Imaging or Adrenal Vein Sampling Approach in Primary Aldosteronism? A Patient-Based Approach
Abstract
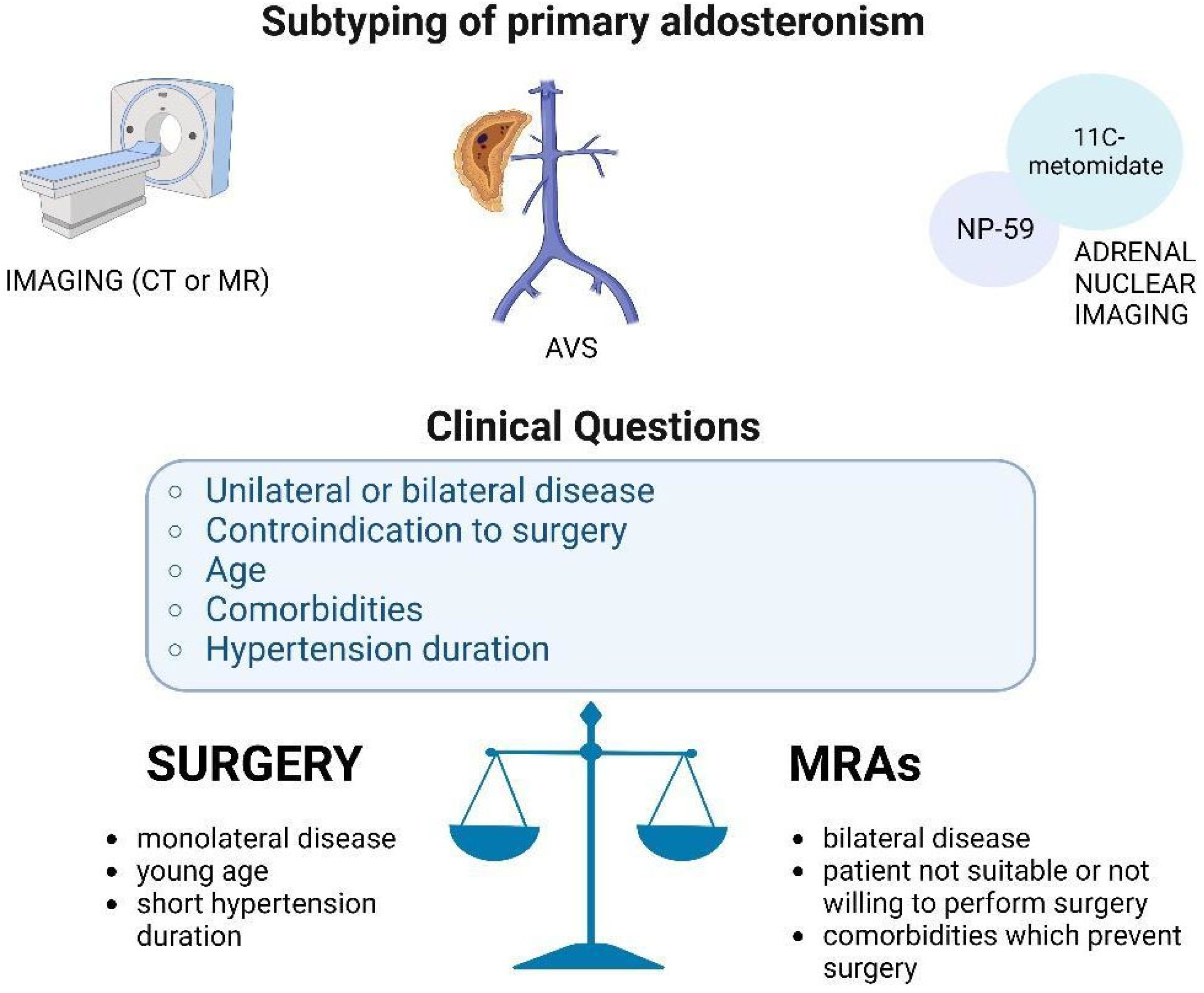
1. Introduction
2. Adrenal Vein Sampling and Unconventional Adrenal Indices
3. Conventional and Nuclear Imaging
4. Imaging Versus AVS in the Diagnosis of Subtyping
5. Clinical Cases Presentation
5.1. Case 1
Does True Unilateral PA Exist?
5.2. Case 2
Are These AVS Results Interpretable?
5.3. Case 3
Can AVS Be Feasible (Safe for Patients and Informative for Physicians) in Patients with Iodine Contrast Media Allergy?
5.4. Case 4
Are MRAs Better Than Surgery?
5.5. Case 5
6. Conclusions
| First Author | Cohort Described | Significant Findings |
|---|---|---|
| AVS is better than imaging to define the subtyping of PA | ||
| Williams TA [27] | 761 patients with unilateral PA (235 with CT management diagnosed from 1994–2016, and 526 with AVS management diagnosed from 1994–2015. | Biochemical remission in 80% (188 of 235) cases after a CT-based treatment decision vs. 93% (491 of 526) after an AVS-based treatment decision (p < 0.001). |
| Rossi GP [28] | 1311 PA patients. | Imaging did not detect the culprit adrenal in 28% of the surgically cured unilateral PA patients. The clinical outcome did not differ significantly between the imaging-positive and imaging-negative patients. |
| Surgery is the suggested treatment for monolateral PA | ||
| Satoh M [34] | 326 PA patients who had received MRA treatment (n = 152) or adrenalectomy (n = 174). | Clinical outcomes were not different in after MRAs or adrenalectomy, except for a reduction in the number of antihypertensive drugs after surgery (p < 0.001). |
| Wu V-C [35] | 858 unilateral PA cases among 1220 PA patients and 1210 essential hypertension controls. | Adrenalectomy was associated with lower all-cause mortality of unilateral PA patients, compared to controls (p = 0.017). More beneficial effect of adrenalectomy over MRA treatment on long-term MACE (p < 0.001), atrial fibrillation (p < 0.001), and congestive heart failure (p < 0.001) in unilateral PA patients. |
| Rossi G. P [52] | 1125 consecutively newly diagnosed hypertensive patients (PA, PH, and IHA). | The medical treatment of PA patients was associated with an increase of 82% of relative risk of atrial fibrillation compared with APA (treated with adrenalectomy) and PH (p = 0.025). |
| MRA is able to reduce cardiovascular risk in patients with PA | ||
| Catena C [49] | 54 consecutive patients who received a diagnosis of PA between 1994 and 2001. | Cardiovascular outcome (myocardial infarction, stroke, any type of revascularization procedure, and arrhythmias) was similar to patients with PA treated with adrenalectomy vs. MRAs (p = 0.71). |
| Interpretation of AVS in selected cases (inadequate catheterization, contrast allergy) | ||
| Younes N [41] | 7 patients with previous allergic reactions to ICM were prepared for AVS with 3 doses of 7.5 mg dexamethasone. | Despite adequate serum cortisol suppression following dexamethasone, the basal and post-ACTH selectivity index confirmed adequate cannulation of both adrenal veins. No allergic reactions were reported. |
| Acharya R [42] | Retrospective review of 8 patients with bilateral adrenal masses and AICS (AVS 2008–2016 for cortisol and epinephrine with dexamethasone suppression). | AVS was useful in excluding unilateral adenoma as the source of AICS among patients with bilateral adrenal masses and AICS. |
| Ceolotto G [43] | 136 patients with biochemically confirmed PA, who wished to pursue the surgical cure. | Biochemical cure after adrenalectomy was used to assess the accuracy of LI calculated by using androstenedione, metanephrine and normetanephrine compared to cortisol. The accuracy of LI calculated with the different biomarkers was high for all biomarkers and showed no significant differences (p < 0.0001). |
| Christou F [44] | 125 PA patients. | Assessment of SIs of cortisol, free metanephrine, and the FTMR indices for the AVS procedure. Confirmation that free metanephrine-based SIs are better than those based on cortisol. |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Sabbadin, C.; Fallo, F. Hyperaldosteronism: Screening and Diagnostic Tests. High Blood Press. Cardiovasc. Prev. 2016, 23, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Chee, M.R.; Hoo, J.; Libianto, R.; Gwini, S.M.; Hamilton, G.; Narayan, O.; Young, M.J.; Fuller, P.J.; Yang, J. Prospective Screening for Primary Aldosteronism in Patients With Suspected Obstructive Sleep Apnea. Hypertension 2021, 77, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Reincke, M.; Bancos, I.; Mulatero, P.; I Scholl, U.; Stowasser, M.; Williams, T.A. Diagnosis and treatment of primary aldosteronism. Lancet Diabetes Endocrinol. 2021, 9, 876–892. [Google Scholar] [CrossRef]
- Cicala, M.V.; Mantero, F. Hypertension in Cushing’s Syndrome: From Pathogenesis to Treatment. Neuroendocrinology 2010, 92, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Colussi, G.; Sechi, L.A. Treatment of Primary Aldosteronism and Organ Protection. Int. J. Endocrinol. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Auchus, R.J.; Brown, M.; Lenders, J.W.; Naruse, M.; Plouin, P.F.; Satoh, F.; Young, W.F. An Expert Consensus Statement on Use of Adrenal Vein Sampling for the Subtyping of Primary Aldosteronism. Hypertension 2014, 63, 151–160. [Google Scholar] [CrossRef]
- Hundemer, G.L.; Curhan, G.C.; Yozamp, N.; Wang, M.; Vaidya, A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: A retrospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 51–59. [Google Scholar] [CrossRef]
- Iacobone, M.; Citton, M.; Viel, G.; Rossi, G.P.; Nitti, D. Approach to the surgical management of primary aldosteronism. Gland Surg. 2015, 4, 69–81. [Google Scholar] [CrossRef]
- Rossi, G.P.; Bernini, G.; Caliumi, C.; Desideri, G.; Fabris, B.; Ferri, C.; Ganzaroli, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; et al. A Prospective Study of the Prevalence of Primary Aldosteronism in 1,125 Hypertensive Patients. J. Am. Coll. Cardiol. 2006, 48, 2293–2300. [Google Scholar] [CrossRef]
- Cicala, M.-V.; Mantero, F. Primary Aldosteronism: What consensus for the diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Monticone, S.; Deinum, J.; Amar, L.; Prejbisz, A.; Zennaro, M.-C.; Beuschlein, F.; Rossi, G.P.; Nishikawa, T.; Morganti, A.; et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: A position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J. Hypertens. 2020, 38, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Sechi, L.A.; Williams, T.A.; Lenders, J.W.; Reincke, M.; Satoh, F.; Januszewicz, A.; Naruse, M.; Doumas, M.; Veglio, F.; et al. Subtype diagnosis, treatment, complications and outcomes of primary aldosteronism and future direction of research: A position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J. Hypertens. 2020, 38, 1929–1936. [Google Scholar] [CrossRef]
- Rossi, G.P.; Barisa, M.; Allolio, B.; Auchus, R.J.; Amar, L.; Cohen, D.; Degenhart, C.; Deinum, J.; Fischer, E.; Gordon, R.; et al. The Adrenal Vein Sampling International Study (AVIS) for Identifying the Major Subtypes of Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2012, 97, 1606–1614. [Google Scholar] [CrossRef]
- Monticone, S.; Satoh, F.; Dietz, A.S.; Goupil, R.; Lang, K.; Pizzolo, F.; Gordon, R.D.; Morimoto, R.; Reincke, M.; Stowasser, M.; et al. Clinical Management and Outcomes of Adrenal Hemorrhage Following Adrenal Vein Sampling in Primary Aldosteronism. Hypertension 2016, 67, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Nanba, A.T.; Wannachalee, T.; Shields, J.J.; Byrd, J.B.; Rainey, W.E.; Auchus, R.J.; Turcu, A.F. Adrenal Vein Sampling Lateralization Despite Mineralocorticoid Receptor Antagonists Exposure in Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2019, 104, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Gomez-Sanchez, C.E. The landscape of molecular mechanism for aldosterone production in aldosterone-producing adenoma. Endocr. J. 2020, 67, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Parasiliti-Caprino, M.; Bioletto, F.; Ceccato, F.; Lopez, C.; Bollati, M.; Voltan, G.; Rossato, D.; Giraudo, G.; Scaroni, C.; Ghigo, E.; et al. The diagnostic accuracy of adjusted unconventional indices for adrenal vein sampling in the diagnosis of primary aldosteronism subtypes. J. Hypertens. 2020, 39, 1025–1033. [Google Scholar] [CrossRef]
- Ebbehoj, A.; Li, D.; Kaur, R.J.; Zhang, C.; Singh, S.; Li, T.; Atkinson, E.; Achenbach, S.; Khosla, S.; Arlt, W.; et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 894–902. [Google Scholar] [CrossRef]
- Blake, M.A.; Cronin, C.G.; Boland, G.W. Adrenal Imaging. AJR Am. J. Roentgenol. 2010, 194, 1450–1460. [Google Scholar] [CrossRef]
- Riester, A.; Fischer, E.; Degenhart, C.; Reiser, M.F.; Bidlingmaier, M.; Beuschlein, F.; Reincke, M.; Quinkler, M. Age below 40 or a Recently Proposed Clinical Prediction Score Cannot Bypass Adrenal Venous Sampling in Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2014, 99, E1035–E1039. [Google Scholar] [CrossRef] [PubMed]
- Powlson, A.S.; Gurnell, M.; Brown, M.J. Nuclear imaging in the diagnosis of primary aldosteronism. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Soinio, M.; Luukkonen, A.-K.; Seppänen, M.; Kemppainen, J.; Seppänen, J.; Pienimäki, J.-P.; Leijon, H.; Vesterinen, T.; Arola, J.; Lantto, E.; et al. Functional Imaging with 11C-Metomidate PET for Subtype Diagnosis in Primary Aldosteronism. Eur. J. Endocrinol. 2020, 183, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Burton, T.J.; Mackenzie, I.S.; Balan, K.; Koo, B.; Bird, N.; Soloviev, D.V.; Azizan, E.A.B.; Aigbirhio, F.; Gurnell, M.; Brown, M.J. Evaluation of the Sensitivity and Specificity of11C-Metomidate Positron Emission Tomography (PET)-CT for Lateralizing Aldosterone Secretion by Conn’s Adenomas. J. Clin. Endocrinol. Metab. 2012, 97, 100–109. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, P.M.; O’Donoghue, D.; Bashari, W.; Senanayake, R.; Joyce, M.B.; Powlson, A.S.; Browne, D.; O’Sullivan, G.J.; Cheow, H.; Mendichovszky, I.; et al. 11C-Metomidate PET/CT is a useful adjunct for lateralization of primary aldosteronism in routine clinical practice. Clin. Endocrinol. 2019, 90, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, T.; Prejbisz, A.; Kool, L.J.S.; Groenewoud, H.J.M.M.; Velema, M.; Spiering, W.; Kołodziejczyk-Kruk, S.; Arntz, M.; Kądziela, J.; Langenhuijsen, J.F.; et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: An outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 2016, 4, 739–746. [Google Scholar] [CrossRef]
- Williams, T.A.; Burrello, J.; Sechi, L.A.; Fardella, C.E.; Matrozova, J.; Adolf, C.; Baudrand, R.; Bernardi, S.; Beuschlein, F.; Catena, C.; et al. Computed Tomography and Adrenal Venous Sampling in the Diagnosis of Unilateral Primary Aldosteronism. Hypertension 2018, 72, 641–649. [Google Scholar] [CrossRef]
- Rossi, G.P.; Crimì, F.; Rossitto, G.; Amar, L.; Azizi, M.; Riester, A.; Reincke, M.; Degenhart, C.; Widimsky, J.; Naruse, M.; et al. Identification of Surgically Curable Primary Aldosteronism by Imaging in a Large, Multiethnic International Study. J. Clin. Endocrinol. Metab. 2021, 106, e4340–e4349. [Google Scholar] [CrossRef]
- Reincke, M.; Williams, T.A. True unilateral primary aldosteronism exists, and unilateral adrenalectomy saves lives. Eur. J. Endocrinol. 2022, 186, C5–C7. [Google Scholar] [CrossRef]
- Gomez-Sanchez, C.E.; Qi, X.; Velarde-Miranda, C.; Plonczynski, M.W.; Parker, C.R.; Rainey, W.; Satoh, F.; Maekawa, T.; Nakamura, Y.; Sasano, H.; et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol. Cell. Endocrinol. 2014, 383, 111–117. [Google Scholar] [CrossRef]
- Meyer, L.S.; Handgriff, L.; Lim, J.S.; Udager, A.M.; Kinker, I.-S.; Ladurner, R.; Wildgruber, M.; Knösel, T.; Bidlingmaier, M.; Rainey, W.E.; et al. Single-Center Prospective Cohort Study on the Histopathology, Genotype, and Postsurgical Outcomes of Patients With Primary Aldosteronism. Hypertension 2021, 78, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, F.; Zhang, Y.; Wen, J.; Dong, D.; Chang, X.; Sun, H.; Ma, X.; Cui, Y.; Chen, S.; et al. Surgical Outcomes of Aldosterone-Producing Adenoma on the Basis of the Histopathological Findings. Front. Endocrinol. 2021, 12, 663096. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Gomez-Sanchez, C.E.; Rainey, W.E.; Giordano, T.J.; Lam, A.K.; Marker, A.; Mete, O.; Yamazaki, Y.; Zerbini, M.C.N.; Beuschlein, F.; et al. International Histopathology Consensus for Unilateral Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2021, 106, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Maruhashi, T.; Yoshida, Y.; Shibata, H. Systematic review of the clinical outcomes of mineralocorticoid receptor antagonist treatment versus adrenalectomy in patients with primary aldosteronism. Hypertens. Res. 2019, 42, 817–824. [Google Scholar] [CrossRef]
- Wu, V.-C.; Wang, S.-M.; Huang, K.-H.; Tsai, Y.C.; Chan, C.-K.; Yang, S.-Y.; Lin, L.-Y.; Chang, C.-C.; Lu, C.-C.; Lin, Y.-H.; et al. Long-term mortality and cardiovascular events in patients with unilateral primary aldosteronism after targeted treatments. Eur. J. Endocrinol. 2022, 186, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, N.; Umakoshi, H.; Seki, T.; Gomez-Sanchez, C.E.; Mukai, K.; Suematsu, M.; Umezawa, Y.; Oya, M.; Kosaka, T.; Seki, M.; et al. Characterization of Aldosterone-producing Cell Cluster (APCC) at Single-cell Resolution. J. Clin. Endocrinol. Metab. 2022, 107, 2439–2448. [Google Scholar] [CrossRef]
- Sasamura, H.; Hashimoto, S.; Kuribayashi, S.; Ueno, K.; Takase, A.; Hayashi, M.; Saruta, T. Use of Gadolinium Contrast Adrenal Venography for the Assessment of Primary Aldosteronism in a Patient with Iodine Allergy. Endocr. J. 2004, 51, 487–492. [Google Scholar] [CrossRef]
- Battistel, M.; Ceolotto, G.; Barbiero, G.; Rossitto, G.; Rossi, G.P. Adrenal venous sampling in dye-allergic primary aldosteronism patients: Prevalence, pitfalls and a possible solution. J. Hypertens. 2018, 36, 1942–1944. [Google Scholar] [CrossRef]
- Saiga, A.; Yokota, H.; Nagano, H.; Sawada, K.; Kubota, Y.; Wada, T.; Horikoshi, T.; Tanaka, T.; Uno, T. 131I-6β-iodomethyl-19-norcholesterol adrenal scintigraphy as an alternative to adrenal venous sampling in differentiating aldosterone-producing adenoma from bilateral idiopathic hyperaldosteronism. Nucl. Med. Commun. 2020, 41, 1226–1233. [Google Scholar] [CrossRef]
- Committee on Drugs and Contrast Media. Published 2022. Available online: https://www.acr.org/-/media/ACR/files/clinical-resources/contrast_media.pdf (accessed on 14 September 2022).
- Younes, N.; Therasse, E.; Bourdeau, I.; Lacroix, A. Successful Adrenal Vein Sampling Using Dexamethasone Premedication in Patients with Iodine Contrast Media Allergy. J. Endocr. Soc. 2022, 6, bvac093. [Google Scholar] [CrossRef]
- Acharya, R.; Dhir, M.; Bandi, R.; Yip, L.; Challinor, S. Outcomes of Adrenal Venous Sampling in Patients with Bilateral Adrenal Masses and ACTH-Independent Cushing’s Syndrome. World J. Surg. 2019, 43, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ceolotto, G.; Antonelli, G.; Caroccia, B.; Battistel, M.; Barbiero, G.; Plebani, M.; Rossi, G.P. Comparison of Cortisol, Androstenedione and Metanephrines to Assess Selectivity and Lateralization of Adrenal Vein Sampling in Primary Aldosteronism. J. Clin. Med. 2021, 10, 4755. [Google Scholar] [CrossRef]
- Christou, F.; Pivin, E.; Denys, A.; Abid, K.A.; Zingg, T.; Matter, M.; Pechère-Bertschi, A.; Maillard, M.; Grouzmann, E.; Wuerzner, G. Accurate Location of Catheter Tip With the Free-to-Total Metanephrine Ratio During Adrenal Vein Sampling. Front. Endocrinol. 2022, 13, 842968. [Google Scholar] [CrossRef] [PubMed]
- Crimì, F.; Quaia, E.; Cabrelle, G.; Zanon, C.; Pepe, A.; Regazzo, D.; Tizianel, I.; Scaroni, C.; Ceccato, F. Diagnostic Accuracy of CT Texture Analysis in Adrenal Masses: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 637. [Google Scholar] [CrossRef] [PubMed]
- Catena, C.; Colussi, G.; Lapenna, R.; Nadalini, E.; Chiuch, A.; Gianfagna, P.; Sechi, L.A. Long-Term Cardiac Effects of Adrenalectomy or Mineralocorticoid Antagonists in Patients With Primary Aldosteronism. Hypertension 2007, 50, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Muth, A.; Ragnarsson, O.; Johannsson, G.; Wängberg, B. Systematic review of surgery and outcomes in patients with primary aldosteronism. Br. J. Surg. 2015, 102, 307–317. [Google Scholar] [CrossRef]
- Rossi, G.-P.; Sechi, L.A.; Giacchetti, G.; Ronconi, V.; Strazzullo, P.; Funder, J.W. Primary aldosteronism: Cardiovascular, renal and metabolic implications. Trends Endocrinol. Metab. 2008, 19, 88–90. [Google Scholar] [CrossRef]
- Catena, C.; Colussi, G.; Nadalini, E.; Chiuch, A.; Baroselli, S.; Lapenna, R.; Sechi, L.A. Cardiovascular Outcomes in Patients With Primary Aldosteronism After Treatment. Arch. Intern. Med. 2008, 168, 80–85. [Google Scholar] [CrossRef]
- Sechi, L.A.; Colussi, G.L.; Novello, M.; Uzzau, A.; Catena, C. Mineralocorticoid Receptor Antagonists and Clinical Outcomes in Primary Aldosteronism: As Good as Surgery? Horm. Metab. Res. 2015, 47, 1000–1006. [Google Scholar] [CrossRef]
- Vaidya, A.; Mulatero, P.; Baudrand, R.; Adler, G.K. The Expanding Spectrum of Primary Aldosteronism: Implications for Diagnosis, Pathogenesis, and Treatment. Endocr. Rev. 2018, 39, 1057–1088. [Google Scholar] [CrossRef]
- Rossi, G.P.; Maiolino, G.; Flego, A.; Belfiore, A.; Bernini, G.; Fabris, B.; Ferri, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; et al. Adrenalectomy Lowers Incident Atrial Fibrillation in Primary Aldosteronism Patients at Long Term. Hypertension 2018, 71, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Deutschbein, T.; Reimondo, G.; Di Dalmazi, G.; Bancos, I.; Patrova, J.; Vassiliadi, D.A.; Nekić, A.B.; Debono, M.; Lardo, P.; Ceccato, F.; et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: An international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022, 10, 499–508. [Google Scholar] [CrossRef]
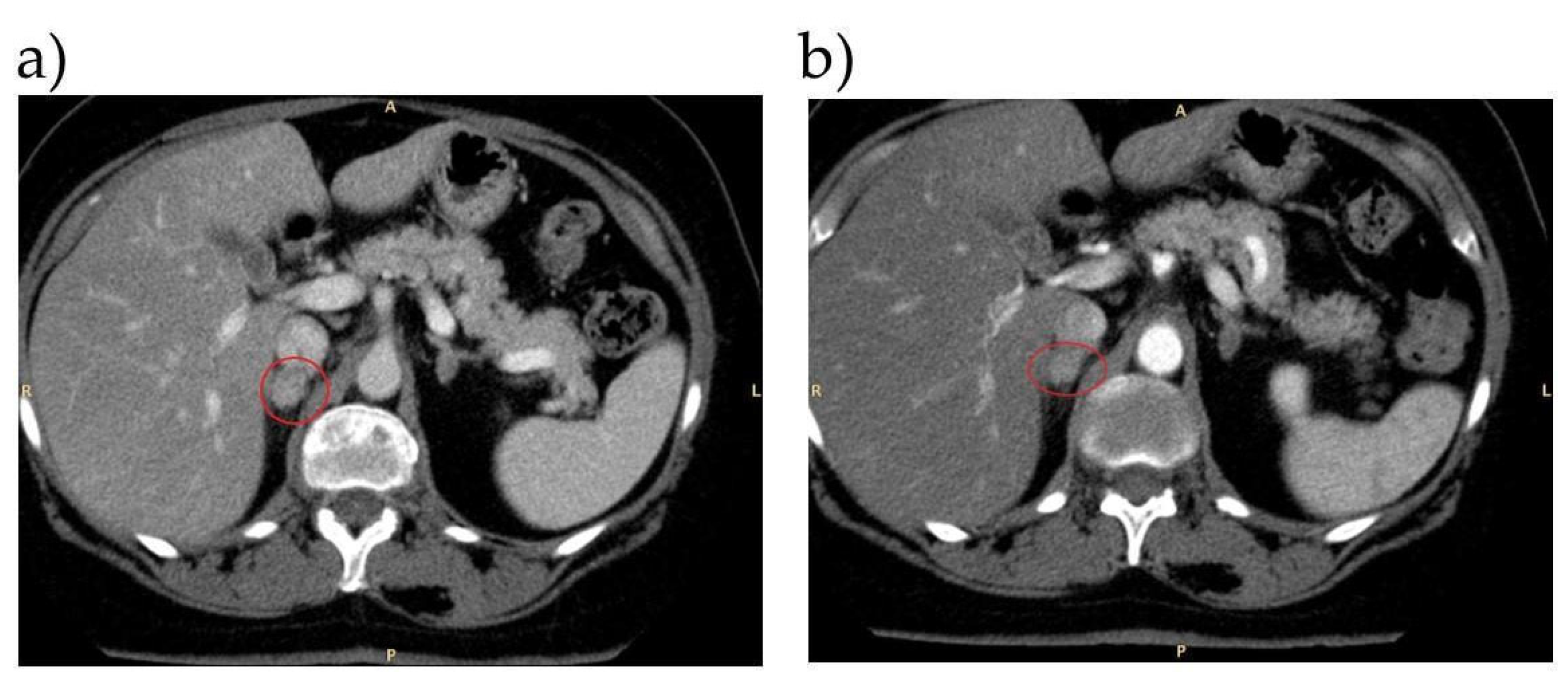
| Case | Confirmatory Test | Imaging | AVS | Treatment | Follow-Up |
|---|---|---|---|---|---|
| 1 | SIT and CCT: both positive | Left adrenal adenoma | Left-sided aldosterone lateralization | Left adrenalectomy | Good control of BP on Olmesartan therapy, renin levels no more suppressed 6 months after surgery |
| 2 | SIT and CCT: both positive | Left adrenal adenoma | AVS not interpretable for inadequate catheterization. Use of unconventional indices (MAI and MI) with demonstration of left aldosterone lateralization | Left adrenalectomy | Normotension and normal potassium levels at last follow-up (3 months) |
| 3 | SIT: positive | CT: left adrenal adenoma Adrenal scintigraphy: bilateral tracer uptake, larger on the left side | Left aldosterone lateralization (AVS performed with previous DEX premedication) | Left adrenalectomy | Normotension without any pharmacological treatment after surgery |
| 4 | CCT: positive | Right adrenal adenoma | AVS not performed because the patient was not suitable for surgery | Medical therapy with MRA (potassium canrenoate) | Good control of BP with MRA since last follow up (30 months); no worsening of the hypertensive cardiomyopathy |
| 5 | CCT: positive | Bilateral adrenal adenoma (20 mm right and 15 mm left) | Right aldosterone lateralization | Scheduled for right adrenalectomy | MRA therapy in association with Ca-antagonist with good pressure control |
| Interpretation | Baseline | 15 min | |
|---|---|---|---|
| Ratio dx/sin | lateralization index > 3 | 0.01 | 0.01 |
| Ratio sin/dx | lateralization index > 3 | 83.36 | 78.31 |
| Cortisol right adrenal/cortisol VCI | selectivity index > 2 | 0.95 | 0.93 |
| Cortisol left adrenal/cortisol VCI | selectivity index > 2 | 3.82 | 5.05 |
| MAI (Monoadrenal Index) | MI (Monolateral Index) | |
|---|---|---|
| left | 52.34 | 30.78 |
| right | 0.6 | 0.78 |
| Interpretation | Baseline | 15 min | |
|---|---|---|---|
| Ratio dx/sin | lateralization index > 3 | 0.01 | 1.16 |
| Ratio sin/dx | lateralization index > 3 | 109.6 | 3.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tizianel, I.; Sabbadin, C.; Mian, C.; Scaroni, C.; Ceccato, F. Imaging or Adrenal Vein Sampling Approach in Primary Aldosteronism? A Patient-Based Approach. Tomography 2022, 8, 2735-2748. https://doi.org/10.3390/tomography8060228
Tizianel I, Sabbadin C, Mian C, Scaroni C, Ceccato F. Imaging or Adrenal Vein Sampling Approach in Primary Aldosteronism? A Patient-Based Approach. Tomography. 2022; 8(6):2735-2748. https://doi.org/10.3390/tomography8060228
Chicago/Turabian StyleTizianel, Irene, Chiara Sabbadin, Caterina Mian, Carla Scaroni, and Filippo Ceccato. 2022. "Imaging or Adrenal Vein Sampling Approach in Primary Aldosteronism? A Patient-Based Approach" Tomography 8, no. 6: 2735-2748. https://doi.org/10.3390/tomography8060228
APA StyleTizianel, I., Sabbadin, C., Mian, C., Scaroni, C., & Ceccato, F. (2022). Imaging or Adrenal Vein Sampling Approach in Primary Aldosteronism? A Patient-Based Approach. Tomography, 8(6), 2735-2748. https://doi.org/10.3390/tomography8060228






