Angiovolume and Peak Enhancement on Preoperative CAD-Derived MRI as Prognostic Factors in Primary Operable Triple-Negative Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
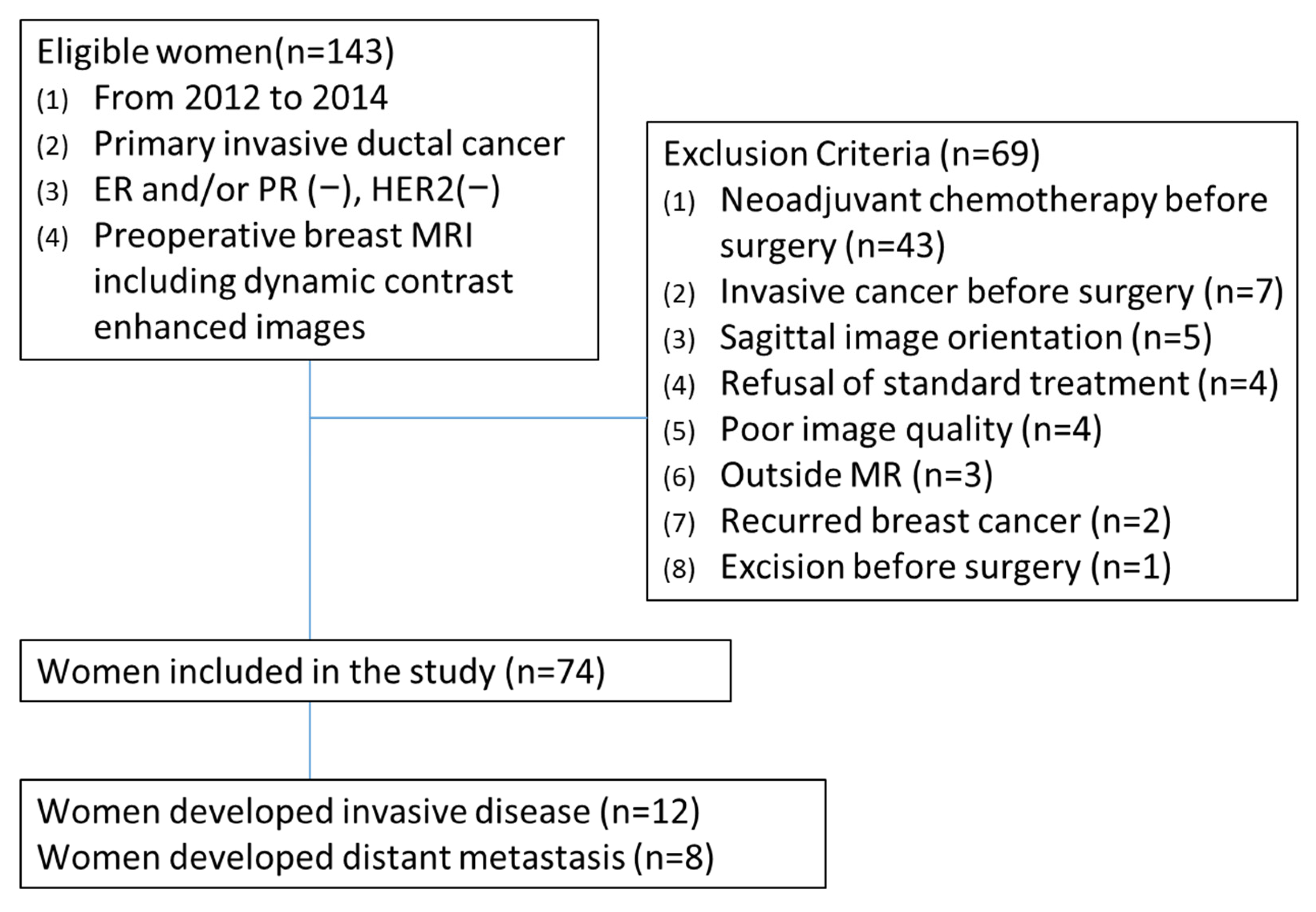
2.1. Study Population
2.2. MRI Acquisition
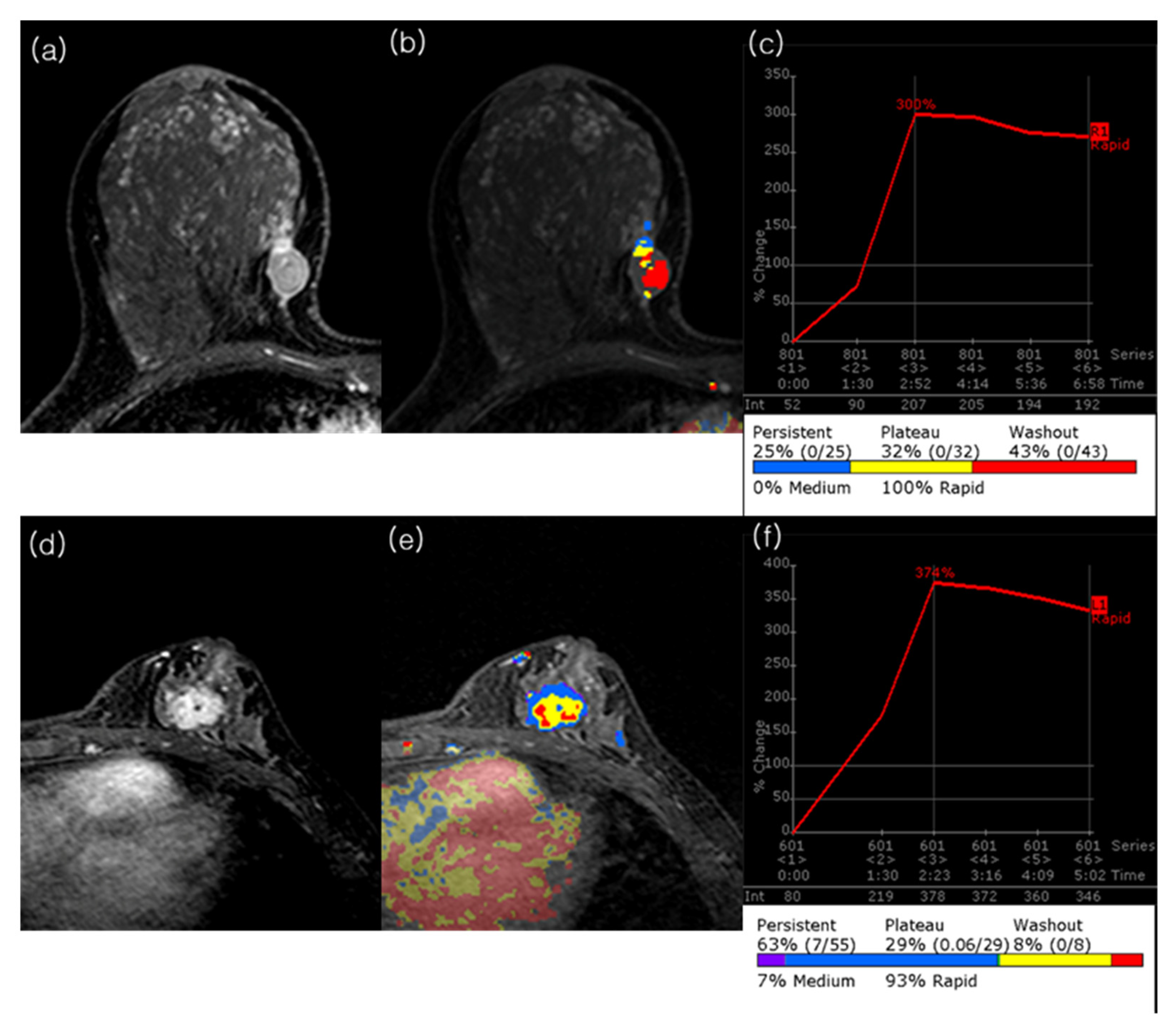
2.3. MRI Analysis
2.4. Clinicopathologic Data and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics and Outcomes
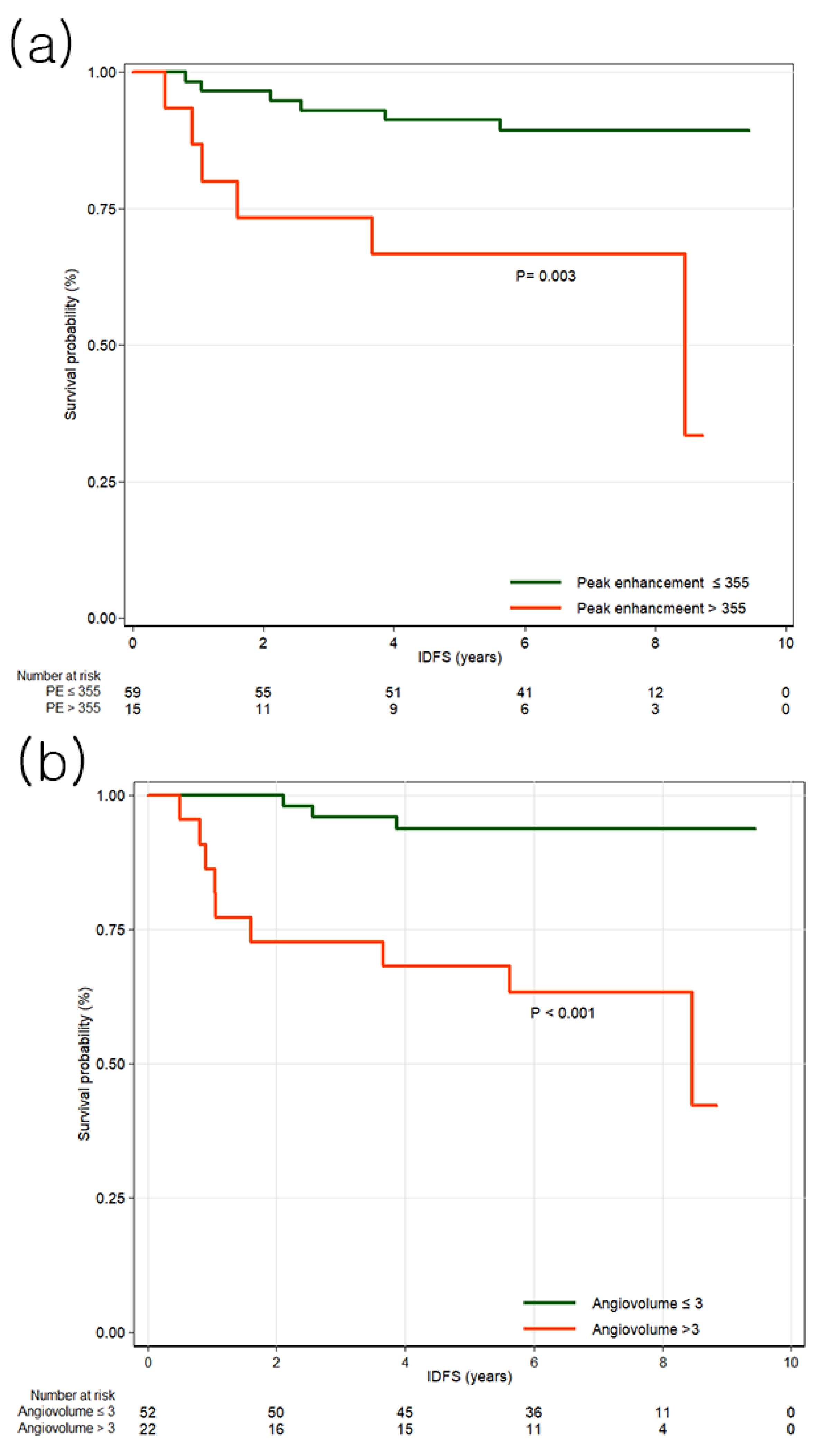
3.2. Invasive Disease-Free Survival
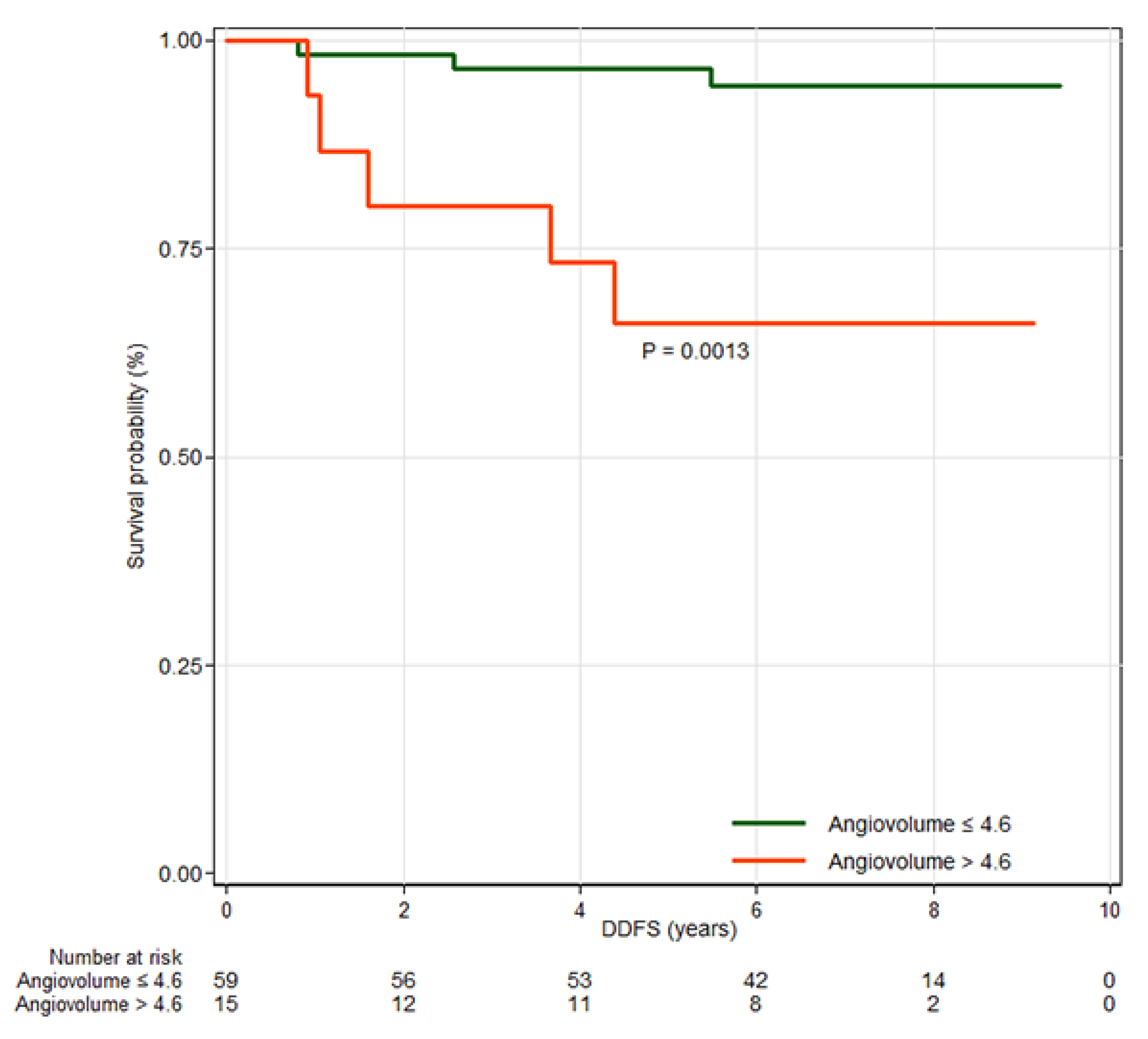
3.3. Distant Metastasis Free Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNBC | triple-negative breast cancer |
| ER | estrogen receptors |
| PR | progesterone receptors |
| HER2 | human epidermal growth factor receptor type 2 |
| DCE | Dynamic contrast-enhanced |
| CAD | computer-assisted diagnosis |
| IDFS | invasive disease-free survival |
| DDFS | distant metastasis-free survival |
| IHC | immunohistochemistry |
| T2WI | T2-weighted imaging |
| T1WI | T1-weighted imaging |
| SPAIR | spectral attenuated inversion recovery |
| TR | repetition time |
| TE | Echo time |
| FOV | Field of view |
| ETL | Echo train length |
| SI | Signal intensity |
| HR | hazard ratio |
| CI | confidence interval |
| BCS | Breast-Conserving Surgery |
| DSS | Disease-Specific Survival |
| MVD | Microvessel Density |
| TIL | Tumor-Infiltrating Lymphocytes |
Appendix A
| All Patients (n = 74) | Patient Without Invasive Cancer (n = 62) | Patient with Invasive Cancer (n = 12) | Hazard Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|---|---|
| Age (years) † | 51 ± 11.7 | 51.4 ± 12.1 | 48.9 ± 9.5 | 0.99 | 0.94–1.04 | 0.66 |
| Type of surgery | ||||||
| BCS | 62 (83.8) | 53 (85.5) | 9 (75) | 0.49 | 0.13–1.81 | 0.286 |
| Mastectomy | 12 (16.2) | 9 (14.5) | 3 (25) | 1 | ||
| Type of Axillary Dissection | ||||||
| Sentinel dissection | 66 (89.2) | 57 (91.9) | 9 (75) | 1 | ||
| Axillary dissection | 8 (10.8) | 5 (8.1) | 3 (25) | 3.55 | 0.94–13.44 | 0.062 |
| Adjuvant chemotherapy | ||||||
| No | 3 (4.1) | 3 (4.8) | 0 (0) | 1 | ||
| Yes | 71 (96) | 59 (95.2) | 12 (100) | 0.61 | 0.08–77.96 | 0.748 * |
| Adjuvant radiation therapy | ||||||
| No | 11 (14.9) | 9 (14.5) | 2 (16.7) | 1 | ||
| Yes | 63 (85.1) | 53 (85.5) | 10 (83.3) | 0.77 | 0.17–3.52 | 0.737 |
| Tumor size (cm) † | 1.8 ± 0.8 | 1.7 ± 0.7 | 2.2 ± 1.3 | 1.67 | 0.94–2.95 | 0.078 |
| Axillary node metastasis | ||||||
| Negative | 62 (83.8) | 54 (87.1) | 8 (66.7) | 1 | ||
| Positive | 12 (16.2) | 8 (12.9) | 4 (33.3) | 3.3 | 0.96–11.31 | 0.057 |
| Histologic grade | ||||||
| 1 or 2 | 15 (20.3) | 13 (21) | 2 (16.7) | 1 | ||
| 3 | 59 (79.7) | 49 (79) | 10 (83.3) | 1.31 | 0.29–5.99 | 0.730 |
| Lymphovascular invasion | ||||||
| Negative | 53 (71.6) | 47 (75.8) | 6 (50) | 1 | ||
| Positive | 21 (28.4) | 15 (24.2) | 6 (50) | 2.75 | 0.88–8.54 | 0.081 |
| Ki-67 index | ||||||
| Low (<20%) | 11 (14.9) | 9 (14.5) | 2 (16.7) | 1 | ||
| High (>20%) | 63 (85.1) | 53 (85.5) | 10 (83.3) | 1.03 | 0.22–4.75 | 0.968 |
| P53 status | ||||||
| Negative | 28 (27.8) | 23 (37.1) | 5 (41.7) | 1 | ||
| Positive | 46 (62.2) | 39 (62.9) | 7 (58.3) | 0.82 | 0.26–2.59 | 0.730 |
| EGFR status | ||||||
| Negative | 53 (71.6) | 44 (71) | 9 (75) | 1 | ||
| Positive | 21 (28.4) | 18 (29) | 3 (25) | 0.8 | 0.22–2.95 | 0.736 |
| CK5/6 status | ||||||
| Negative | 21 (28.4) | 18 (29) | 3 (25) | 1 | ||
| Positive | 53 (71.6) | 44 (71) | 9 (75) | 1.16 | 0.32–4.3 | 0.819 |
| Basal-like tumor | ||||||
| Negative | 18 (24.3) | 15 (24.2) | 3 (25) | 1 | ||
| Positive | 56 (75.7) | 47 (75.8) | 9 (75) | 0.94 | 0.25–3.46 | 0.921 |
| Variable | Achieva (n = 63) | Ingenia (n = 11) | p Value |
|---|---|---|---|
| CAD parameters | |||
| Peak enhancement (%) | 290.1 ± 170.3 | 252.7 ± 162.7 | 0.502 |
| Angiovolume (mL) | 3.7 ± 5.2 | 2.8 ± 3.6 | 0.622 |
| Maximum diameter (cm) | 2.3 ± 1.5 | 2.2 ± 1.1 | 0.766 |
| Calculated Volume (cm3) | 7.2 ± 18.5 | 4.2 ± 5.6 | 0.602 |
| Early enhancement pattern † | |||
| %V of medium | 23.8 ± 29.6 | 20.5 ± 8.2 | 0.735 |
| %V of fast | 76.3 ± 29.6 | 79.5 ± 27.2 | 0.732 |
| Delay enhancement pattern † | |||
| %V of persistent | 53.4 ± 27.6 | 49.6 ± 36.9 | 0.691 |
| %V of plateau | 32.7 ± 17.3 | 34.8 ± 23.5 | 0.719 |
| %V of washout | 14.0 ± 18.1 | 15.5 ± 19.7 | 0.807 |
| Kinetic heterogeneity | 0.677 ± 0.276 | 0.570 ± 0.249 | 0.230 |
| Variable | All Patients (n = 74) | Patient Without Invasive Cancer (n = 62) | Patient with Invasive Cancer (n = 12) | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|---|
| T2 weighted image | ||||||
| Peritumoral edema | ||||||
| Negative | 29 (39.2) | 24 (28.7) | 5 (41.7) | 1 | ||
| Positive | 45 (60.8) | 38 (61.3) | 7 (58.3) | 0.89 | 0.28–2.81 | 0.845 |
| Intratumoral T2 high SI | ||||||
| Negative | 35 (47.3) | 32 (51.6) | 3 (25) | 1 | ||
| Positive | 39 (52.7) | 30 (48.4) | 9 (75) | 2.87 | 0.77–10.65 | 0.115 |
| CAD parameters | ||||||
| Peak enhancement, per increase of 100% † | 284.5 ± 168.6 | 266.6 ± 148.8 | 377.3 ± 233.8 | 1.3 | 1.02–1.66 | 0.033 |
| Angiovolume, per increase of 5 mL † | 3.5 ± 5.0 | 2.6 ± 3.5 | 8.2 ± 8.1 | 1.72 | 1.23–2.41 | 0.002 |
| Maximum diameter (cm) † | 2.3 ± 1.5 | 2.1 ± 1.1 | 3.5 ± 2.4 | 1.31 | 1.06–1.62 | 0.013 |
| Calculated tumor Volume, per increase of 5 cm3 † | 6.8 ± 17.2 | 3.7 ± 5 | 22.9 ± 38.5 | 1.08 | 1.01–1.16 | 0.017 |
| Early enhancement pattern † | ||||||
| %V of medium | 23.3 ± 29.1 | 23.4 ± 29.3 | 22.9 ± 29.2 | 1 | 0.98–1.02 | 0.86 |
| %V of fast | 76.7 ± 29.1 | 76.7 ± 29.3 | 77.2 ± 29.2 | 1 | 0.98–1.02 | 0.86 |
| Delay enhancement patter † | ||||||
| %V of persistent | 52.9 ± 28.9 | 53.8 ± 28.4 | 47.8 ± 32.2 | 0.99 | 0.96–1.01 | 0.356 |
| %V of plateau | 33 ± 18.1 | 33.1 ± 18.7 | 32.2 ± 15.3 | 1 | 0.97–1.03 | 0.965 |
| %V of washout | 14.2 ± 18.2 | 13.1 ± 17 | 20.1 ± 23.6 | 1 | 0.98–1.02 | 0.812 |
| Kinetic heterogeneity † | 0.661 ± 0.273 | 0.656 ± 0.279 | 0.687 ± 0.247 | 1.33 | 0.15–11.61 | 0.796 |
| All Patients (n = 74) | Patient Without Distant Metastasis (n = 66) | Patient with Distant Metastasis (n = 8) | Hazard Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|---|---|
| Age (years) † | 51 ± 11.7 | 50.9 ± 11.9 | 51.8 ±10.2 | 1.01 | 0.95–1.07 | 0.823 |
| Type of surgery | ||||||
| Breast conserving surgery | 62 (83.8) | 56 (84.9) | 6 (75) | 0.48 | 0.1–2.39 | 0.371 |
| Mastectomy | 12 (16.2) | 10 (15.2) | 2 (25) | 1 | ||
| Type of Axillary Dissection | ||||||
| Sentinel dissection | 66 (89.2) | 61 (92.4) | 5 (62.5) | 1 | ||
| Axillary dissection | 8 (10.8) | 5 (7.6) | 3 (37.5) | 6.19 | 1.46–26.19 | 0.013 |
| Adjuvant chemotherapy | ||||||
| No | 3 (4.1) | 3 (4.6) | 0 (0) | 1 | ||
| Yes | 71 (96) | 63 (95.5) | 8 (100) | 0.42 | 0.05–53.97 | 0.595 * |
| Adjuvant radiation therapy | ||||||
| No | 11 (14.9) | 10 (15.2) | 1 (12.5) | 1 | ||
| Yes | 63 (85.1) | 56 (84.9) | 7 (87.5) | 1.06 | 0.13–8.62 | 0.956 |
| Tumor size (cm) † | 1.8 ± 0.8 | 1.7 ± 0.7 | 2.3 ± 1.5 | 1.97 | 1.03–3.76 | 0.040 |
| Axillary node metastasis | ||||||
| Negative | 62 (83.8) | 58 (87.9) | 4 (50) | 1 | ||
| Positive | 12 (16.2) | 8 (12.1) | 4 (50) | 5.83 | 1.45–23.4 | 0.013 |
| Histologic grade | ||||||
| 1 or 2 | 15 (20.3) | 14 (21.2) | 1 (12.5) | 1 | ||
| 3 | 59 (79.7) | 52 (78.8) | 7 (87.5) | 1.96 | 0.24–15.92 | 0.530 |
| Lymphovascular invasion | ||||||
| Negative | 53 (71.6) | 51 (77.3) | 2 (25) | 1 | ||
| Positive | 21 (28.4) | 15 (22.7) | 6 (75) | 7.87 | 1.59–39.04 | 0.012 |
| Ki-67 index | ||||||
| Low (<20%) | 11 (14.9) | 10 (15.2) | 1 (12.5) | 1 | ||
| High (>20%) | 63 (85.1) | 56 (84.9) | 7 (87.5) | 1.36 | 0.17–11.07 | 0.773 |
| P53 status | ||||||
| Negative | 28 (27.8) | 25 (37.9) | 3 (37.5) | 1 | ||
| Positive | 46 (62.2) | 41 (62.1) | 5 (62.5) | 1.02 | 0.24–4.26 | 0.982 |
| EGFR status | ||||||
| Negative | 53 (71.6) | 47 (71.2) | 6 (75) | 1 | ||
| Positive | 21 (28.4) | 19 (28.8) | 2 (25) | 0.83 | 0.17–4.1 | 0.816 |
| CK5/6 status | ||||||
| Negative | 21 (28.4) | 19 (28.8) | 2 (25) | 1 | ||
| Positive | 53 (71.6) | 47 (71.2) | 6 (75) | 1.28 | 0.26–6.32 | 0.766 |
| Basal-like tumor type | ||||||
| Negative | 18 (24.3) | 16 (24.2) | 2 (25) | 1 | ||
| Positive | 56 (75.7) | 50 (75.8) | 6 (75) | 1.02 | 0.21–5.04 | 0.983 |
| Variable | All Patients (n = 74) | Patient Without Distant Metastasis (n = 66) | Patient with Distant Metastasis (n = 8) | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|---|
| T2 weighted image | ||||||
| Peritumoral edema | ||||||
| Negative | 29 (39.2) | 26 ± 39.4 | 3 ± 37.5 | 1 | ||
| Positive | 45 (60.8) | 40 ± 60.6 | 5 ± 62.5 | 1.04 | 0.25–4.33 | 0.962 |
| Intratumoral T2 high SI | ||||||
| Negative | 35 (47.3) | 34 (51.5) | 1 (12.5) | 1 | ||
| Positive | 39 (52.7) | 32 (48.5) | 7 (87.5) | 6.72 | 0.83–54.63 | 0.075 |
| CAD parameters | ||||||
| Peak enhancement, per increase of 100% † | 284.5 ± 168.6 | 267.0 ± 146.6 | 429 ± 264.9 | 1.42 | 1.08–1.85 | 0.012 |
| Angiovolume, per increase of 5 mL † | 3.5 ± 5.0 | 3.0 ± 4.3 | 8.1 ± 7.8 | 1.76 | 1.16–2.68 | 0.008 |
| Maximum diameter (cm) † | 2.3 ± 1.5 | 2.2 ± 1.4 | 3.2 ± 1.6 | 1.28 | 0.96–1.7 | 0.088 |
| Calculated Volume, per increase of 5 cm3 † | 6.8 ± 17.2 | 5.7 ± 16.8 | 15.2 ± 19.4 | 1.07 | 0.97–1.18 | 0.182 |
| Early enhancement pattern † | ||||||
| %V of medium | 23.3 ± 29.1 | 23.8 ± 29.3 | 19.3 ± 29.1 | 0.99 | 0.97–1.02 | 0.610 |
| %V of fast | 76.7 ± 29.1 | 76.3 ± 29.3 | 80.8 ± 29.1 | 1.01 | 0.98–1.03 | 0.610 |
| Delay enhancement pattern † | ||||||
| %V of persistent | 52.9 ± 28.9 | 53.6 ± 28.5 | 47 ± 33.6 | 0.98 | 0.94–1.01 | 0.189 |
| %V of plateau | 33 ± 18.1 | 32.9 ± 18.4 | 33.5 ± 17 | 1.01 | 0.97–1.05 | 0.587 |
| %V of washout | 14.2 ± 18.2 | 13.6 ± 17.8 | 19.8 ± 22.3 | 0.99 | 0.97–1.02 | 0.659 |
| Kinetic heterogeneity † | 0.661 ± 0.273 | 0.658 ± 0.272 | 0.685 ± 0.299 | 1.05 | 0.80–1.36 | 0.743 |
References
- Coates, A.S.; Winer, E.P.; Goldhirsch, A.; Gelber, R.D.; Gnant, M.; Piccart-Gebhart, M.; Thurlimann, B.; Senn, H.J.; Panel, M. Tailoring therapies—Improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann. Oncol. 2015, 26, 1533–1546. [Google Scholar] [CrossRef]
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Bailey, J.; Burstein, H.J.; et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; Andre, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C.; et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanovic, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef]
- Cheang, M.C.; Voduc, D.; Bajdik, C.; Leung, S.; McKinney, S.; Chia, S.K.; Perou, C.M.; Nielsen, T.O. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin. Cancer Res. 2008, 14, 1368–1376. [Google Scholar] [CrossRef]
- Schulmeyer, C.E.; Fasching, P.A.; Haberle, L.; Meyer, J.; Schneider, M.; Wachter, D.; Ruebner, M.; Poschke, P.; Beckmann, M.W.; Hartmann, A.; et al. Expression of the Immunohistochemical Markers CK5, CD117, and EGFR in Molecular Subtypes of Breast Cancer Correlated with Prognosis. Diagnostics 2023, 13, 372. [Google Scholar] [CrossRef]
- Tischkowitz, M.; Brunet, J.S.; Begin, L.R.; Huntsman, D.G.; Cheang, M.C.; Akslen, L.A.; Nielsen, T.O.; Foulkes, W.D. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer 2007, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hong, Y.-J.; Zhang, F.; Li, Y.-K. Computer-aided evaluation of the correlation between MRI morphology and immunohistochemical biomarkers or molecular subtypes in breast cancer. Sci. Rep. 2017, 7, 13818. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Ko, E.S.; Lim, Y.; Han, B.K.; Ko, E.Y.; Choi, J.S.; Lee, J.E. Preoperative dynamic breast magnetic resonance imaging kinetic features using computer-aided diagnosis: Association with survival outcome and tumor aggressiveness in patients with invasive breast cancer. PLoS ONE 2018, 13, e0195756. [Google Scholar] [CrossRef] [PubMed]
- Song, S.E.; Cho, K.R.; Seo, B.K.; Woo, O.H.; Jung, S.P.; Sung, D.J. Kinetic Features of Invasive Breast Cancers on Computer-Aided Diagnosis Using 3T MRI Data: Correlation with Clinical and Pathologic Prognostic Factors. Korean J. Radiol. 2019, 20, 411–421. [Google Scholar] [CrossRef]
- Dietzel, M.; Zoubi, R.; Vag, T.; Gajda, M.; Runnebaum, I.B.; Kaiser, W.A.; Baltzer, P.A. Association between survival in patients with primary invasive breast cancer and computer aided MRI. J. Magn. Reson. Imaging 2013, 37, 146–155. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.J.; Hwangbo, L.; Suh, H.B.; Kim, S.; Choo, K.S.; Nam, K.J.; Kang, T. Kinetic heterogeneity of breast cancer determined using computer-aided diagnosis of preoperative MRI scans: Relationship to distant metastasis-free survival. Radiology 2020, 295, 517–526. [Google Scholar] [CrossRef]
- Kim, J.Y.J.; Kim, J.Y.J.; Kang, H.J.; Shin, J.K.; Kang, T.; Lee, S.W.; Bae, Y.T. Computer-aided Diagnosis?generated Kinetic Features of Breast Cancer at Preoperative MR Imaging: Association with Disease-free Survival of Patients with Primary Operable Invasive Breast Cancer. Radiology 2017, 284, 45–54. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.H.; Kang, B.J. Prognostic Factors of Disease Recurrence in Breast Cancer Using Quantitative and Qualitative Magnetic Resonance Imaging (MRI) Parameters. Sci. Rep. 2020, 10, 7598. [Google Scholar] [CrossRef]
- Hayashi, Y.; Satake, H.; Ishigaki, S.; Ito, R.; Kawamura, M.; Kawai, H.; Iwano, S.; Naganawa, S. Kinetic volume analysis on dynamic contrast-enhanced MRI of triple-negative breast cancer: Associations with survival outcomes. Br. J. Radiol. 2020, 93, 20190712. [Google Scholar] [CrossRef] [PubMed]
- Park, V.Y.; Kim, E.K.; Kim, M.J.; Yoon, J.H.; Moon, H.J. Perfusion Parameters on Breast Dynamic Contrast-Enhanced MRI Are Associated With Disease-Specific Survival in Patients With Triple-Negative Breast Cancer. Am. J. Roentgenol. 2017, 208, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Shin, S.U.; Ryu, H.S.; Han, W.; Im, S.A.; Park, I.A.; Noh, D.Y.; Moon, W.K. Pretreatment MR Imaging Features of Triple-Negative Breast Cancer: Association with Response to Neoadjuvant Chemotherapy and Recurrence-Free Survival. Radiology 2016, 281, 392–400. [Google Scholar] [CrossRef]
- Carlson, R.W.; Allred, D.C.; Anderson, B.O.; Burstein, H.J.; Carter, W.B.; Edge, S.B.; Erban, J.K.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. Invasive breast cancer. J. Natl. Compr. Cancer Netw. 2011, 9, 136–222. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Garrett-Mayer, E.; White, J.; Blinder, V.S.; Foster, J.C.; Amiri-Kordestani, L.; Hwang, E.S.; Bliss, J.M.; Rakovitch, E.; Perlmutter, J.; et al. Updated Standardized Definitions for Efficacy End Points (STEEP) in Adjuvant Breast Cancer Clinical Trials: STEEP Version 2.0. J. Clin. Oncol. 2021, 39, 2720–2731. [Google Scholar] [CrossRef]
- Pickles, M.D.; Lowry, M.; Manton, D.J.; Turnbull, L.W. Prognostic value of DCE-MRI in breast cancer patients undergoing neoadjuvant chemotherapy: A comparison with traditional survival indicators. Eur. Radiol. 2015, 25, 1097–1106. [Google Scholar] [CrossRef]
- Tuncbilek, N.; Tokatli, F.; Altaner, S.; Sezer, A.; Ture, M.; Omurlu, I.K.; Temizoz, O. Prognostic value DCE-MRI parameters in predicting factor disease free survival and overall survival for breast cancer patients. Eur. J. Radiol. 2012, 81, 863–867. [Google Scholar] [CrossRef]
- Tofts, P.S.; Brix, G.; Buckley, D.L.; Evelhoch, J.L.; Henderson, E.; Knopp, M.V.; Larsson, H.B.; Lee, T.Y.; Mayr, N.A.; Parker, G.J.; et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reson. Imaging 1999, 10, 223–232. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schild, H.H. Dynamic image interpretation of MRI of the breast. J. Magn. Reson. Imaging 2000, 12, 965–974. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Uzzan, B.; Nicolas, P.; Cucherat, M.; Perret, G.Y. Microvessel density as a prognostic factor in women with breast cancer: A systematic review of the literature and meta-analysis. Cancer Res. 2004, 64, 2941–2955. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Rahbar, H.; Hippe, D.S.; Rendi, M.H.; Parker, E.U.; Shekar, N.; Hirano, M.; Cheung, K.J.; Partridge, S.C. Dynamic contrast-enhanced breast MRI features correlate with invasive breast cancer angiogenesis. NPJ Breast Cancer 2021, 7, 42. [Google Scholar] [CrossRef]
- Choi, W.J.; Kim, Y.; Cha, J.H.; Shin, H.J.; Chae, E.Y.; Yoon, G.Y.; Kim, H.H. Correlation between magnetic resonance imaging and the level of tumor-infiltrating lymphocytes in patients with estrogen receptor-negative HER2-positive breast cancer. Acta Radiol. 2020, 61, 3–10. [Google Scholar] [CrossRef]
- Ku, Y.J.; Kim, H.H.; Cha, J.H.; Shin, H.J.; Chae, E.Y.; Choi, W.J.; Lee, H.J.; Gong, G. Predicting the level of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: Usefulness of breast MRI computer-aided detection and diagnosis. J. Magn. Reson. Imaging 2018, 47, 760–766. [Google Scholar] [CrossRef]
- Lamplugh, Z.; Fan, Y. Vascular Microenvironment, Tumor Immunity and Immunotherapy. Front. Immunol. 2021, 12, 811485. [Google Scholar] [CrossRef]
- Michaelson, J.S.; Silverstein, M.; Wyatt, J.; Weber, G.; Moore, R.; Halpern, E.; Kopans, D.B.; Hughes, K. Predicting the survival of patients with breast carcinoma using tumor size. Cancer 2002, 95, 713–723. [Google Scholar] [CrossRef]
- Min, S.K.; Lee, S.K.; Woo, J.; Jung, S.M.; Ryu, J.M.; Yu, J.; Lee, J.E.; Kim, S.W.; Chae, B.J.; Nam, S.J. Relation Between Tumor Size and Lymph Node Metastasis According to Subtypes of Breast Cancer. J. Breast Cancer 2021, 24, 75–84. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Grainge, M.J.; Rakha, E.A.; Green, A.R.; Ellis, I.O. Tumor size is an unreliable predictor of prognosis in basal-like breast cancers and does not correlate closely with lymph node status. Breast Cancer Res. Treat. 2009, 117, 199–204. [Google Scholar] [CrossRef]
- Koh, H.W.; Jung, J.J.; Kim, H.K.; Moon, H.G.; Han, W.; Kim, E.K.; Lee, H.B.; Shin, H.C. Tumor volume as a predictor of recurrence-free survival, but not overall survival, in early breast cancer. Eur. J. Surg. Oncol. 2025, 51, 110024. [Google Scholar] [CrossRef]
- Song, S.E.; Woo, O.H.; Cho, Y.; Cho, K.R.; Park, K.H.; Kim, J.W. Prediction of Axillary Lymph Node Metastasis in Early-stage Triple-Negative Breast Cancer Using Multiparametric and Radiomic Features of Breast MRI. Acad. Radiol. 2023, 30 (Suppl. S2), S25–S37. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.J.; Hwangbo, L.; Kang, T.; Park, H. Diffusion-weighted Imaging of Invasive Breast Cancer: Relationship to Distant Metastasis-free Survival. Radiology 2019, 291, 300–307. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.J.; Kim, E.K.; Yoon, J.H.; Park, V.Y. MRI Radiomic Features: Association with Disease-Free Survival in Patients with Triple-Negative Breast Cancer. Sci. Rep. 2020, 10, 3750. [Google Scholar] [CrossRef]
- Koh, J.; Lee, E.; Han, K.; Kim, S.; Kim, D.K.; Kwak, J.Y.; Yoon, J.H.; Moon, H.J. Three-dimensional radiomics of triple-negative breast cancer: Prediction of systemic recurrence. Sci. Rep. 2020, 10, 2976. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef]
| Variable | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Peak enhancement, per increase of 100% | 1.40 | 1.06–1.84 | 0.019 |
| Angiovolume, per increase of 5 mL | 2.86 | 1.26–6.47 | 0.012 |
| Maximum 3D diameter (cm) | 0.68 | 0.27–1.69 | 0.404 |
| Calculated tumor volume, per increase of 5 cm3 | 1.01 | 0.81–1.27 | 0.898 |
| Variable | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Tumor size | 0.66 | 0.27–1.62 | 0.368 |
| Type of lymph node dissection | |||
| Sentinel dissection | 1 | ||
| Axillary dissection | 1.27 | 0.13–12.48 | 0.840 |
| Axillary node metastasis | |||
| Negative | 1 | ||
| Positive | 2.83 | 0.32–24.85 | 0.347 |
| Lymphovascular invasion | |||
| Negative | 1 | ||
| Positive | 10.08 | 0.86–118.03 | 0.066 |
| Peak enhancement, per increase of 100% | 1.31 | 0.93–1.85 | 0.123 |
| Angiovolume, per increase of 5 mL | 2.47 | 1.28–4.78 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, B.L.; Kim, S.M.; Shin, S.U.; Cho, S.M.; Choi, Y.Y.; Jang, M. Angiovolume and Peak Enhancement on Preoperative CAD-Derived MRI as Prognostic Factors in Primary Operable Triple-Negative Breast Cancer. Tomography 2025, 11, 137. https://doi.org/10.3390/tomography11120137
Yun BL, Kim SM, Shin SU, Cho SM, Choi YY, Jang M. Angiovolume and Peak Enhancement on Preoperative CAD-Derived MRI as Prognostic Factors in Primary Operable Triple-Negative Breast Cancer. Tomography. 2025; 11(12):137. https://doi.org/10.3390/tomography11120137
Chicago/Turabian StyleYun, Bo La, Sun Mi Kim, Sung Ui Shin, Su Min Cho, Yoon Yeong Choi, and Mijung Jang. 2025. "Angiovolume and Peak Enhancement on Preoperative CAD-Derived MRI as Prognostic Factors in Primary Operable Triple-Negative Breast Cancer" Tomography 11, no. 12: 137. https://doi.org/10.3390/tomography11120137
APA StyleYun, B. L., Kim, S. M., Shin, S. U., Cho, S. M., Choi, Y. Y., & Jang, M. (2025). Angiovolume and Peak Enhancement on Preoperative CAD-Derived MRI as Prognostic Factors in Primary Operable Triple-Negative Breast Cancer. Tomography, 11(12), 137. https://doi.org/10.3390/tomography11120137






