Electrocardiographic Changes in Patients with Type 2 Diabetes Mellitus—A Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Objective
3. Materials and Methods
- Initially, a simple search was conducted using the terms “diabetes electrocardiogram” and “diabetes electrocardiography”.
- Using MeSH, “diabetes” was entered, and “electrocardiogram changes OR electrocardiographic changes” was selected. Filters such as publication period, study type, and target population were applied.
- Keywords such as “diabetes”, “electrocardiogram”, “changes”, “complications”, “cardiovascular”, and “disease” were combined using operators like OR, AND, and NOT.
- A final comprehensive search was conducted using all the selected keywords and filters.
3.1. Inclusion and Exclusion Criteria
- Were published before 2017;
- Were clinical case reports or trials;
- Focused on patients with type 1 diabetes;
- Were written in languages other than English;
- Provided insufficient data for analysis;
- Were not observational in nature;
- Involved patients who had electrocardiographic abnormalities diagnosed before their diabetes diagnosis;
- Did not adhere to high research standards or presented a high risk of bias.
3.2. Data Extraction
3.3. Statistical Analysis
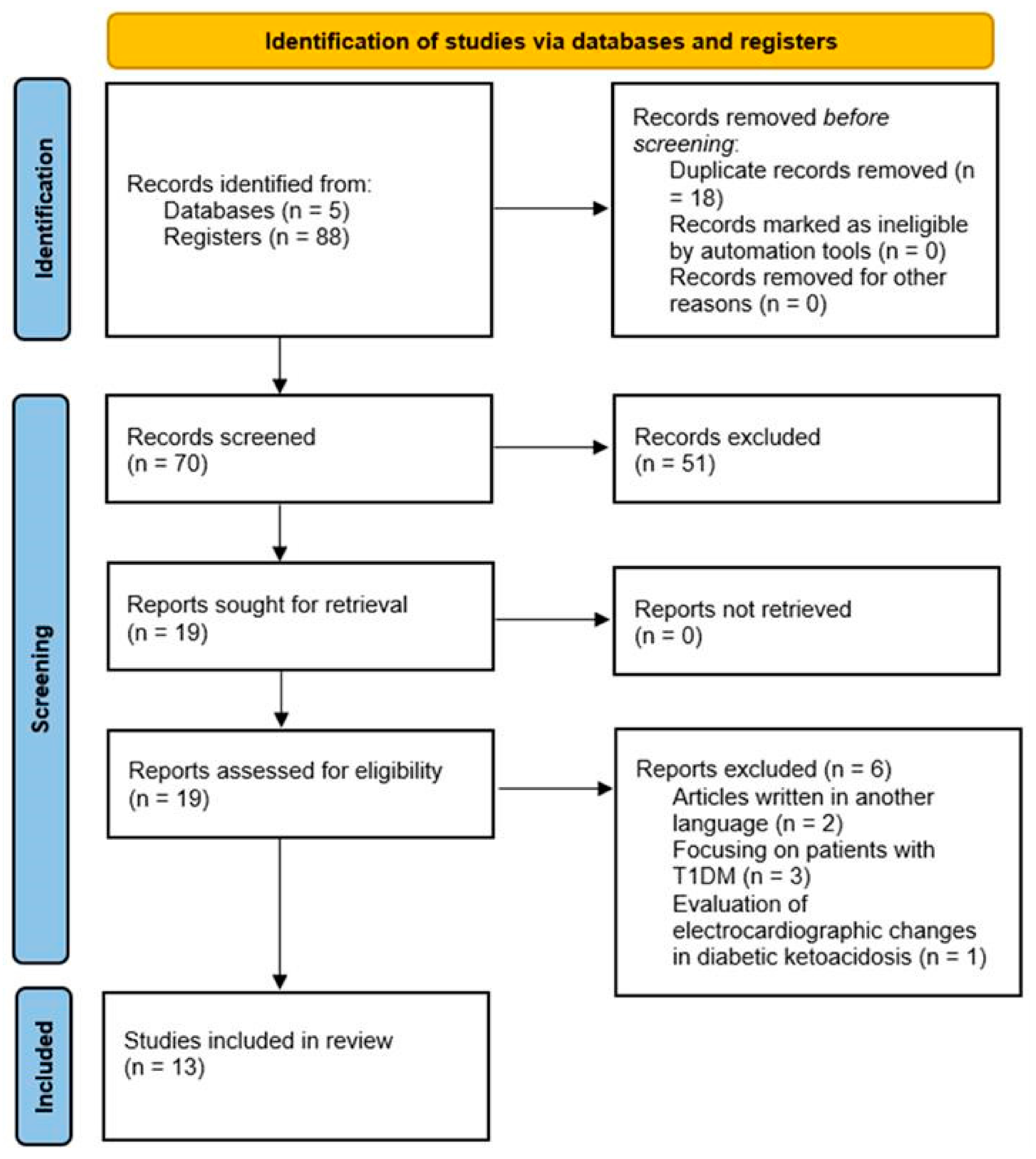
4. Results
4.1. Sensitivity Analysis: Exploration of Heterogeneity
4.2. Included Studies
4.3. Narrative Summary
4.4. Quantitative Summary
4.5. Major Electrocardiographic Changes
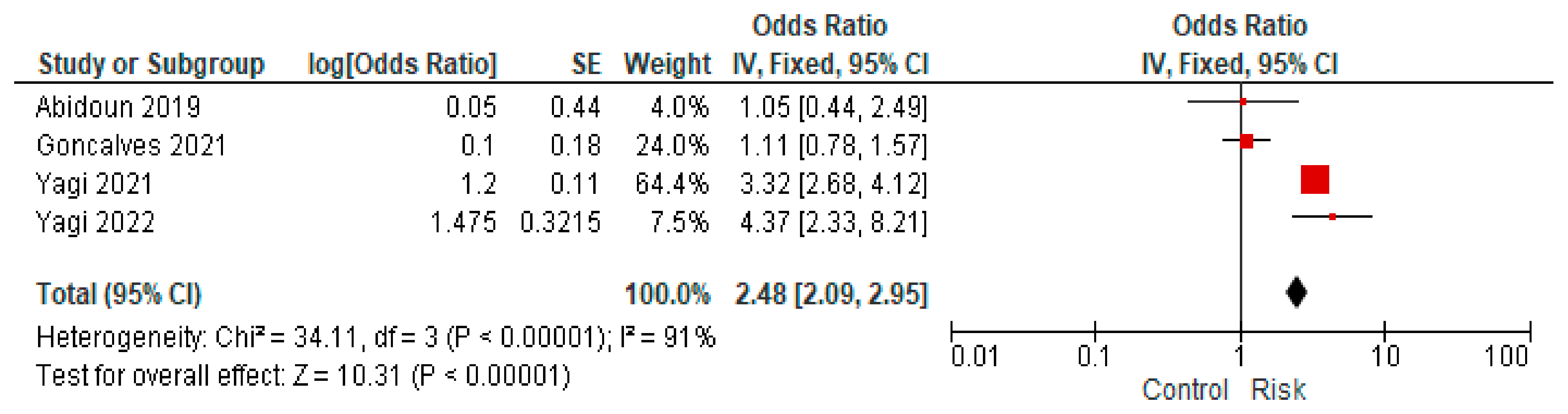
4.6. fQRS Complex Changes
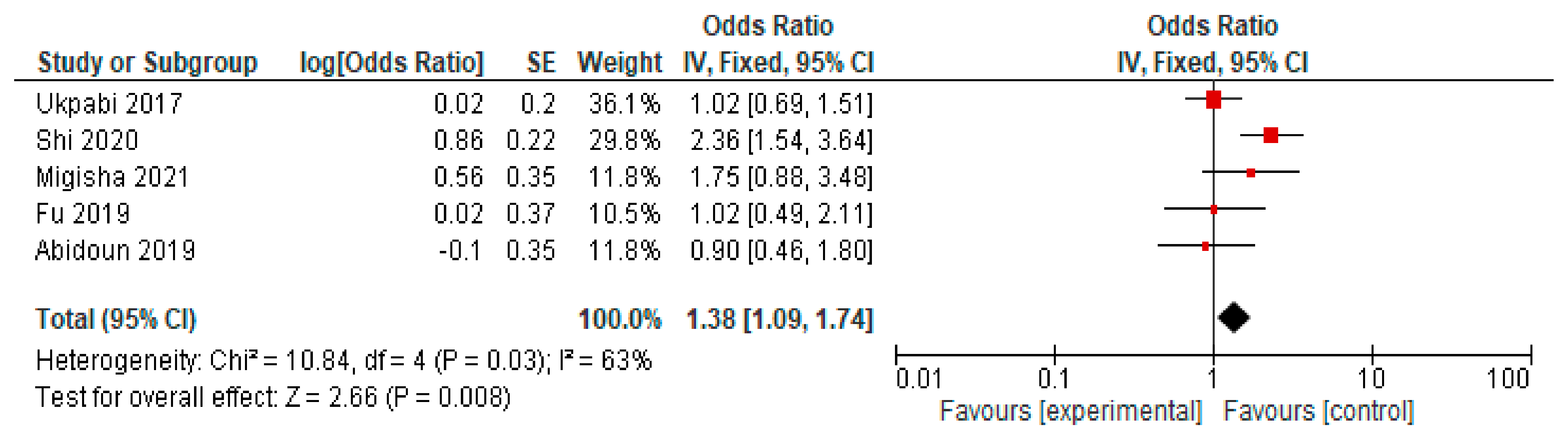
4.7. Prolonged QTc Interval Changes
5. Discussions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. 1), S20–S42.
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, Å.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care 2023, 46, e151–e199. [Google Scholar] [PubMed]
- Choi, J.H.; Lee, K.A.; Moon, J.H.; Chon, S.; Kim, D.J.; Kim, H.J.; Kim, N.H.; Seo, J.A.; Kim, M.K.; Lim, J.H.; et al. 2023 Clinical Practice Guidelines for Diabetes Mellitus of the Korean Diabetes Association. Diabetes Metab. J. 2023, 47, 575–594. [Google Scholar] [CrossRef]
- Bae, J.H.; Han, K.D.; Ko, S.H.; Yang, Y.S.; Choi, J.H.; Choi, K.M.; Kwon, H.S.; Won, K.C. Diabetes Fact Sheet in Korea 2021. Diabetes Metab. J. 2022, 46, 417–426. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Care in Diabetes—2023 Abridged for Primary Care Providers. Clin. Diabetes 2023, 41, 4–31. [Google Scholar]
- Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Gregg, E.W.; Cheng, Y.J.; Srinivasan, M.; Lin, J.; Geiss, L.S.; Albright, A.L.; Imperatore, G. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: An epidemiological analysis of linked national survey and vital statistics data. Lancet 2018, 391, 2430–2440. [Google Scholar] [CrossRef]
- Goldberg, R.B.; Orchard, T.J.; Crandall, J.P.; Boyko, E.J.; Budoff, M.; Dabelea, D.; Gadde, K.M.; Knowler, W.C.; Lee, C.G.; Nathan, D.M.; et al. Effects of Long-term Metformin and Lifestyle Interventions on Cardiovascular Events in the Diabetes Prevention Program and Its Outcome Study. Circulation 2022, 145, 1632–1641. [Google Scholar]
- Zhang, J.; Yang, J.; Liu, L.; Li, L.; Cui, J.; Wu, S.; Tang, K. Significant abnormal glycemic variability increased the risk for arrhythmias in elderly type 2 diabetic patients. BMC Endocr. Disord. 2021, 21, 83. [Google Scholar]
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3047. [Google Scholar]
- Cowie, C.C.; Casagrande, S.S.; Menke, A.; Cissell, M.A.; Eberhardt, M.S.; Meigs, J.B.; Gregg, E.W.; Knowler, W.C.; Barrett-Connor, E.; Becker, D.J.; et al. Diabetes in America; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MD, USA, 2018. [Google Scholar] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023, 44, 4043–4140. [Google Scholar] [PubMed]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Das, S.R.; Ekhlaspour, L.; Hilliard, M.E.; Johnson, E.L.; et al. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S179–S218. [Google Scholar]
- Nichols, G.A.; Reinier, K.; Chugh, S.S. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care 2009, 32, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Nijpels, G.; van der Heijden, A.A.W.A.; Elders, P.; Beulens, J.W.J.; de Vet, H.C.W. The interobserver agreement of ECG abnormalities using Minnesota codes in people with type 2 diabetes. PLoS ONE 2021, 16, e0255466. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating Guidance for Reporting Systematic Reviews: Development of the PRISMA 2020 Statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Harms, P.P.; van der Heijden, A.A.; Rutters, F.; Tan, H.L.; Beulens, J.W.; Nijpels, G.; Elders, P. Prevalence of ECG abnormalities in people with type 2 diabetes: The Hoorn Diabetes Care System cohort. J. Diabetes Its Complicat. 2021, 35, 107810. [Google Scholar] [CrossRef]
- Kersten, D.J.; D’Angelo, K.; Vargas, J.; Verma, G.; Malik, U.; Shavolian, S.; Zeltser, R.; Hai, O.; Makaryus, A.N. Determining the clinical significance of computer interpreted electrocardiography conclusions. Am. J. Cardiovasc. Dis. 2021, 11, 375–381. [Google Scholar]
- Abiodun, A.; Oladimeji, A.; Bamidele, T.; Adewole, A.; Mayowa, O. Prevalence of ECG abnormalities among adults with metabolic syndrome in a Nigerian Teaching Hospital. Afr. Health Sci. 2019, 19, 2829–2838. [Google Scholar] [CrossRef]
- Bedane, D.A.; Tadesse, S.; Bariso, M.; Reta, W.; Desu, G. Assessment of electrocardiogram abnormality and associated factors among apparently healthy adult type 2 diabetic patients on follow-up at Jimma Medical Center, Southwest Ethiopia: Cross-sectional study. BMC Cardiovasc. Disord. 2021, 21, 312. [Google Scholar] [CrossRef]
- Gonçalves, M.A.A.; Pedro, J.M.; Silva, C.; Magalhães, P.; Brito, M. Prevalence of major and minor electrocardiographic abnormalities and their relationship with cardiovascular risk factors in Angolans. Int. J. Cardiol. Heart Vasc. 2022, 39, 100965. [Google Scholar] [CrossRef] [PubMed]
- Sardesai, V.; Kokane, H.; Mukherjee, S.; Sangle, S. A study of electrocardiographic and 2D echocardiographic changes in type 2 diabetes mellitus patients without cardiovascular symptoms. J. Family Med. Prim. Care 2022, 11, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Sinamaw, D.; Getnet, M.; Abdulkadir, M.; Abebaw, K.; Ebrahim, M.; Diress, M.; Akalu, Y.; Ambelu, A.; Dagnew, B. Patterns and associated factors of electrocardiographic abnormality among type 2 diabetic patients in Amhara National Regional State Referral Hospitals, Ethiopia: A multicenter institution-based cross-sectional study. BMC Cardiovasc. Disord. 2022, 22, 230. [Google Scholar]
- Yagi, K.; Nagata, Y.; Yamagami, T.; Chujo, D.; Kamigishi, M.; Yokoyama-Nakagawa, M.; Shikata, M.; Enkaku, A.; Takikawa-Nishida, A.; Honoki, H.; et al. High prevalence of fragmented QRS on electrocardiography in Japanese patients with diabetes irrespective of metabolic syndrome. J. Diabetes Investig. 2021, 12, 1680–1688. [Google Scholar] [PubMed]
- Yagi, K.; Imamura, T.; Tada, H.; Liu, J.; Miyamoto, Y.; Ohbatake, A.; Ito, N.; Shikata, M.; Enkaku, A.; Takikawa, A.; et al. Fragmented QRS on electrocardiography as a predictor for diastolic cardiac dysfunction in type 2 diabetes. J. Diabetes Investig. 2022, 13, 1052–1061. [Google Scholar]
- Fu, L.; Deng, H.; Lin, W.D.; He, S.F.; Liu, F.Z.; Liu, Y.; Zhan, X.Z.; Fang, X.H.; Liao, H.T.; Wei, W.; et al. Association between elevated blood glucose level and non-valvular atrial fibrillation: A report from the Guangzhou heart study. BMC Cardiovasc. Disord. 2019, 19, 270. [Google Scholar]
- Shi, H.; Jiang, X. Correlation Between QTc Prolongation and Obstructive Sleep Apnea in Patients with Type 2 Diabetes Mellitus. Med. Sci. Monit. 2020, 26, e926954-1. [Google Scholar]
- Ukpabi, O.J.; Onwubere, B.J.C. QTc prolongation in Black diabetic subjects with cardiac autonomic neuropathy. Afr. Health Sci. 2017, 17, 1092–1100. [Google Scholar]
- Migisha, R.; Agaba, D.C.; Katamba, G.; Miranda, S.L.; Muyingo, A.; Siedner, M.J. High prevalence of prolonged QTc interval among individuals in ambulatory diabetic care in southwestern Uganda. Int. J. Diabetes Dev. Ctries. 2021, 41, 614–620. [Google Scholar]
- Harms, P.P.; Herings, R.A.R.; Remmelzwaal, S.; Rutters, F.; Beulens, J.W.J.; Nijpels, G.; Elders, P.P.J.M.; Blom, M.T. The added value of ECG abnormalities in predicting incident cardiovascular disease risk for people with type 2 diabetes: The Hoorn Diabetes Care System cohort. Eur. J. Prev. Cardiol. Eur. J. Prev. Cardiol. 2025, zwaf033. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, R.K.; Kulshrestha, M.; Chaudhary, R.R. Evaluation of ECG Abnormalities in Patients with Asymptomatic Type 2 Diabetes Mellitus. J. Clin. Diagn. Res. 2017, 11, OC39–OC41. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.R.; Baradaran, M.; Rostamzadeh, A.; Masudi, S.; Aghamiri, A.; Hajizadeh, R. Assessment of the fragmented QRS relative frequency and its predictive value in patients with pulmonary embolism. Caspian J. Intern. Med. 2024, 16, 83–89. [Google Scholar] [PubMed]
- Soflaei Saffar, S.; Nazar, E.; Sahranavard, T.; Fayedeh, F.; Moodi Ghalibaf, A.; Ebrahimi, M.; Alimi, H.; Shahri, B.; Izadi-Moud, A.; Ferns, G.A.; et al. Association of T-wave electrocardiogram changes and type 2 diabetes: A cross-sectional sub-analysis of the MASHAD cohort population using the Minnesota coding system. BMC Cardiovasc. Disord. 2024, 24, 48. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, K.; Pakravan, S.; Azadbakht, K. The most common electrocardiographic abnormalities in patients with diabetes mellitus. J. Parathyroid Dis. 2022, 10, e9142. [Google Scholar]
- Vaidya, A.; Bentley-Lewis, R.; Jeunemaitre, X.; Adler, G.K.; Williams, J.S. Dietary sodium alters the prevalence of electrocardiogram determined left ventricular hypertrophy in hypertension. Am. J. Hypertens. 2009, 22, 669–673. [Google Scholar] [CrossRef]

| Study | Author | Year of Publication | Study Type | Characteristics of Studied Subjects | Number of Patients | Type of Intervention | Followed Effect |
|---|---|---|---|---|---|---|---|
| 1 | Harms et al. [18] | 2021 | Cohort | Average age: 67.7 ± 11.0 Male sex: 55.6% Female sex: 44.4% | 8068 Participants without a history of cardiovascular diseases: 6494 | Electrocardiogram abnormalities were defined using the Minnesota classification. These were classified into different types of abnormalities (minor and major electrocardiogram abnormalities). | Electrocardiogram abnormalities were very commonly found in the population with type II diabetes, including among those without a history of cardiovascular diseases. Among patients with diabetes diagnosed for more than 10 years, the prevalence was of 48.8% minor abnormalities and 48.9% significant abnormalities. |
| 2 | Kersten et al. [19] | 2021 | Cohort | Average age: 57 ± 16 Male sex: 41% Female sex: 59% | 517 | The electrocardiograms were performed using the Nihon Kohden Cardiofax V Electrocardiograph ECG-1550A (Nihon Kohden, Shinjuku, Japan) and were interpreted using the ECAPS 12C software. | It was found that the appearance of electrocardiographic abnormalities in the context of advanced age, coronary artery disease, or diabetes mellitus is very likely to be accompanied by changes in echocardiography. |
| 3 | Abiodun et al. [20] | 2019 | Cross-sectional | Average age: 53.72 ± 15.2 Male sex: 51.7% Female sex: 48.3% | 491 | An electrocardiogram was performed. An assessment of the risk of metabolic syndrome in the cardiovascular system was conducted. Questionnaires were used to collect data about the patients. | There was a high prevalence of metabolic syndrome and the occurrence of electrocardiographic changes in the studied population. Electrocardiogram abnormalities appeared more frequently in the male population, with no significant difference between those with or without metabolic syndrome. |
| 4 | Bedane et al. [21] | 2021 | Cross-sectional | Average age: 53.34 ± 11.07 Male sex: 61% Female sex: 39% | 344 | A standard twelve-lead electrocardiogram was performed. | Most of the studied population had electrocardiographic abnormalities (3 out of 5). Factors such as a duration of diabetes of more than 10 years or the use of solid oils were associated with the appearance of abnormalities on electrocardiography. |
| 5 | Goncalves et al. [22] | 2021 | Cross-sectional | Average age: 35.0 ± 14.5 Male sex: 37% Female sex: 63% | 2379 | A standard twelve-lead electrocardiogram was performed on all patients and then coded using the Minnesota classification. | Minor electrocardiographic abnormalities were more frequently observed in the male population, while significant abnormalities were more common in the female population. The prevalence was as follows: 22.3% minor abnormalities and 4.58% significant abnormalities. |
| 6 | Sardesai et al. [23] | 2022 | Cross-sectional | Average age: 56.3 ± 8.60 Male sex: 58.5% Female sex: 41.5% | 130 | The results of the electrocardiogram and 2D echocardiography were correlated with blood glucose levels. | Electrocardiogram abnormalities were strongly correlated with postprandial glucose levels, while they were not correlated with fasting glucose levels. |
| 7 | Sinamaw et al. [24] | 2021 | Cross-sectional | Average age: 56.7 (±12.7, range = 28–80) Male sex: 51.55% Female sex: 48.45 | 258 | A digital electrocardiograph was used to measure electrocardiographic parameters, and other data were collected using a questionnaire. | Half of the patients had at least one change observed on the electrocardiogram. Factors such as the long duration of disease (type II diabetes), high postprandial glucose, or hypertension were associated with electrocardiographic abnormalities. |
| 8 | Yagi et al. [25] | 2021 | Cross-sectional | Average age: 51 ± 8 Male sex: 76% Female sex: 24% | 702 | An electrocardiogram was performed, and fQRS-type changes were sought. | fQRS-type changes were more frequently observed in patients with diabetes than in those with metabolic syndrome or the control group. |
| 9 | Yagi et al. [26] | 2022 | Cross-sectional | Average age: 67.3 ± 12.6 Male sex: 60.3% Female sex: 39.7% | 320 | An electrocardiogram was performed, and fQRS-type changes were sought to associate them with diastolic cardiac dysfunction in the context of diabetes mellitus. | The appearance of fQRS could be a promising predictor for the onset of diastolic cardiac dysfunction, but the results should be confirmed through a more extensive cohort study. |
| 10 | Fu et al. [27] | 2019 | Cross-sectional | Average age: 58.2 Male sex: 35.2% Female sex: 64.8% | 11488 | Each study participant completed a questionnaire and underwent a physical examination, blood collection for laboratory tests, electrocardiography, and other tests. | Increased blood glucose levels are highly prevalent in those over 35 years old and are associated with an increased prevalence of non-valvular atrial fibrillation. |
| 11 | Shi et al. [28] | 2020 | Cross-sectional | Average age: 52 Male sex: 70.11% Female sex: 29.88% | 358 | A standard twelve-lead electrocardiogram was performed to identify QTc. Tests were also performed to diagnose obstructive sleep apnea and then correlated with QTc changes. | The severity of obstructive sleep apnea was strongly correlated with QTc prolongation in 358 patients with type II diabetes. Factors such as advanced age, body mass index, and female sex are independent factors for the occurrence of obstructive sleep apnea or cardiovascular diseases. |
| 12 | Ukpabi et al. [29] | 2017 | Cross-sectional | Average age: 46.09 ± 9.51 Male sex: 49.43% Female sex: 50.56% | 176 | A control group of non-diabetic patients was included. Tests for cardiac autonomic function were performed to diagnose cardiac autonomic neuropathy. | The prevalence of prolonged QTc in patients with type II diabetes and cardiac autonomic neuropathy was 12%. |
| 13 | Migisha et al. [30] | 2021 | Cross-sectional | Average age: 50.1 years (SD ± 9.8) Male sex: 30.4% Female sex: 69.6% | 299 | A standard twelve-lead electrocardiogram was performed. A linear progression analysis was conducted to identify QTc correlations. | The prevalence of QTc abnormalities was very high. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexescu, T.-G.; Nechita, A.; Alexander, P.; Perné, M.-G.; Milaciu, M.-V.; Ciulei, G.; Para, I.; Negrean, V.; Chiș, A.-F.; Todea, D.-A.; et al. Electrocardiographic Changes in Patients with Type 2 Diabetes Mellitus—A Meta-Analysis. J. Mind Med. Sci. 2025, 12, 14. https://doi.org/10.3390/jmms12010014
Alexescu T-G, Nechita A, Alexander P, Perné M-G, Milaciu M-V, Ciulei G, Para I, Negrean V, Chiș A-F, Todea D-A, et al. Electrocardiographic Changes in Patients with Type 2 Diabetes Mellitus—A Meta-Analysis. Journal of Mind and Medical Sciences. 2025; 12(1):14. https://doi.org/10.3390/jmms12010014
Chicago/Turabian StyleAlexescu, Teodora-Gabriela, Antonia Nechita, Paula Alexander, Mirela-Georgiana Perné, Mircea-Vasile Milaciu, George Ciulei, Ioana Para, Vasile Negrean, Ana-Florica Chiș, Doina-Adina Todea, and et al. 2025. "Electrocardiographic Changes in Patients with Type 2 Diabetes Mellitus—A Meta-Analysis" Journal of Mind and Medical Sciences 12, no. 1: 14. https://doi.org/10.3390/jmms12010014
APA StyleAlexescu, T.-G., Nechita, A., Alexander, P., Perné, M.-G., Milaciu, M.-V., Ciulei, G., Para, I., Negrean, V., Chiș, A.-F., Todea, D.-A., Vălean, D., Țărmure, S.-F., & Orășan, O.-H. (2025). Electrocardiographic Changes in Patients with Type 2 Diabetes Mellitus—A Meta-Analysis. Journal of Mind and Medical Sciences, 12(1), 14. https://doi.org/10.3390/jmms12010014








