Association of True Positivity with Serum Prostate-Specific Antigen Levels and Other Clinical Factors in Indeterminate PSMA-RADS-3A Lesions Identified on 18F-DCFPyL PET/CT Scans
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Data Collection
2.3. Image Acquisition
2.4. Image Analysis
- Follow-up 18F-DCFPyL PET/CT scan showing significantly increased or decreased uptake of the radiotracer in the PSMA-RADS-3A lesion signified by a SUVmax change of greater than 30%.
- Follow-up CT/MRI scan showing more than 2 mm increase or decrease in the diameters of the PSMA-RADS-3A lesions (Figure 1).
2.5. Statistical Analysis
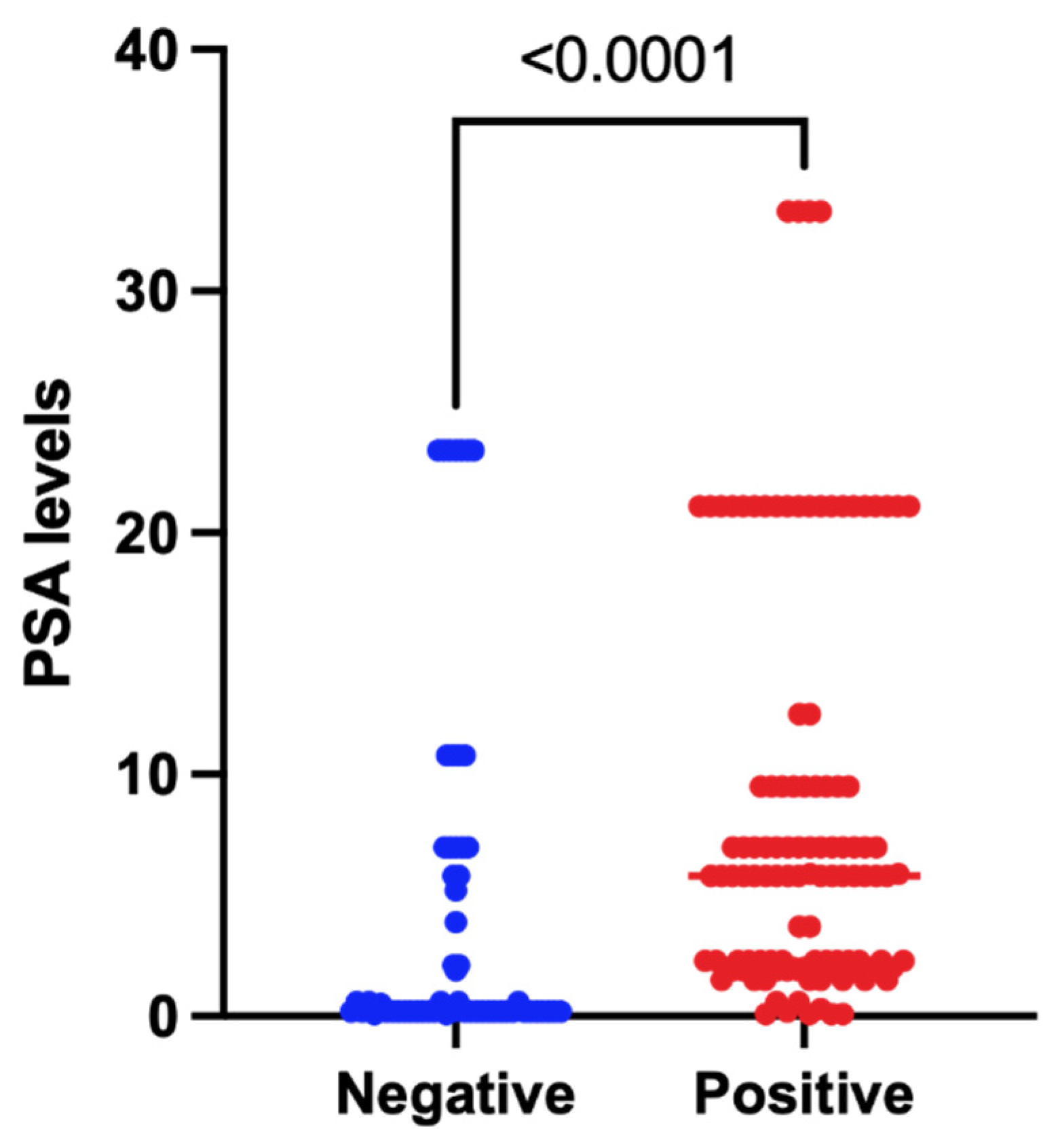
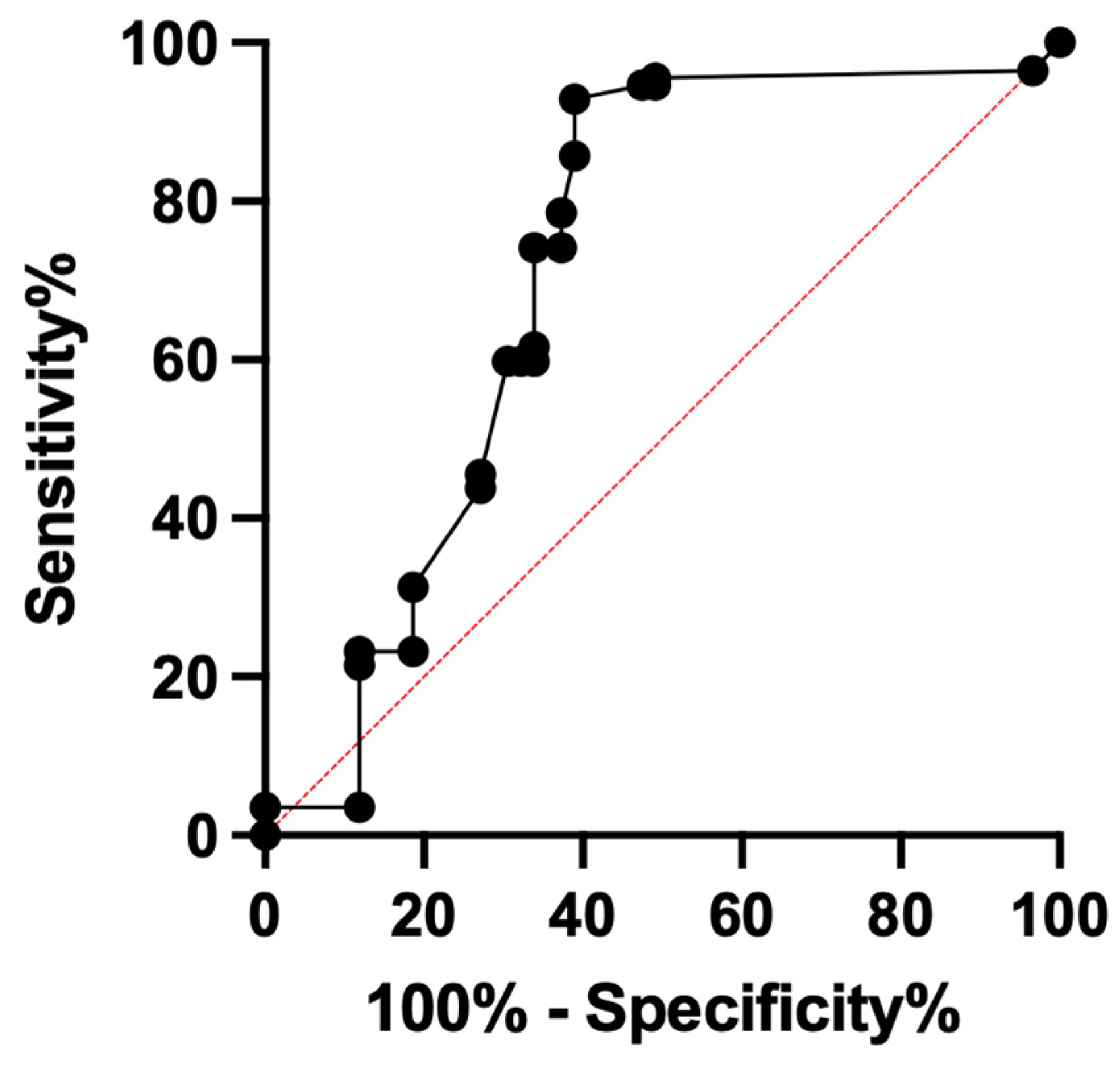
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer of the Prostate—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/prost.html (accessed on 4 July 2022).
- SEER*Explorer Application. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=66&data_type=1&graph_type=2&compareBy=race&chk_race_1=1&chk_race_6=6&chk_race_5=5&chk_race_4=4&chk_race_9=9&chk_race_8=8&rate_type=2&hdn_sex=2&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&advopt_display=1 (accessed on 4 July 2022).
- Rowe, S.P.; Macura, K.J.; Mena, E.; Blackford, A.L.; Nadal, R.; Antonarakis, E.S.; Eisenberger, M.; Carducci, M.; Fan, H.; Dannals, R.F.; et al. PSMA-Based [(18)F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Mol. Imaging Biol. 2016, 18, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET Imaging with a (68) Ga-Labelled PSMA Ligand and (18)F-Choline-Based PET/CT for the Diagnosis of Recurrent Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Papa, N.; Christidis, D.; Wetherell, D.; Hofman, M.S.; Murphy, D.G.; Bolton, D.; Lawrentschuk, N. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. 2016, 70, 926–937. [Google Scholar] [CrossRef]
- Murphy, D.G.; Sweeney, C.J.; Tombal, B. “Gotta Catch’em All”, or Do We? Pokemet Approach to Metastatic Prostate Cancer. Eur. Urol. 2017, 72, 1–3. [Google Scholar] [CrossRef]
- Giesel, F.L.; Will, L.; Kesch, C.; Freitag, M.; Kremer, C.; Merkle, J.; Neels, O.C.; Cardinale, J.; Hadaschik, B.; Hohenfellner, M.; et al. Biochemical Recurrence of Prostate Cancer: Initial Results with [18F]PSMA-1007 PET/CT. J. Nucl. Med. 2018, 59, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Czernin, J.; Cao, M.; Kishan, A.U.; Hegde, J.V.; Shaverdian, N.; Sandler, K.; Chu, F.-I.; King, C.R.; Steinberg, M.L.; et al. 68Ga-PSMA-11 PET/CT Mapping of Prostate Cancer Biochemical Recurrence After Radical Prostatectomy in 270 Patients with a PSA Level of Less Than 1.0 Ng/ML: Impact on Salvage Radiotherapy Planning. J. Nucl. Med. 2018, 59, 230–237. [Google Scholar] [CrossRef]
- Szabo, Z.; Mena, E.; Rowe, S.P.; Plyku, D.; Nidal, R.; Eisenberger, M.A.; Antonarakis, E.S.; Fan, H.; Dannals, R.F.; Chen, Y.; et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Mol. Imaging Biol. 2015, 17, 565–574. [Google Scholar] [CrossRef]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.-P.; Kübler, H.; Haberhorn, U.; Eisenhut, M.; et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence after Radical Prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef]
- Rowe, S.P.; Gorin, M.A.; Hammers, H.J.; Som Javadi, M.; Hawasli, H.; Szabo, Z.; Cho, S.Y.; Pomper, M.G.; Allaf, M.E. Imaging of Metastatic Clear Cell Renal Cell Carcinoma with PSMA-Targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 2015, 29, 877–882. [Google Scholar] [CrossRef]
- Chakraborty, P.S.; Tripathi, M.; Agarwal, K.K.; Kumar, R.; Vijay, M.K.; Bal, C. Metastatic Poorly Differentiated Prostatic Carcinoma with Neuroendocrine Differentiation: Negative on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2015, 40, e163–e166. [Google Scholar] [CrossRef]
- Wright, G.L.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of Prostate-Specific Membrane Antigen in Normal, Benign, and Malignant Prostate Tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Rischpler, C.; Maurer, T.; Schwaiger, M.; Eiber, M. Intense PSMA-Expression Using 68Ga-PSMA PET/CT in a Paravertebral Schwannoma Mimicking Prostate Cancer Metastasis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 193–194. [Google Scholar] [CrossRef]
- Werner, R.A.; Bundschuh, R.A.; Bundschuh, L.; Javadi, M.S.; Leal, J.P.; Higuchi, T.; Pienta, K.J.; Buck, A.K.; Pomper, M.G.; Gorin, M.A.; et al. Interobserver Agreement for the Standardized Reporting System PSMA-RADS 1.0 on 18F-DCFPyL PET/CT Imaging. J. Nucl. Med. 2018, 59, 1857–1864. [Google Scholar] [CrossRef]
- Rowe, S.P.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A. Proposal for a Structured Reporting System for Prostate-Specific Membrane Antigen–Targeted PET Imaging: PSMA-RADS Version 1.0. J. Nucl. Med. 2018, 59, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Werner, R.A.; Higuchi, T.; Lapa, C.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A.; Rowe, S.P. Follow-up of Lesions with Equivocal Radiotracer Uptake on PSMA-Targeted PET in Patients with Prostate Cancer: Predictive Values of the PSMA-RADS-3A and PSMA-RADS-3B Categories. J. Nucl. Med. 2019, 60, 511–516. [Google Scholar] [CrossRef]
- Khatri, W.; Chung, H.W.; Werner, R.A.; Leal, J.P.; Pienta, K.J.; Lodge, M.A.; Gorin, M.A.; Pomper, M.G.; Rowe, S.P. Effect of Point-Spread Function Reconstruction for Indeterminate PSMA-RADS-3A Lesions on PSMA-Targeted PET Imaging of Men with Prostate Cancer. Diagnostics 2021, 11, 665. [Google Scholar] [CrossRef]
- Ravert, H.T.; Holt, D.P.; Chen, Y.; Mease, R.C.; Fan, H.; Pomper, M.G.; Dannals, R.F. An Improved Synthesis of the Radiolabeled Prostate-Specific Membrane Antigen Inhibitor, [18F]DCFPyL: Radiolabeled PSMA Inhibitor, [18F]DCFPyL. J. Label Compd. Radiopharm. 2016, 59, 439–450. [Google Scholar] [CrossRef]
- Froehner, M.; Koch, R.; Farahzadi, S.; Heberling, U.; Borkowetz, A.; Twelker, L.; Baretton, G.B.; Wirth, M.P.; Thomas, C. Long-Term Mortality in Patients with Positive Lymph Nodes at the Time of Radical Prostatectomy. Urol. Int. 2019, 103, 427–432. [Google Scholar] [CrossRef]
- Walz, J.; Chun, F.K.-H.; Klein, E.A.; Reuther, A.; Saad, F.; Graefen, M.; Huland, H.; Karakiewicz, P.I. Nomogram Predicting the Probability of Early Recurrence After Radical Prostatectomy for Prostate Cancer. J. Urol. 2009, 181, 601–608. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Hilton, J.F.; Carroll, P.R. The CAPRA-S Score: A Straightforward Tool for Improved Prediction of Outcomes after Radical Prostatectomy. Cancer 2011, 117, 5039–5046. [Google Scholar] [CrossRef] [PubMed]

| Pre-18F-DCFPyL PET/CT Scan Therapy | Number (%) |
|---|---|
| Radical prostatectomy | 23 (82.1) |
| Radiation therapy | 12 (42.9) |
| Androgen deprivation therapy | 6 (21.4) |
| Chemotherapy | 3 (10.7) |
| Vaccine therapy | 1 (3.6) |
| Salvage radiation | 1 (3.6) |
| Salvage pelvic lymph node dissection | 1 (3.6) |
| None | 3 (10.7) |
| Post-18F-DCFPyL PET/CT scan therapy | |
| Androgen deprivation therapy | 20 (71.4) |
| Radiation therapy | 14 (50) |
| Chemotherapy | 9 (32.1) |
| Radical prostatectomy | 2 (7.1) |
| Cryoablation | 1 (3.6) |
| Salvage pelvic lymph node dissection | 1 (3.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, T.; Werner, R.A.; Chung, H.W.; Khatri, W.; Pienta, K.J.; Pomper, M.G.; Gorin, M.A.; Saad, E.; Rowe, S.P. Association of True Positivity with Serum Prostate-Specific Antigen Levels and Other Clinical Factors in Indeterminate PSMA-RADS-3A Lesions Identified on 18F-DCFPyL PET/CT Scans. Tomography 2022, 8, 2639-2647. https://doi.org/10.3390/tomography8060220
Garg T, Werner RA, Chung HW, Khatri W, Pienta KJ, Pomper MG, Gorin MA, Saad E, Rowe SP. Association of True Positivity with Serum Prostate-Specific Antigen Levels and Other Clinical Factors in Indeterminate PSMA-RADS-3A Lesions Identified on 18F-DCFPyL PET/CT Scans. Tomography. 2022; 8(6):2639-2647. https://doi.org/10.3390/tomography8060220
Chicago/Turabian StyleGarg, Tushar, Rudolf A. Werner, Hyun Woo Chung, Wajahat Khatri, Kenneth J. Pienta, Martin G. Pomper, Michael A. Gorin, Elie Saad, and Steven P. Rowe. 2022. "Association of True Positivity with Serum Prostate-Specific Antigen Levels and Other Clinical Factors in Indeterminate PSMA-RADS-3A Lesions Identified on 18F-DCFPyL PET/CT Scans" Tomography 8, no. 6: 2639-2647. https://doi.org/10.3390/tomography8060220
APA StyleGarg, T., Werner, R. A., Chung, H. W., Khatri, W., Pienta, K. J., Pomper, M. G., Gorin, M. A., Saad, E., & Rowe, S. P. (2022). Association of True Positivity with Serum Prostate-Specific Antigen Levels and Other Clinical Factors in Indeterminate PSMA-RADS-3A Lesions Identified on 18F-DCFPyL PET/CT Scans. Tomography, 8(6), 2639-2647. https://doi.org/10.3390/tomography8060220







