; Togo, S. Results of surgical treatment for multiple (> or =5 nodules) bi-lobar hepatic metastases from colorectal cancer. Langenbeck’s Arch. Surg. 2004, 389, 114–121. [Google Scholar] [CrossRef]
- Gruenberger, T.; Morris, D.L. Staged treatment of bilobar hepatic metastases from colorectal cancer. Eur. J. Surg. 2002, 168, 516–518. [Google Scholar] [CrossRef]
- Birrer, D.L.; Tschuor, C.; Reiner, C.S.; Fritsch, R.; Pfammatter, T.; Schüler, H.G.; Pavic, M.; De Oliveira, M.; Petrowsky, H.; Dutkowski, P.; et al. Multimodal treatment strategies for colorectal liver metastases. Swiss Med. Wkly. 2021, 5, 151. [Google Scholar] [CrossRef]
- Boudiaf, Z.; Bouzid, C.; Ait-Arab, M.R.; Cherchar, K.; Kheloufi, M.; Chibane, A.; Boutekedjiret, I.H.; Hattou, Z.; Gouaref, F.; Bentabak, K. La chirurgie laparoscopique de downstaging pour cancer colorectal avec métastases hépatiques synchrones: Quel intérêt dans les hépatectomies en deux temps? à propos d’une série de 6 cas [Laparoscopic downstaging surgery for colorectal cancer with synchronous liver metastases: What value in two-stage hepatectomies?]. Pan. Afr. Med. J. 2023, 27, 38. [Google Scholar]
- Ono, Y.; Inoue, Y.; Kobayashi, K.; Sato, S.; Kitano, Y.; Oba, A.; Sato, T.; Ito, H.; Takahashi, Y. Laparoscopic Portal Vein Ligation and Embolization During First-Stage Hepatectomy for Initially Unresectable Colorectal Liver Metastases. Ann. Surg. Oncol. 2024, 31, 3069–3070. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Wicherts, D.A.; De Haas, R.J.; Aloia, T.; Lévi, F.; Paule, B.; Castaing, D. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: Myth or reality? J. Clin. Oncol. 2008, 26, 1635. [Google Scholar] [CrossRef] [PubMed]
- Blazer, D.G., 3rd; Kishi, Y.; Maru, D.M.; Kopetz, S.; Chun, Y.S.; Overman, M.J.; Vauthey, J.N. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J. Clin. Oncol. 2008, 26, 5344. [Google Scholar] [CrossRef]
- Benoist, S.; Brouquet, A.; Penna, C.; Julié, C.; El Hajjam, M.; Chagnon, S.; Nordlinger, B. Complete response of colorectal liver metastases after chemotherapy: Does it mean cure? J. Clin. Oncol. 2006, 24, 3939. [Google Scholar] [CrossRef]
- Song, K.D.; Kim, Y.K.; Kim, H.C.; Huh, J.W.; Park, Y.S.; Park, J.O.; Kim, S.T. Disappearing or residual tiny (≤5 mm) colorectal liver metastases after chemotherapy on gadoxetic acid-enhanced liver MRI and diffusion-weighted imaging: Is local treatment required? Eur. Radiol. 2017, 27, 3088–3096. [Google Scholar]
- Kepenekian, V.; Muller, A.; Valette, P.J.; Rousset, P.; Chauvenet, M.; Phelip, G.; Walter, T.; Adham, M.; Glehen, O.; Passot, G. Evaluation of a strategy using pretherapeutic fiducial marker placement to avoid missing liver metastases. BJS Open 2019, 22, 344–353. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Paredes, A.Z.; Moris, D.; Gavriatopoulou, M.; Cloyd, J.M.; Pawlik, T.M. Disappearing liver metastases: A systematic review of the current evidence. Surg. Oncol. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Alberts, S.R.; Horvath, W.L.; Sternfeld, W.C.; Goldberg, R.M.; Mahoney, M.R.; Dakhil, S.R.; Donohue, J.H. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: A North Central Cancer Treatment Group phase II study. J. Clin. Oncol. 2005, 23, 9243. [Google Scholar] [CrossRef]
- Hayashi, H.; Miyamoto, Y.; Higashi, T.; Hiyoshi, Y.; Yamao, T.; Uemura, N.; Matsumura, K.; Imai, K.; Yamashita, Y.I.; Baba, H. CD44 expression enhances chemoresistance and implies occult micrometastases after conversion hepatectomy for initially unresectable colorectal liver metastases. Am. J. Transl. Res. 2020, 15, 5955–5966. [Google Scholar]
- Bridgewater, J.; Pugh, S.A.; Maishman, T.; Eminton, Z.; Mellor, J.; Whitehead, A.; Stanton, L.; Radford, M.; Corkhill, A.; O Griffiths, G.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): Long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, S.; Modest, D.P.; Rossius, L.; Lerch, M.M.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016, 17, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.A.; Meade, A.M.; Seymour, M.T.; Wilson, R.H.; Madi, A.; Fisher, D.; Kenny, S.L.; Kay, E.; Hodgkinson, E.; Pope, M.; et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet Oncol. 2011, 12, 642–653. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Lang, I.; Folprecht, G.; Nowacki, M.; Barone, C.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Celik, I.; Kohne, C. Cetuximab plus FOLFIRI: Final data from the CRYSTAL study on the association of KRAS and BRAF biomarker status with treatment outcome. J. Clin. Oncol. 2010, 28, 3570. [Google Scholar] [CrossRef]
- Tournigand, C.; Cervantes, A.; Figer, A.; Lledo, G.; Flesch, M.; Buyse, M.; Mineur, L.; Carola, E.; Etienne, P.-L.; Rivera, F.; et al. OPTIMOX1: A randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-go fashion in advanced colorectal cancer—A Gercor study. J. Clin. Oncol. 2006, 24, 394–400. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.-L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A randomized, multicenter Phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 Metastatic colorectal Cancer. J. Clin. Oncol. 2014, 20, 2240–2247. [Google Scholar]
- Mise, Y.; Hasegawa, K.; Saiura, A.; Oba, M.; Yamamoto, J.; Nomura, Y.; Kokudo, N. A Multicenter Phase 2 Trial to Evaluate the Efficacy of mFOLFOX6 + Cetuximab as Induction Chemotherapy to Achieve R0 Surgical Resection for Advanced Colorectal Liver Metastases (NEXTO Trial). Ann. Surg. Oncol. 2020, 27, 4188. [Google Scholar] [CrossRef]
- Masi, G.; Loupakis, F.; Pollina, L.; Vasile, E.; Cupini, S.; Ricci, S.; Brunetti, I.M.; Ferraldeschi, R.; Naso, G.; Filipponi, F.; et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann. Surg. 2009, 249, 420. [Google Scholar] [CrossRef]
- Ychou, M.; Viret, F.; Kramar, A.; Desseigne, F.; Mitry, E.; Guimbaud, R.; Delpero, J.R.; Rivoire, M.; Quénet, F.; Portier, G.; et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): A phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother. Pharmacol. 2008, 62, 195. [Google Scholar] [CrossRef]
- Chrabaszcz, S.; Rajeev, R.M.; Witmer, H.D.M.; Dhiman, A.M.; Klooster, B.; Gamblin, T.C.M.; Banerjee, A.; Johnston, F.M.M.; Turaga, K.K. A Systematic Review of Conversion to Resectability in Unresectable Metastatic Colorectal Cancer Chemotherapy Trials. Am. J. Clin. Oncol. 2022, 45, 366. [Google Scholar] [CrossRef] [PubMed]
- Karaoğlan, B.B.; Öz, D.K.; Araz, M.S.; Akyol, C.; Utkan, G. Advancements in the Management of Synchronous Colorectal Liver Metastases: A Comprehensive Review of Surgical, Systemic, and Local Treatment Modalities. Curr. Oncol. Rep. 2024, 26, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Wagman, L.D. Importance of response to neoadjuvant therapy in patients with liver-limited MCRC when the intent is resection and/or ablation. Clin. Color. Cancer 2013, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Damato, A.; Ghidini, M.; Dottorini, L.; Tomasello, G.; Iaculli, A.; Ghidini, A.; Luciani, A.; Petrelli, F. Chemotherapy Duration for Various Indications in Colorectal Cancer: A Review. Curr. Oncol. Rep. 2023, 25, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.J.G.; Bolhuis, K.; Loosveld, O.J.L.; de Groot, J.W.B.; Droogendijk, H.; Helgason, H.H.; Hendriks, M.P.; Klaase, J.M.; Kazemier, G.; Liem, M.S.L.; et al. First-line systemic treatment strategies in patients with initially unresectable colorectal cancer liver metastases (CAIRO5): An open-label, multicentre, randomised, controlled, phase 3 study from the Dutch Colorectal Cancer Group. Lancet Oncol. 2023, 24, 757–771. [Google Scholar] [CrossRef]
- D’angelica, M.; Kornprat, P.; Gonen, M.; Chung, K.-Y.; Jarnagin, W.R.; DeMatteo, R.P.; Fong, Y.; Kemeny, N.; Blumgart, L.H.; Saltz, L.B. Lack of evidence for increased operative morbidity after hepatectomy with perioperative use of bevacizumab: A matched case-control study. Ann. Surg. Oncol. 2007, 14, 759. [Google Scholar] [CrossRef]
- Reddy, S.K.; Morse, M.A.; Hurwitz, H.I.; Bendell, J.C.; Gan, T.J.; Hill, S.E.; Clary, B.M. Addition of bevacizumab to irinotecan- and oxaliplatin-based preoperative chemotherapy regimens does not increase morbidity after resection of colorectal liver metastases. J. Am. Coll. Surg. 2008, 206, 96. [Google Scholar] [CrossRef]
- Okines, A.; del Puerto, O.; Cunningham, D.; Chau, I.; Van Cutsem, E.; Saltz, L.; Cassidy, J. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-III NO16966 trial. Br. J. Cancer 2009, 101, 1033. [Google Scholar] [CrossRef]
- Kesmodel, S.B.; Ellis, L.M.; Lin, E.; Chang, G.J.; Abdalla, E.K.; Kopetz, S.; Vauthey, J.-N.; Rodriguez-Bigas, M.A.; Curley, S.A.; Feig, B.W. Preoperative bevacizumab does not significantly increase postoperative complication rates in patients undergoing hepatic surgery for colorectal cancer liver metastases. J. Clin. Oncol. 2008, 26, 5254. [Google Scholar] [CrossRef]
- Tamandl, D.; Gruenberger, B.; Klinger, M.; Herberger, B.; Kaczirek, K.; Fleischmann, E.; Gruenberger, T. Liver resection remains a safe procedure after neoadjuvant chemotherapy including bevacizumab: A case-controlled study. Ann. Surg. 2010, 252, 124. [Google Scholar] [CrossRef]
- Wicherts, D.A.; de Haas, R.J.; Sebagh, M.; Corrales, E.S.; Gorden, D.L.; Lévi, F.; Paule, B.; Azoulay, D.; Castaing, D.; Adam, R. Impact of bevacizumab on functional recovery and histology of the liver after resection of colorectal metastases. Br. J. Surg. 2011, 98, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pool, A.E.; Marsman, H.A.; Verheij, J.; Ten Kate, F.J.; Eggermont, A.M.; Ijzermans, J.N.; Verhoef, C. Effect of bevacizumab added preoperatively to oxaliplatin on liver injury and complications after resection of colorectal liver metastases. J. Surg. Oncol. 2012, 106, 892. [Google Scholar] [CrossRef] [PubMed]
- Wicherts, D.A.; de Haas, R.J.; Andreani, P.; Sotirov, D.; Salloum, C.; Castaing, D.; Adam, R.; Azoulay, D. Impact of portal vein embolization on long-term survival of patients with primarily unresectable colorectal liver metastases. Br. J. Surg. 2010, 97, 240. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, D.; Chun, Y.S.; Madoff, D.C.; Abdalla, E.K.; Vauthey, J.-N. Chemotherapy with bevacizumab does not affect liver regeneration after portal vein embolization in the treatment of colorectal liver metastases. Ann. Surg. Oncol. 2008, 15, 2765. [Google Scholar] [CrossRef]
- O’connell, R.M.; Hoti, E. Challenges and Opportunities for Precision Surgery for Colorectal Liver Metastases. Cancers 2024, 28, 2379. [Google Scholar] [CrossRef]
- Ychou, M.; Rivoire, M.; Thezenas, S.; Guimbaud, R.; Ghiringhelli, F.; Mercier-Blas, A.; Mineur, L.; Francois, E.; Khemissa, F.; Chauvenet, M.; et al. Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br. J. Cancer 2022, 126, 1264. [Google Scholar] [CrossRef]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004, 22, 229. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nagase, M.; Tamagawa, H.; Ueda, S.; Tamura, T.; Murata, K.; Nakajima, T.E.; Baba, E.; Tsuda, M.; Moriwaki, T.; et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann. Oncol. 2016, 27, 1539. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Van Cutsem, E.; Rougier, P.; Ciardiello, F.; Heeger, S.; Schlichting, M.; Celik, I.; Köhne, C.-H. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer 2012, 48, 1466–1475. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009, 360, 1408. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Makhson, A.; Hartmann, J.T.; Aparicio, J.; de Braud, F.; Koralewski, P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic col orectal cancer. J. Clin. Oncol. 2009, 27, 663. [Google Scholar] [CrossRef]
- Primrose, J.; Falk, S.; Finch-Jones, M.; Valle, J.; O’Reilly, D.; Siriwardena, A.; Hornbuckle, J.; Peterson, M.; Rees, M.; Iveson, T.; et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: The New EPOC randomised controlled trial. Lancet Oncol. 2014, 15, 601. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007. [Google Scholar] [CrossRef] [PubMed]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B.; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Mizusawa, J.; Inaba, Y.; Hamaguchi, T.; Shida, D.; Ohue, M.; Komori, K.; Shiomi, A.; Shiozawa, M.; Watanabe, J.; et al. Hepatectomy Followed by mFOLFOX6 Versus Hepatectomy Alone for Liver-Only Metastatic Colorectal Cancer (JCOG0603): A Phase II or III Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 3789. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).