[18F]fluciclovine vs. [18F]fluorocholine Positron Emission Tomography/Computed Tomography: A Head-to-Head Comparison for Early Detection of Biochemical Recurrence in Prostate Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Imaging Protocol and Analysis
2.3. Image Analysis
2.4. Data Analysis
2.5. Statistical Analysis
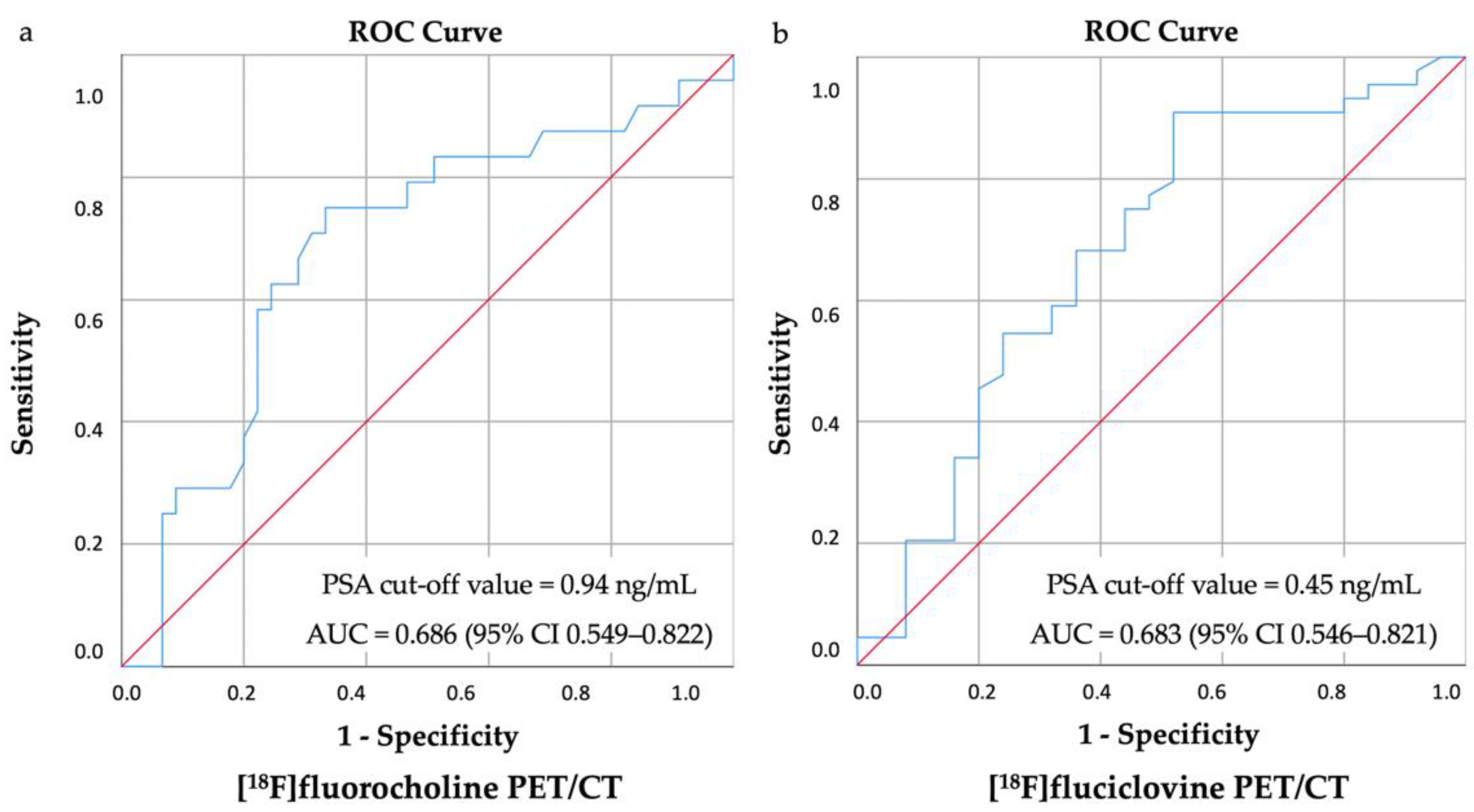
3. Results
3.1. Per-Patient and Per-Region Detection Rate
3.2. Biochemical Parameters
3.3. Clinical and Histological Parameters
3.4. Recurrent Lesions’ Number: A Subanalysis
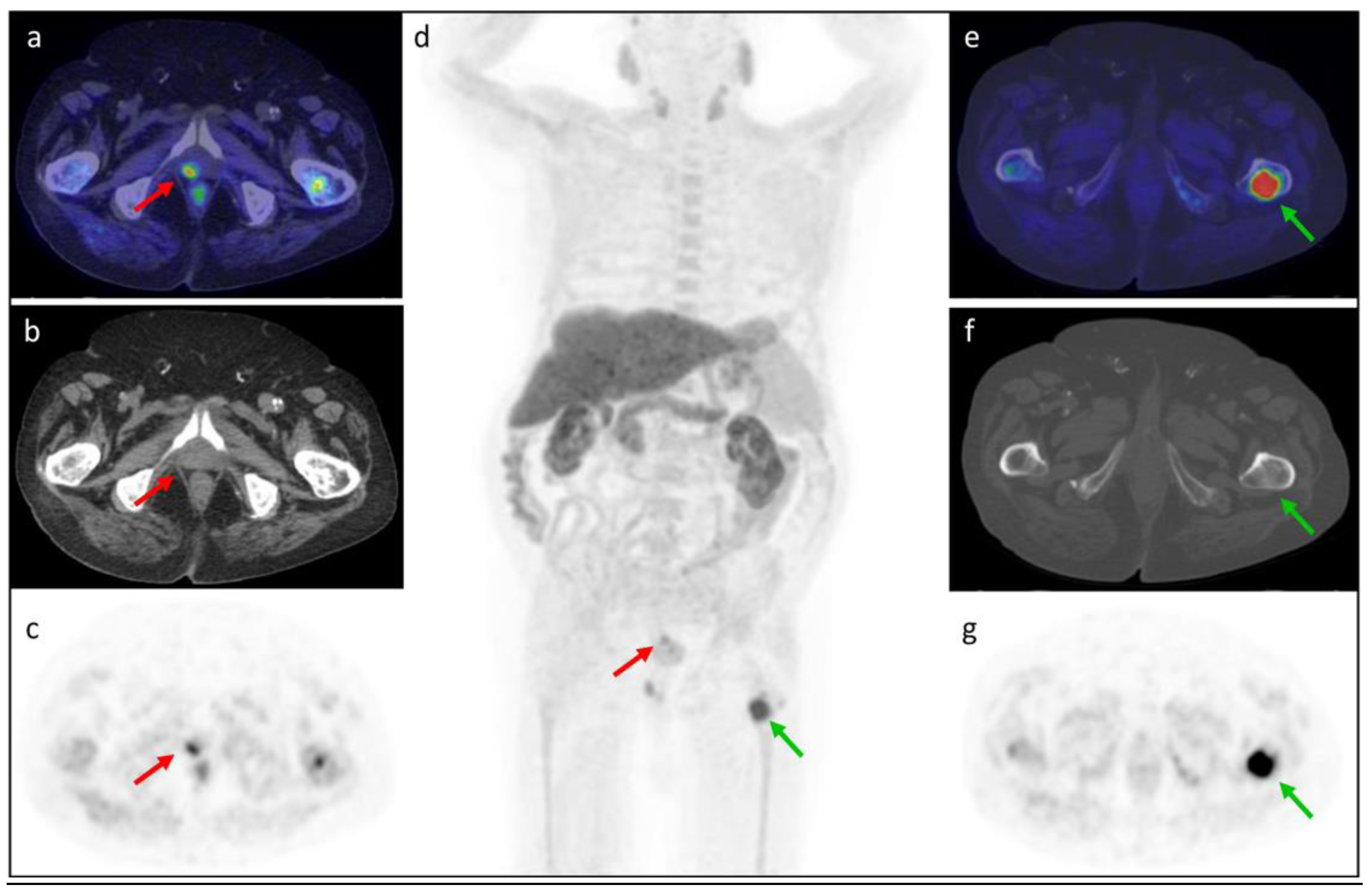
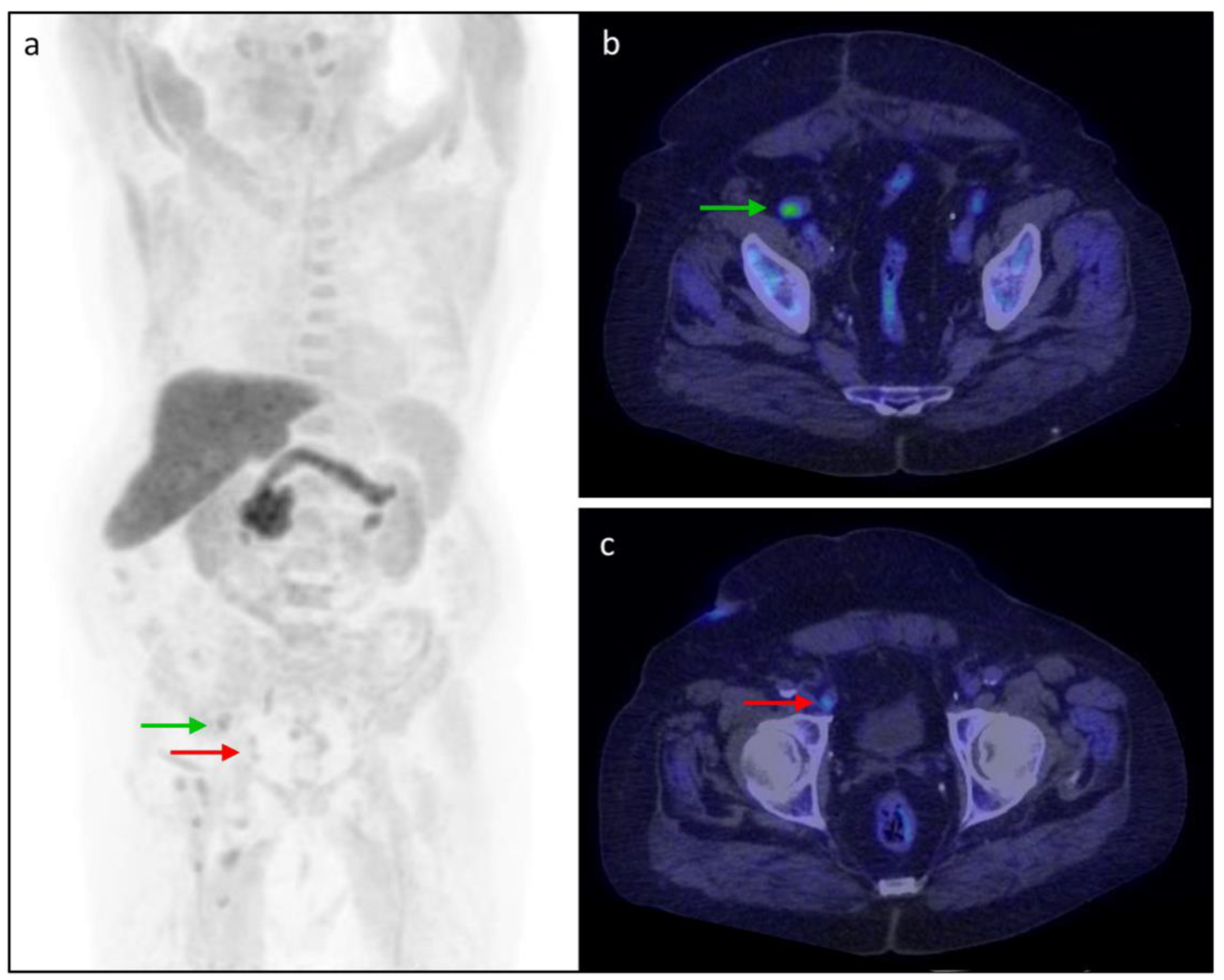
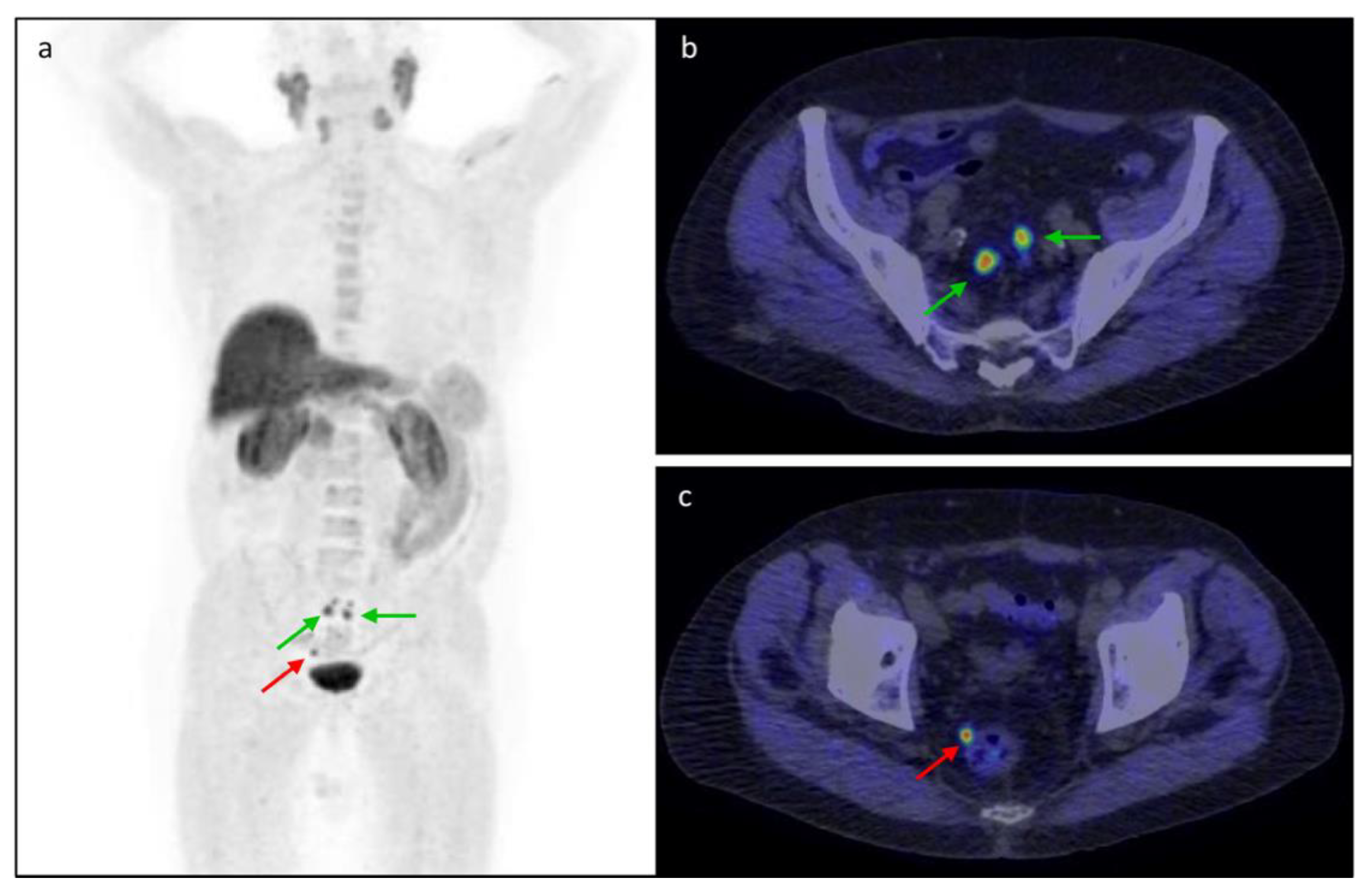
3.5. Clinical Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Mottet, N.; Schmid, H.P.; Van Der Kwast, T.; Wiegel, T.; et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 2011, 59, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.J.; Scardino, P.T.; Eastham, J.A.; Bianco, F.J.; Dotan, Z.A.; Fearn, P.A.; Kattan, M.W. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J. Natl. Cancer Inst. 2006, 98, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Triviño-Ibáñez, E.M.; Puche-Sanz, I.; Gómez-Río, M.; Cózar Olmo, J.M.; Llamas-Elvira, J.M.; Rodríguez-Fernández, A. Usefulness of 18F-fluorocoline PET/CT in prostate cancer patients with biochemical recurrence: Influence of PSA kinetics and hormone therapy. Med. Clin. 2019, 153, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; van der Kwast, T.H.; Lam, T.B.; et al. EAU-EANM-ESTRO-ESUR-ISUP_SIOG Guidelines on Prostate Cancer 2022. Eur. Urol. 2022, 79, 243–262. [Google Scholar] [CrossRef]
- Altini, C.; Asabella, A.N.; Lavelli, V.; Bianco, G.; Ungaro, A.; Pisani, A.; Merenda, N.; Ferrari, C.; Rubini, G. Role of 18F-FDG PET/CT in comparison with CECT for whole-body assessment of patients with esophageal cancer. Recent. Prog. Med. 2019, 110, 144–150. [Google Scholar] [CrossRef]
- Jadvar, H. Is There Use for FDG-PET in Prostate Cancer? Semin. Nucl. Med. 2016, 46, 502–506. [Google Scholar] [CrossRef]
- Fanti, S.; Minozzi, S.; Castellucci, P.; Balduzzi, S.; Herrmann, K.; Krause, B.J.; Oyen, W.; Chiti, A. PET/CT with (11)C-choline for evaluation of prostate cancer patients with biochemical recurrence: Meta-analysis and critical review of available data. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 55–69. [Google Scholar] [CrossRef]
- Nappi, A.G.; Ferrari, C.; Mammucci, P.; Rubini, D.; Lavelli, V.; Sardaro, A.; Pisani, A.R.; Rubini, G. [18F]Fluciclovine PET/CT Improves the Clinical Management of Early Recurrence Prostate Cancer Patients. Cancers 2022, 14, 1461. [Google Scholar] [CrossRef]
- García Garzón, J.R.; de Arcocha Torres, M.; Delgado-Bolton, R.; Ceci, F.; Alvarez Ruiz, S.; Orcajo Rincón, J.; Caresia Aróztegui, A.P.; García Velloso, M.J.; García Vicente, A.M. 68Ga-PSMA PET/CT in prostate cancer. Rev. Esp. Med. Nucl. Imagen Mol. 2018, 37, 130–138. [Google Scholar] [CrossRef]
- Zanoni, L.; Mei, R.; Bianchi, L.; Giunchi, F.; Maltoni, L.; Pultrone, C.V.; Nanni, C.; Bossert, I.; Matti, A.; Schiavina, R.; et al. The Role of [18 F]Fluciclovine PET/CT in the Characterization of High-Risk Primary Prostate Cancer: Comparison with [11C]Choline PET/CT and Histopathological Analysis. Cancers 2021, 13, 1575. [Google Scholar] [CrossRef]
- Memorial Sloan Kettering Cancer Center. Prostate Cancer Nomograms: PSA Doubling Time. Available online: https://www.mskcc.org/nomograms/prostate/psa_doubling_time (accessed on 30 August 2022).
- Tilki, D.; Preisser, F.; Graefen, M.; Huland, H.; Pompe, R.S. External Validation of the European Association of Urology Biochemical Recurrence Risk Groups to Predict Metastasis and Mortality After Radical Prostatectomy in a European Cohort. Eur. Urol. 2019, 75, 896–900. [Google Scholar] [CrossRef]
- Nanni, C.; Zanoni, L.; Bach-Gansmo, T.; Minn, H.; Willoch, F.; Bogsrud, T.V.; Edward, E.P.; Savir-Baruch, B.; Teoh, E.; Ingram, F.; et al. [18 F]Fluciclovine PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging-version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 579–591. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Dreicer, R.; Paciorek, A.; Carroll, P.R.; Konety, B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J. Urol. 2008, 179, 906–910. [Google Scholar] [CrossRef]
- Schuster, D.M.; Nanni, C.; Fanti, S.; Oka, S.; Okudaira, H.; Inoue, Y.; Sörensen, J.; Owenius, R.; Choyke, P.; Turkbey, B.; et al. Anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid: Physiologic uptake patterns, incidental findings, and variants that may simulate disease. J. Nucl. Med. 2014, 55, 1986–1992. [Google Scholar] [CrossRef]
- Rouvière, O.; Vitry, T.; Lyonnet, D. Imaging of prostate cancer local recurrences: Why and how? Eur. Radiol. 2010, 20, 1254–1266. [Google Scholar] [CrossRef]
- Treglia, G.; Ceriani, L.; Sadeghi, R.; Giovacchini, G.; Giovanella, L. Relationship between prostate-specific antigen kinetics and detection rate of radiolabelled choline PET/CT in restaging prostate cancer patients: A meta-analysis. Clin. Chem. Lab. Med. 2014, 52, 725–733. [Google Scholar] [CrossRef]
- Evangelista, L.; Zattoni, F.; Guttilla, A.; Saladini, G.; Zattoni, F.; Colletti, P.M.; Rubello, D. Choline PET or PET/CT and biochemical relapse of prostate cancer: A systematic review and meta-analysis. Clin. Nucl. Med. 2013, 38, 305–314. [Google Scholar] [CrossRef]
- Picchio, M.; Spinapolice, E.G.; Fallanca, F.; Crivellaro, C.; Giovacchini, G.; Gianolli, L.; Messa, C. [11C]Choline PET/CT detection of bone metastases in patients with PSA progression after primary treatment for prostate cancer: Comparison with bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 13–26. [Google Scholar] [CrossRef]
- Castellucci, P.; Ceci, F.; Graziani, T.; Schiavina, R.; Brunocilla, E.; Mazzarotto, R.; Pettinato, C.; Celli, M.; Lodi, F.; Fanti, S. Early biochemical relapse after radical prostatectomy: Which prostate cancer patients may benefit from a restaging 11C-Choline PET/CT scan before salvage radiation therapy? J. Nucl. Med. 2014, 55, 1424–1429. [Google Scholar] [CrossRef]
- Bach-Gansmo, T.; Nanni, C.; Nieh, P.T.; Zanoni, L.; Bogsrud, T.V.; Sletten, H.; Korsan, K.A.; Kieboom, J.; Tade, F.I.; Odewole, O.; et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine (18 F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J. Urol. 2017, 197, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, A.D.; Ahn, G.S.; Barsky, A.R.; Gillman, J.A.; Vapiwala, N.; Pantel, A.R. 18F-Fluciclovine PET/CT in Therapeutic Decision Making for Prostate Cancer: A Large Single-Center Practice-Based Analysis. Clin. Nucl. Med. 2021, 46, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.; Abiodun-Ojo, O.A.; Jani, A.B.; Schuster, D.M. Clinical utility of 18F-Fluciclovine PET/CT in recurrent prostate cancer with very low (≤0.3 ng/mL) prostate-specific antigen levels. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 406–414. [Google Scholar] [PubMed]
- Filippi, L.; Bagni, O.; Crisafulli, C.; Cerio, I.; Brunotti, G.; Chiaravalloti, A.; Schillaci, O.; Dore, F. Detection Rate and Clinical Impact of PET/CT with 18F-FACBC in Patients with Biochemical Recurrence of Prostate Cancer: A Retrospective Bicentric Study. Biomedicines 2022, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.M.; Martin, C.R.; Dechet, C.; Morton, K.; Evans, D.; Ambrose, J.; Maughan, B.L.; O’Neil, B.; Lowrance, W. 18F-fluciclovine PET CT detection of biochemical recurrent prostate cancer at specific PSA thresholds after definitive treatment. Urol. Oncol. 2020, 38, 636.e1–636.e6. [Google Scholar] [CrossRef]
- Wang, Y.; Chow, D.Z.; Ebert, E.; Tajmir, S.; Scott, J.A.; Palmer, E.L. Utility of 18F-Fluciclovine PET/CT for Detecting Prostate Cancer Recurrence in Patients with Low (<1 ng/mL) or Very Low (<0.3 ng/mL) Prostate-Specific Antigen Levels. Am. J. Roentgenol. 2020, 215, 997–1001. [Google Scholar] [CrossRef]
- Nanni, C.; Schiavina, R.; Boschi, S.; Ambrosini, V.; Pettinato, C.; Brunocilla, E.; Martorana, G.; Fanti, S. Comparison of 18F-FACBC and 11C-choline PET/CT in patients with radically treated prostate cancer and biochemical relapse: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2013, 40 (Suppl. S1), 11–17. [Google Scholar] [CrossRef]
- Nanni, C.; Schiavina, R.; Brunocilla, E.; Borghesi, M.; Ambrosini, V.; Zanoni, L.; Gentile, G.; Vagnoni, V.; Romagnoli, D.; Martorana, G.; et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: A prospective study in 28 patients. Clin. Genitourin. Cancer 2014, 12, 106–110. [Google Scholar] [CrossRef]
- Nanni, C.; Schiavina, R.; Brunocilla, E.; Boschi, S.; Borghesi, M.; Zanoni, L.; Pettinato, C.; Martorana, G.; Fanti, S. 18F-Fluciclovine PET/CT for the Detection of Prostate Cancer Relapse: A Comparison to 11C-Choline PET/CT. Clin. Nucl. Med. 2015, 40, e386–e391. [Google Scholar] [CrossRef]
- Wang, R.; Shen, G.; Huang, M.; Tian, R. The Diagnostic Role of 18F-Choline, 18F-Fluciclovine and 18F-PSMA PET/CT in the Detection of Prostate Cancer with Biochemical Recurrence: A Meta-Analysis. Front. Oncol. 2021, 11, 684629. [Google Scholar] [CrossRef]
- Ma, W.; Mao, J.; Yang, J.; Wang, T.; Zhao, Z.H. Comparing the diagnostic performance of radiotracers in prostate cancer biochemical recurrence: A systematic review and meta-analysis. Eur. Radiol. 2022. [Google Scholar] [CrossRef]
- Pernthaler, B.; Kvaternik, H.; Aigner, R.M. A Prospective Head-to-Head Comparison of 18F-Fluciclovine with 68Ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT: A Special Aspect in Imaging Local Recurrence: Reply. Clin. Nucl. Med. 2020, 45, 498–499. [Google Scholar] [CrossRef]
- Tsechelidis, I.; Vrachimis, A. PSMA PET in imaging prostate cancer. Front. Oncol. 2022, 12, 831429. [Google Scholar] [CrossRef]


| Patients’ Characteristics | ||||
|---|---|---|---|---|
| Variables | Total (n = 138) | [18F]fluorocholine (n = 69) | [18F]fluciclovine (n = 69) | p-Value |
| Age—y | ||||
| Mean ± SD | 71.22 ± 6.72 | 71.80 ± 6.46 | 70.65 ± 6.96 | 0.705 |
| Median (Range) | 71.50 (50–87) | 72 (52–87) | 71 (50–83) | |
| PSA—ng/mL | ||||
| Mean ± SD | 1.20 ± 1.17 | 1.25 ± 1.22 | 1.17 ± 1.11 | 0.244 |
| PSA value—no. (%) | ||||
| <0.5 ng/mL | 49 (36%) | 25 (36%) | 23 (34%) | 0.508 |
| 0.5–1 ng/mL | 36 (26%) | 16 (23%) | 21 (30%) | |
| >1 ng/mL | 53 (38%) | 28 (41%) | 25 (36%) | |
| PSAdt—months | ||||
| Mean ± SD | 37.22 ± 221.03 | 12.92 ± 13.70 | 50.14 ± 273.70 | 0.131 |
| Median (Range) | 10.70 (0.10–2241.10) | 8.20 (0.10–70.90) | 10.75 (1.10–2241.10) | |
| PSAdt—no. (%) | ||||
| ≤12 months | 58 (51%) | 22 (48%) | 36 (53%) | 0.356 |
| >12 months | 56 (49%) | 24 (52%) | 32 (47%) | |
| GS—no. (%) | ||||
| <8 | 96 (70%) | 49 (71%) | 47 (68%) | 0.427 |
| ≥8 | 42 (30%) | 20 (29%) | 22 (32%) | |
| EAU BCR risk group—no. (%) | ||||
| Low | 65 (47%) | 38 (55%) | 27 (39%) | 0.060 |
| High | 73 (53%) | 31 (45%) | 42 (61%) | |
| Primary Treatment—no. (%) | ||||
| Prostatectomy Only | 77 (58%) | 42 (61%) | 35 (55%) | 0.105 |
| Radiotherapy Only | 13 (10%) | 9 (13%) | 4 (6%) | |
| Prostatectomy + Radiotherapy | 43 (32%) | 18 (26%) | 25 (39%) | |
| Ongoing HT—no. (%) | ||||
| Yes | 29 (21%) | 17 (25%) | 12 (17%) | 0.202 |
| No | 109 (79%) | 52 (75%) | 57 (83%) | |
| Per-Patient Analysis | |||
| PSA level | [18F]fluorocholine DR | [18F]fluciclovine DR | p-value |
| <0.5 ng/mL | 5/26 (19%) | 10/23 (43%) | 0.063 |
| 0.5–1 ng/mL | 4/15 (27%) | 14/21 (67%) | 0.018 |
| >1 ng/mL | 15/28 (54%) | 20/25 (80%) | 0.040 |
| Per-Region Analysis: Prostate/Prostate bed | |||
| PSA level | [18F]fluorocholine DR | [18F]fluciclovine DR | p-value |
| <0.5 ng/mL | 4/26 (15%) | 7/23 (30%) | 0.180 |
| 0.5–1 ng/mL | 3/15 (20%) | 11/21 (52%) | 0.049 |
| >1 ng/mL | 3/28 (11%) | 17/25 (68%) | <0.0001 |
| Per-Region Analysis: Lymph Node | |||
| PSA level | [18F]fluorocholine DR | [18F]fluciclovine DR | p-value |
| <0.5 ng/mL | 1/26 (4%) | 5/23 (22%) | 0.057 |
| 0.5–1 ng/mL | 1/15 (7%) | 5/21 (24%) | 0.174 |
| >1 ng/mL | 7/28 (25%) | 9/25 (36%) | 0.284 |
| Per-Region Analysis: Bone | |||
| PSA level | [18F]fluorocholine DR | [18F]fluciclovine DR | p-value |
| <0.5 ng/mL | 1/26 (4%) | 1/23 (4%) | 0.724 |
| 0.5–1 ng/mL | 0/15 (0%) | 2/21 (10%) | 0.219 |
| >1 ng/mL | 6/28 (21%) | 2/25 (8%) | 0.164 |
| Per-Patient Analysis | |||
|---|---|---|---|
| PSAdoubling time | [18F]fluorocholine DR | [18F]fluciclovine DR | p-value |
| ≤12 months | 12/22 (55%) | 22/36 (61%) | 0.412 |
| >12 months | 5/24 (21%) | 21/32 (66%) | 0.001 |
| Per-Patient Analysis | |||
|---|---|---|---|
| Gleason Score | [18F]fluorocholine DR | [18F]fluciclovine DR | p-value |
| <8 | 14/49 (29%) | 30/46 (65%) | <0.0001 |
| ≥8 | 10/20 (50%) | 14/23 (61%) | 0.342 |
| EAU BCR Risk Group | |||
| Low | 9/36 (25%) | 18/26 (69%) | 0.001 |
| High | 15/33 (45%) | 26/43 (60%) | 0.143 |
| Ongoing HT | |||
| Yes | 9/17 (53%) | 7/12 (58%) | 0.537 |
| No | 15/52 (29%) | 37/57 (65%) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, C.; Mammucci, P.; Lavelli, V.; Pisani, A.R.; Nappi, A.G.; Rubini, D.; Sardaro, A.; Rubini, G. [18F]fluciclovine vs. [18F]fluorocholine Positron Emission Tomography/Computed Tomography: A Head-to-Head Comparison for Early Detection of Biochemical Recurrence in Prostate Cancer Patients. Tomography 2022, 8, 2709-2722. https://doi.org/10.3390/tomography8060226
Ferrari C, Mammucci P, Lavelli V, Pisani AR, Nappi AG, Rubini D, Sardaro A, Rubini G. [18F]fluciclovine vs. [18F]fluorocholine Positron Emission Tomography/Computed Tomography: A Head-to-Head Comparison for Early Detection of Biochemical Recurrence in Prostate Cancer Patients. Tomography. 2022; 8(6):2709-2722. https://doi.org/10.3390/tomography8060226
Chicago/Turabian StyleFerrari, Cristina, Paolo Mammucci, Valentina Lavelli, Antonio Rosario Pisani, Anna Giulia Nappi, Dino Rubini, Angela Sardaro, and Giuseppe Rubini. 2022. "[18F]fluciclovine vs. [18F]fluorocholine Positron Emission Tomography/Computed Tomography: A Head-to-Head Comparison for Early Detection of Biochemical Recurrence in Prostate Cancer Patients" Tomography 8, no. 6: 2709-2722. https://doi.org/10.3390/tomography8060226
APA StyleFerrari, C., Mammucci, P., Lavelli, V., Pisani, A. R., Nappi, A. G., Rubini, D., Sardaro, A., & Rubini, G. (2022). [18F]fluciclovine vs. [18F]fluorocholine Positron Emission Tomography/Computed Tomography: A Head-to-Head Comparison for Early Detection of Biochemical Recurrence in Prostate Cancer Patients. Tomography, 8(6), 2709-2722. https://doi.org/10.3390/tomography8060226





