DECT Numbers in Upper Abdominal Organs for Differential Diagnosis: A Feasibility Study
Abstract
:1. Introduction
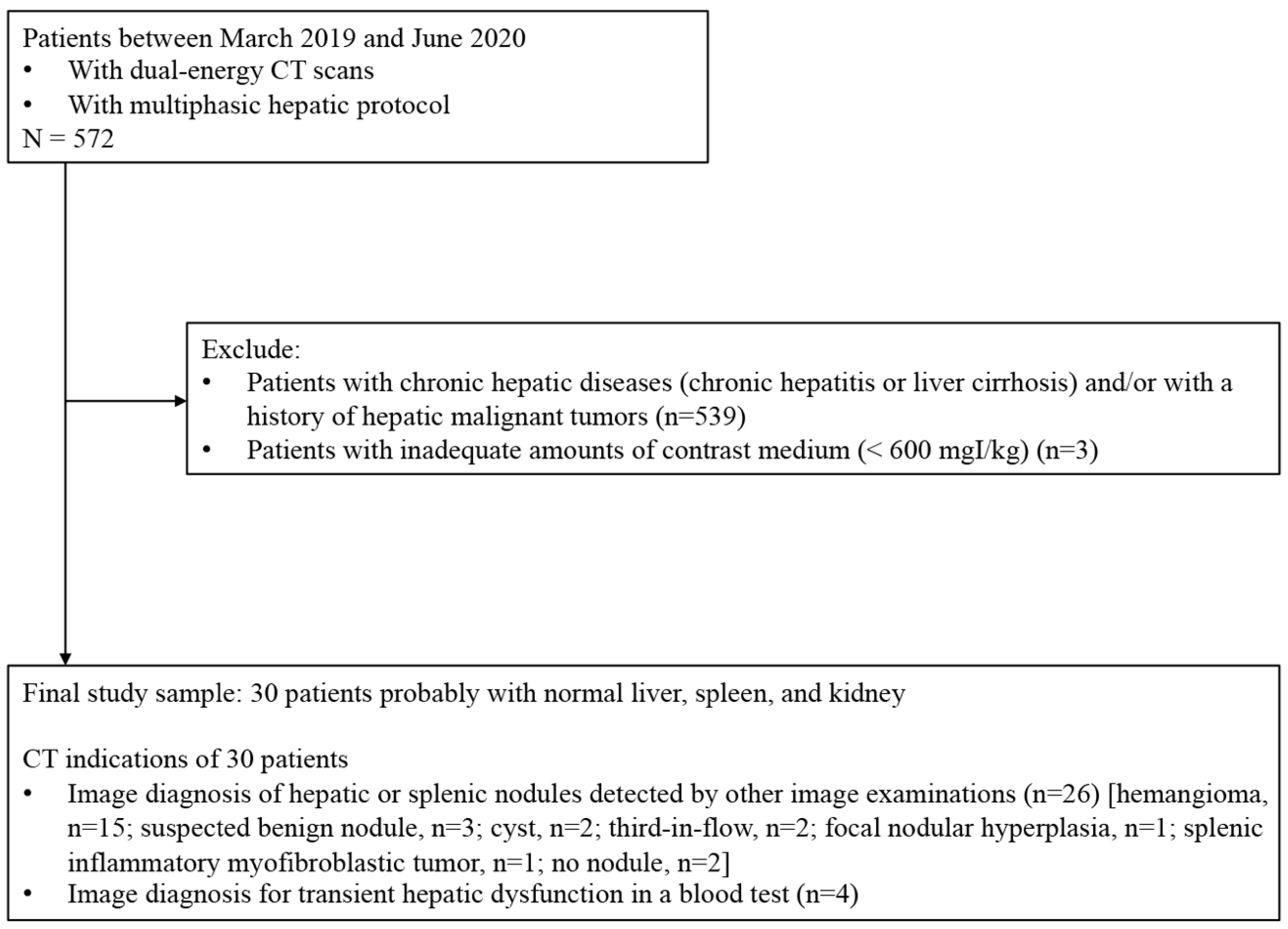
2. Methods
2.1. Study Population
2.2. DECT Protocol
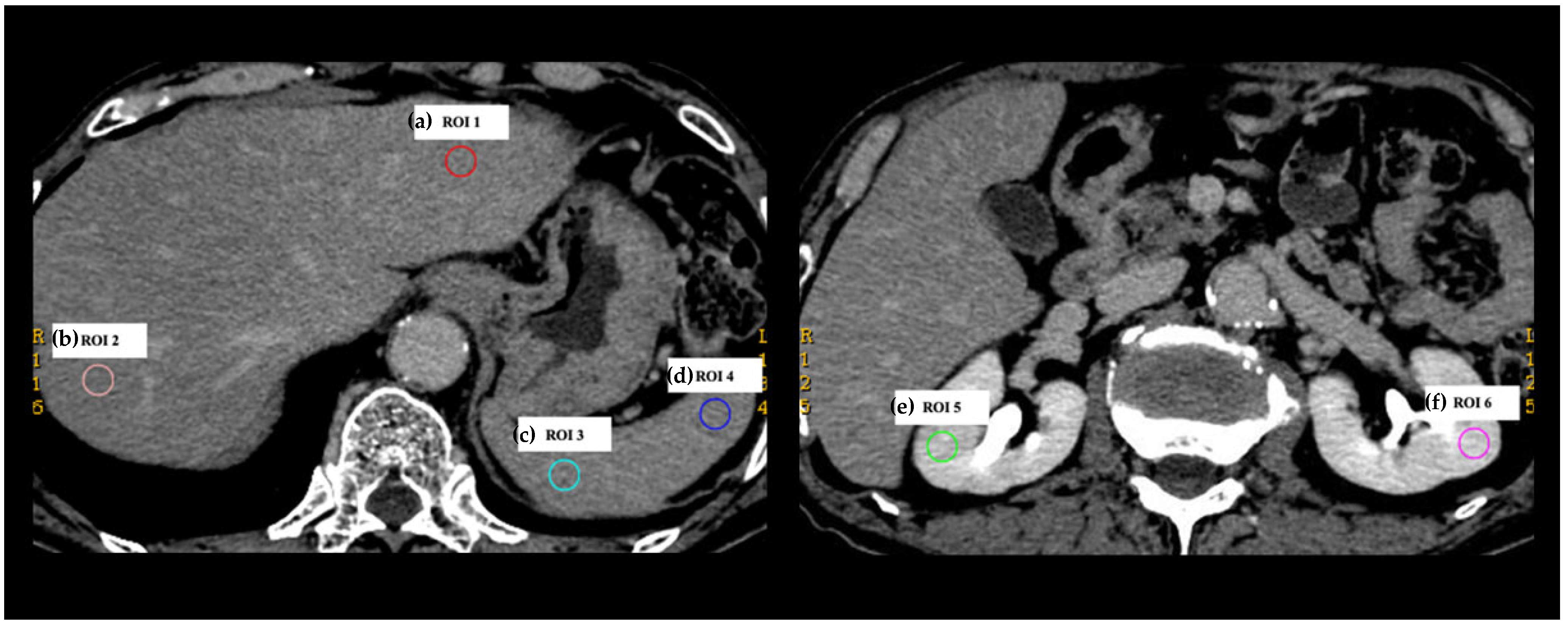
2.3. Collection of DECT Numbers
2.4. Analyses of DECT Numbers
2.5. Statistical Analyses
3. Results
3.1. Patient and CT Imaging Characteristics
3.2. Agreement between Two DECT Numbers within the Same Organ
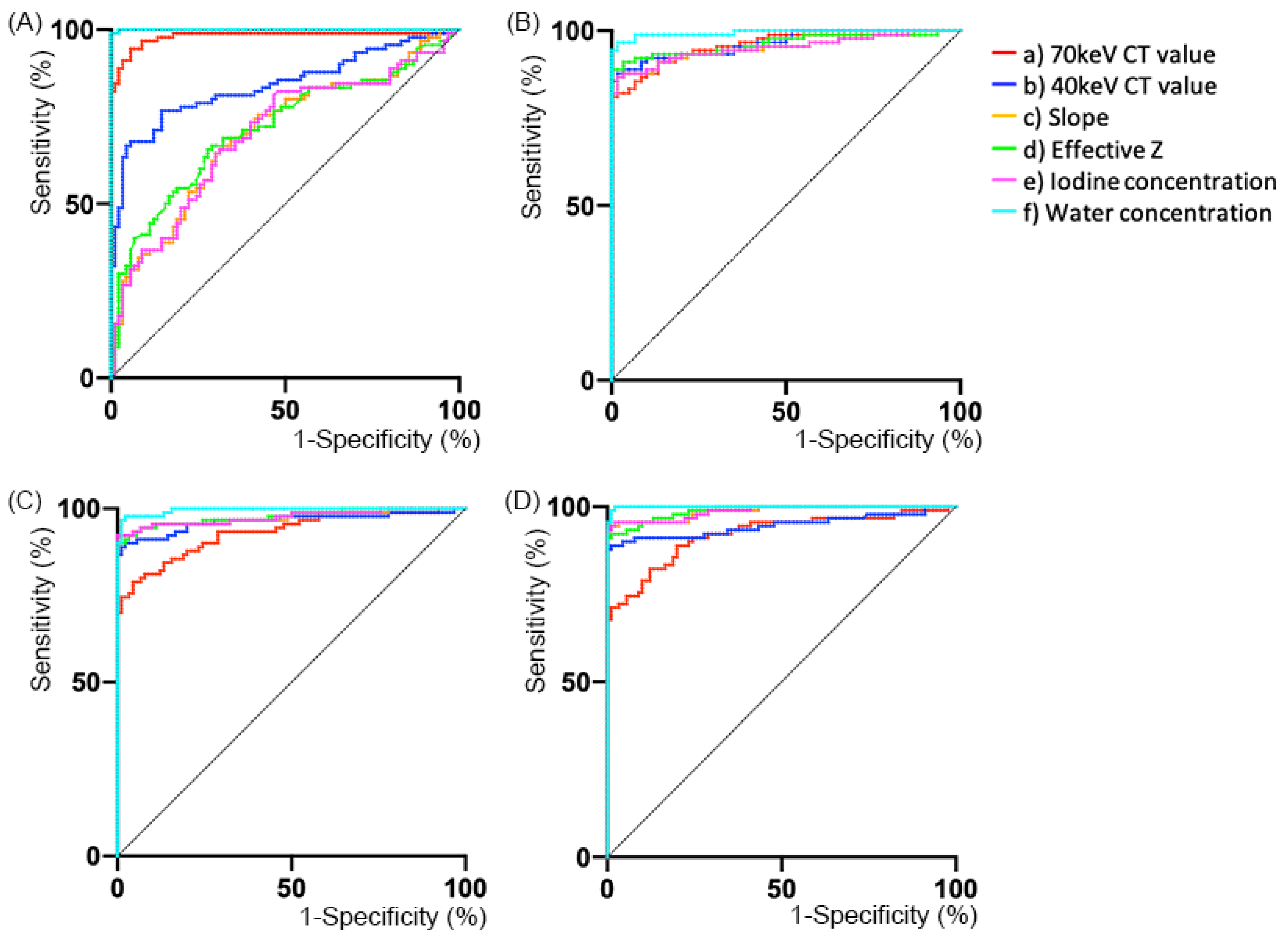
3.3. Diagnostic Ability
4. Discussion
5. Conclusions
6. Take Away in a Sentence
- The value ranges of DECT numbers within the same abdominal organs were particularly narrow on DP images.
- Diagnostic ability to distinguish between the same or different organs was notably high when using the differences in DECT numbers on PVP or DP images.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rutherford, R.A.; Pullan, B.R.; Isherwood, I. Measurement of effective atomic number and electron density using an EMI scanner. Neuroradiology 1976, 11, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; Vogl, T.J.; Martin, S.S.; Nance, J.W.; Duguay, T.M.; Wichmann, J.L.; De Cecco, C.N.; Varga-Szemes, A.; van Assen, M.; Tesche, C.; et al. Review of clinical applications for virtual monoenergetic dual-energy CT. Radiology 2019, 293, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Shuman, W.P.; Green, D.E.; Busey, J.M.; Mitsumori, L.M.; Choi, E.; Koprowicz, K.M.; Kanal, K.M. Dual-energy liver CT: Effect of monochromatic imaging on lesion detection, conspicuity, and contrast-to-noise ratio of hypervascular lesions on late arterial phase. AJR Am. J. Roentgenol. 2014, 203, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, D.; Parakh, A.; Patino, M.; Kambadakone, A.; Rummeny, E.J.; Sahani, D.V. Iodine material density images in du-al-energy CT: Quantification of contrast uptake and washout in HCC. Abdom. Radiol. NY 2018, 43, 3317–3323. [Google Scholar] [CrossRef]
- Muenzel, D.; Lo, G.C.; Yu, H.S.; Parakh, A.; Patino, M.; Kambadakone, A.; Rummeny, E.J.; Sahani, D.V. Material density iodine images in dual-energy CT: Detection and characterization of hypervascular liver lesions compared to magnetic res-onance imaging. Eur. J. Radiol. 2017, 95, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Tanoue, S.; Inoue, T.; Oda, S.; Nakaura, T.; Utsunomiya, D.; Yamashita, Y. Dual-layer spectral CT improves image quality of multiphasic pancreas CT in patients with pancreatic ductal adenocarcinoma. Eur. Radiol. 2020, 30, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Tochigi, T.; Parakh, A.; Joseph, E.; Hahn, P.F.; Kambadakone, A. Low keV portal venous phase as a surrogate for pancreatic phase in a pancreatic protocol dual-energy CT: Feasibility, image quality, and lesion conspicuity. Eur. Radiol. 2021, 31, 6898–6908. [Google Scholar] [CrossRef]
- Tabari, A.; Gee, M.S.; Singh, R.; Lim, R.; Nimkin, K.; Primak, A.; Schmidt, B.; Kalra, M.K. Reducing radiation dose and contrast medium volume with application of dual-energy CT in children and young adults. AJR Am. J. Roentgenol. 2020, 214, 1199–1205. [Google Scholar] [CrossRef]
- Choi, Y.; Shin, N.Y.; Jang, J.; Ahn, K.J.; Kim, B.S. Dual-energy CT for differentiating acute intracranial hemorrhage from contrast staining or calcification: A meta-analysis. Neuroradiology 2020, 62, 1617–1626. [Google Scholar] [CrossRef]
- Monti, C.B.; Zanardo, M.; Cozzi, A.; Schiaffino, S.; Spagnolo, P.; Secchi, F.; De Cecco, C.N.; Sardanelli, F. Dual-energy CT performance in acute pulmonary embolism: A meta-analysis. Eur. Radiol. 2021, 31, 6248–6258. [Google Scholar] [CrossRef]
- McGrath, T.A.; Frank, R.A.; Schieda, N.; Blew, B.; Salameh, J.P.; Bossuyt, P.M.M.; Mcinnes, M.D.F. Diagnostic accuracy of dual-energy computed tomography (DECT) to differentiate uric acid from non-uric acid calculi: Systematic review and me-ta-analysis. Eur. Radiol. 2020, 30, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Gamala, M.; Jacobs, J.W.G.; van Laar, J.M. The diagnostic performance of dual energy CT for diagnosing gout: A systematic literature review and meta-analysis. Rheumatology 2019, 58, 2117–2121. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Beltramello, A.; Minerva, G.; Catania, M.; Guerriero, M.; Albanese, S.; Carbognin, G. Identidfication of residu-al-recurrent cholesteatoma in operated ears: Diagnostic accuracy of dual-energy CT and MRI. Radiol. Med. 2019, 124, 478–486. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, T.; Albrecht, M.H.; Caudo, D.; Mazziotti, S.; Vogl, T.J.; Wichmann, J.L.; Martin, S.; Yel, I.; Ascenti, G.; Koch, V.; et al. Virtual non-calcium dual-energy CT: Clinical applications. Eur. Radiol. Exp. 2021, 5, 38. [Google Scholar] [CrossRef]
- Foti, G.; Beltramello, A.; Catania, M.; Rigotti, S.; Serra, G.; Carbognin, G. Diagnostic accuracy of dual-energy CT and virtual non-calcium techniques to evaluate bone marrow edema in vertebral compression fractures. Radiol. Med. 2019, 124, 487–494. [Google Scholar] [CrossRef]
- Foti, G.; Catania, M.; Caia, S.; Romano, L.; Beltramello, A.; Zorzi, C.; Carbognin, G. Identification of bone marrow edema of the ankle: Diagnostic accuracy of dual-energy CT in comparison with MRI. Radiol. Med. 2019, 124, 1028–1036. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, C.; Yang, Z.; Cheng, Z.; Deng, H.; Chen, M.; Duan, X.; Mao, J.; Shen, J. Axillary sentinel lymph nodes in breast cancer: Quantitative evaluation at dual-energy CT. Radiology 2018, 289, 337–346. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, D.; Li, H.; Zhang, R.; Lv, Y.; Yang, A.; Xie, C. Papillary thyroid cancer: Dual-energy spectral CT quantitative parameters for preoperative diagnosis of metastasis to the cervical lymph nodes. Radiology 2015, 275, 167–176. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wei, C.; Wang, C.B.; Li, N.Y.; Zhang, P.; Dong, J.N. Preoperative prediction of cervical nodal metastasis in pa-pillary thyroid carcinoma: Value of quantitative dual-energy CT parameters and qualitative morphologic features. AJR Am. J. Roentgenol. 2021, 216, 1335–1343. [Google Scholar] [CrossRef]
- Pan, Z.; Pang, L.; Ding, B.; Yan, C.; Zhang, H.; Du, L.; Wang, B.; Song, Q.; Chen, K.; Yan, F. Gastric cancer staging with dual energy spectral CT imaging. PLoS ONE 2013, 8, e53651. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Fang, M.; Li, G.; Duan, X.; Mao, J.; Shen, J. Preoperative diagnosis of regional lymph node metastasis of colorectal cancer with quantitative parameters from dual-energy CT. AJR Am. J. Roentgenol. 2019, 213, W17–W25. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Inoue, T.; Oda, S.; Tanoue, S.; Nakaura, T.; Ikeda, O.; Yamashita, Y. Adrenal adenomas versus metastases: Diagnostic performance of dual-energy spectral CT virtual noncontrast imaging and iodine maps. Radiology 2020, 296, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ju, Y.; Wu, J.; Liu, A.; Chen, A.; Liu, J.; Liu, Y.; Li, J. Differentiation of liver abscess from liver metastasis using dual-energy spectral CT quantitative parameters. Eur. J. Radiol. 2019, 113, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Terada, K.; Kawashima, H.; Yoneda, N.; Toshima, F.; Hirata, M.; Kobayashi, S.; Gabata, T. Predicting axillary lymph node metastasis in breast cancer using the similarity of quantitative dual-energy CT parameters between the primary lesion and axillary lymph node. Jpn. J. Radiol. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Jinzaki, M.; Tanami, Y.; Ueno, A.; Yamada, M.; Kuribayashi, S. Virtual monochromatic spectral imaging with fast kilovoltage switching: Improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 2011, 259, 257–262. [Google Scholar] [CrossRef] [PubMed]
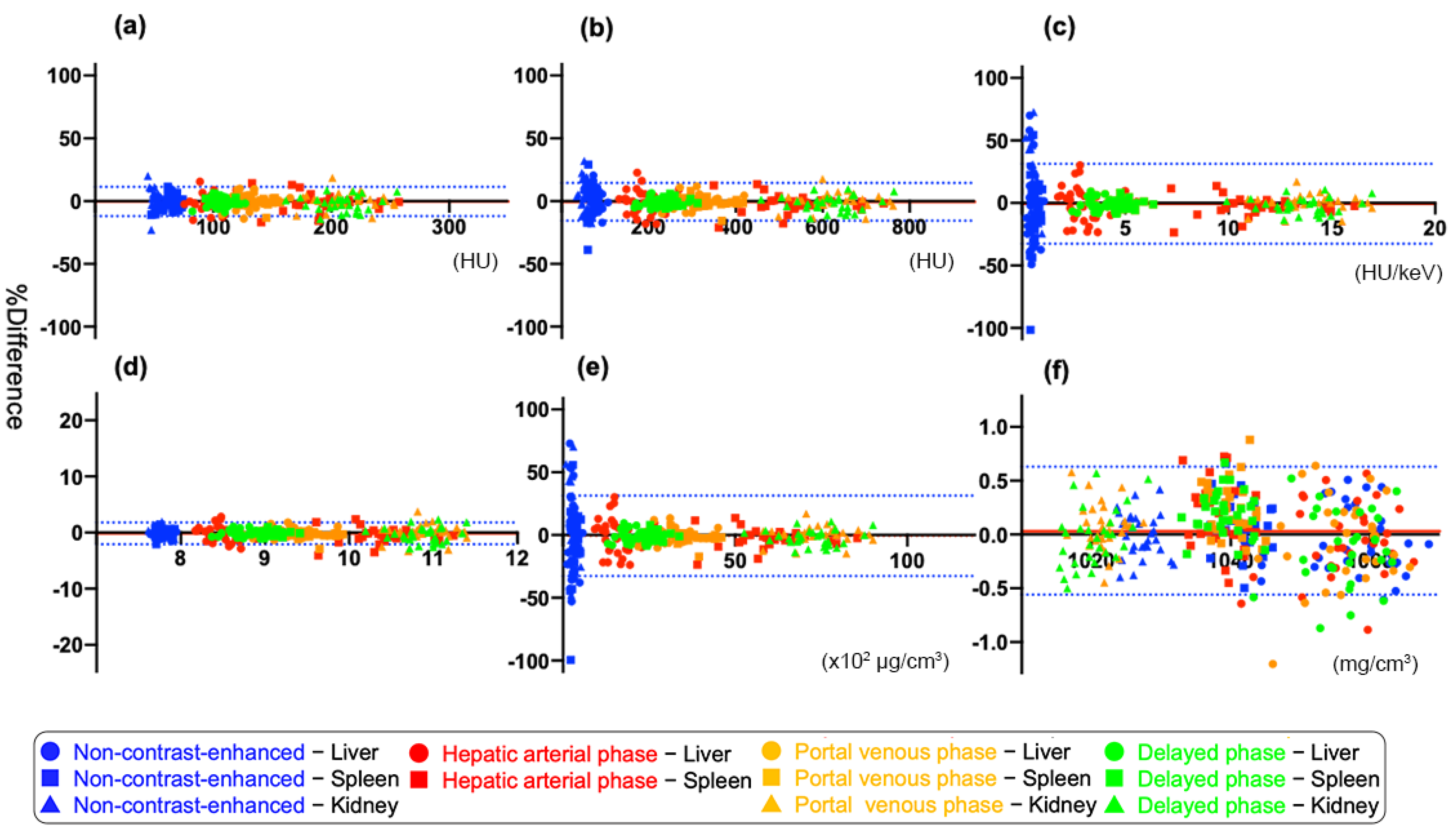
| Mean Bias (%) | 95% Limits of Agreement (%) | |
|---|---|---|
| (a) 70 keV CT value (n = 330) * | 5.9 | −12.0, 11.3 |
| Non-contrast-enhanced (n = 90) | 7.3 | −14.0, 14.5 |
| HAP (n = 60) | 6.8 | −14.5, 12.0 |
| PVP (n = 90) | 5.1 | −9.9, 10.0 |
| DP (n = 90) | 4.4 | −9.5, 7.8 |
| (b) 40 keV CT value (n = 330) * | 7.7 | −15.7, 14.4 |
| Non-contrast-enhanced (n = 90) | 11.1 | −21.3, 22.0 |
| HAP (n = 60) | 8.6 | −18.4, 15.1 |
| PVP (n = 90) | 5.1 | −10.5, 9.6 |
| DP (n = 90) | 4.3 | −9.7, 7.3 |
| (c) Slope (n = 330) * | 16.3 | −32.6, 31.2 |
| Non-contrast-enhanced (n = 90) | 28.8 | −55.6, 57.5 |
| HAP (n = 60) | 11.5 | −24.3, 20.7 |
| PVP (n = 90) | 5.4 | −11.4, 9.9 |
| DP (n = 90) | 5.0 | −11.2, 8.3 |
| (d) Effective Z (n = 330) * | 1.0 | −2.1, 1.8 |
| Non-contrast-enhanced (n = 90) | 0.7 | −1.4, 1.4 |
| HAP (n = 60) | 1.4 | −3.0, 2.3 |
| PVP (n = 90) | 1.0 | −2.1, 1.8 |
| DP (n = 90) | 0.9 | −2.0, 1.5 |
| (e) IC (n = 330) * | 16.3 | −32.7, 31.3 |
| Non-contrast-enhanced (n = 90) | 29.0 | −55.9, 57.8 |
| HAP (n = 60) | 11.5 | −24.3, 20.7 |
| PVP (n = 90) | 5.4 | −11.5, 9.9 |
| DP (n = 90) | 5.0 | −11.3, 8.4 |
| (f) WC (n = 330) * | 0.31 | −0.56, 0.63 |
| Non-contrast-enhanced (n = 90) | 0.27 | −0.52, 0.52 |
| HAP (n = 60) | 0.34 | −0.61, 0.72 |
| PVP (n = 90) | 0.32 | −0.55, 0.70 |
| DP (n = 90) | 0.31 | −0.59, 0.62 |
| AUC | Cut-Off 1 * (%) | Sens (%) | Spec (%) | Cut-Off 2 * (%) | Sens (%) | Spec (%) | ||
|---|---|---|---|---|---|---|---|---|
| Non-contrast-enhanced | (a) 70 keV CT value | 0.980 (0.963, 0.996) | 12.5 | 94.4 | 94.4 | 13.9 | 95.6 | 91.1 |
| (b) 40 keV CT value | 0.837 (0.790, 0.884) | 19.1 | 93.3 | 67.8 | 21 | 95.6 | 66.7 | |
| (c) Slope | 0.692 (0.626, 0.759) | 24.2 | 68.9 | 65.6 | 55.1 | 95.6 | 28.9 | |
| (d) Effective Z | 0.717 (0.656, 0.779) | 0.65 | 72.2 | 65.6 | 1.34 | 95.6 | 32.2 | |
| (e) IC | 0.689 (0.623, 0.756) | 23.4 | 68.9 | 65.6 | 56.4 | 95.6 | 26.7 | |
| (f) WC | 1.000 (0.999, 1.000) | 0.66 | 100 | 98.9 | 0.51 | 95.6 | 100 | |
| HAP | (a) 70 keV CT value | 0.953 (0.928, 0.977) | 16.8 | 100 | 81.1 | 15.4 | 95 | 83.3 |
| (b) 40 keV CT value | 0.958 (0.936, 0.980) | 20.9 | 98.3 | 87.8 | 18.2 | 95 | 88.9 | |
| (c) Slope | 0.943 (0.915, 0.970) | 25.1 | 98.3 | 86.7 | 23.6 | 95 | 87.8 | |
| (d) Effective Z | 0.962 (0.940, 0.984) | 4.05 | 100 | 88.9 | 2.85 | 95 | 91.1 | |
| (e) IC | 0.942 (0.914, 0.970) | 25.4 | 98.3 | 86.7 | 23.7 | 95 | 87.8 | |
| (f) WC | 0.994 (0.988, 1.000) | 0.72 | 98.3 | 96.7 | 0.71 | 95 | 96.7 | |
| PVP | (a) 70 keV CT value | 0.932 (0.903, 0.960) | 11.6 | 95.6 | 78.9 | 11.6 | 95.6 | 78.9 |
| (b) 40 keV CT value | 0.963 (0.941, 0.985) | 14.2 | 97.8 | 90 | 12.8 | 95.6 | 90 | |
| (c) Slope | 0.975 (0.958, 0.992) | 17.2 | 100 | 92.2 | 13.9 | 95.6 | 93.3 | |
| (d) Effective Z | 0.977 (0.961, 0.992) | 3.79 | 100 | 90 | 2.71 | 95.6 | 92.2 | |
| (e) IC | 0.975 (0.959, 0.992) | 17.2 | 100 | 92.2 | 13.9 | 95.6 | 93.3 | |
| (f) WC | 0.996 (0.992, 1.000) | 0.88 | 98.9 | 96.7 | 0.64 | 95.6 | 97.8 | |
| DP | (a) 70 keV CT value | 0.920 (0.887, 0.952) | 11.1 | 98.9 | 71.1 | 8.6 | 95.6 | 72.2 |
| (b) 40 keV CT value | 0.947 (0.919, 0.974) | 13.1 | 98.9 | 87.8 | 10.1 | 95.6 | 90 | |
| (c) Slope | 0.987 (0.977, 0.997) | 12 | 98.9 | 93.3 | 10.5 | 95.6 | 95.6 | |
| (d) Effective Z | 0.988 (0.979, 0.996) | 2.55 | 98.9 | 92.2 | 2.22 | 95.6 | 92.2 | |
| (e) IC | 0.987 (0.977, 0.997) | 11.9 | 98.9 | 93.3 | 10.5 | 95.6 | 95.6 | |
| (f) WC | 0.999 (0.998, 1.000) | 0.72 | 98.9 | 97.8 | 0.61 | 95.6 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toshima, F.; Yoneda, N.; Terada, K.; Inoue, D.; Gabata, T. DECT Numbers in Upper Abdominal Organs for Differential Diagnosis: A Feasibility Study. Tomography 2022, 8, 2698-2708. https://doi.org/10.3390/tomography8060225
Toshima F, Yoneda N, Terada K, Inoue D, Gabata T. DECT Numbers in Upper Abdominal Organs for Differential Diagnosis: A Feasibility Study. Tomography. 2022; 8(6):2698-2708. https://doi.org/10.3390/tomography8060225
Chicago/Turabian StyleToshima, Fumihito, Norihide Yoneda, Kanako Terada, Dai Inoue, and Toshifumi Gabata. 2022. "DECT Numbers in Upper Abdominal Organs for Differential Diagnosis: A Feasibility Study" Tomography 8, no. 6: 2698-2708. https://doi.org/10.3390/tomography8060225
APA StyleToshima, F., Yoneda, N., Terada, K., Inoue, D., & Gabata, T. (2022). DECT Numbers in Upper Abdominal Organs for Differential Diagnosis: A Feasibility Study. Tomography, 8(6), 2698-2708. https://doi.org/10.3390/tomography8060225






