Clinical Value of Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS) for Staging of Patients with Suspected Head and Neck Cancer
Abstract
1. Introduction
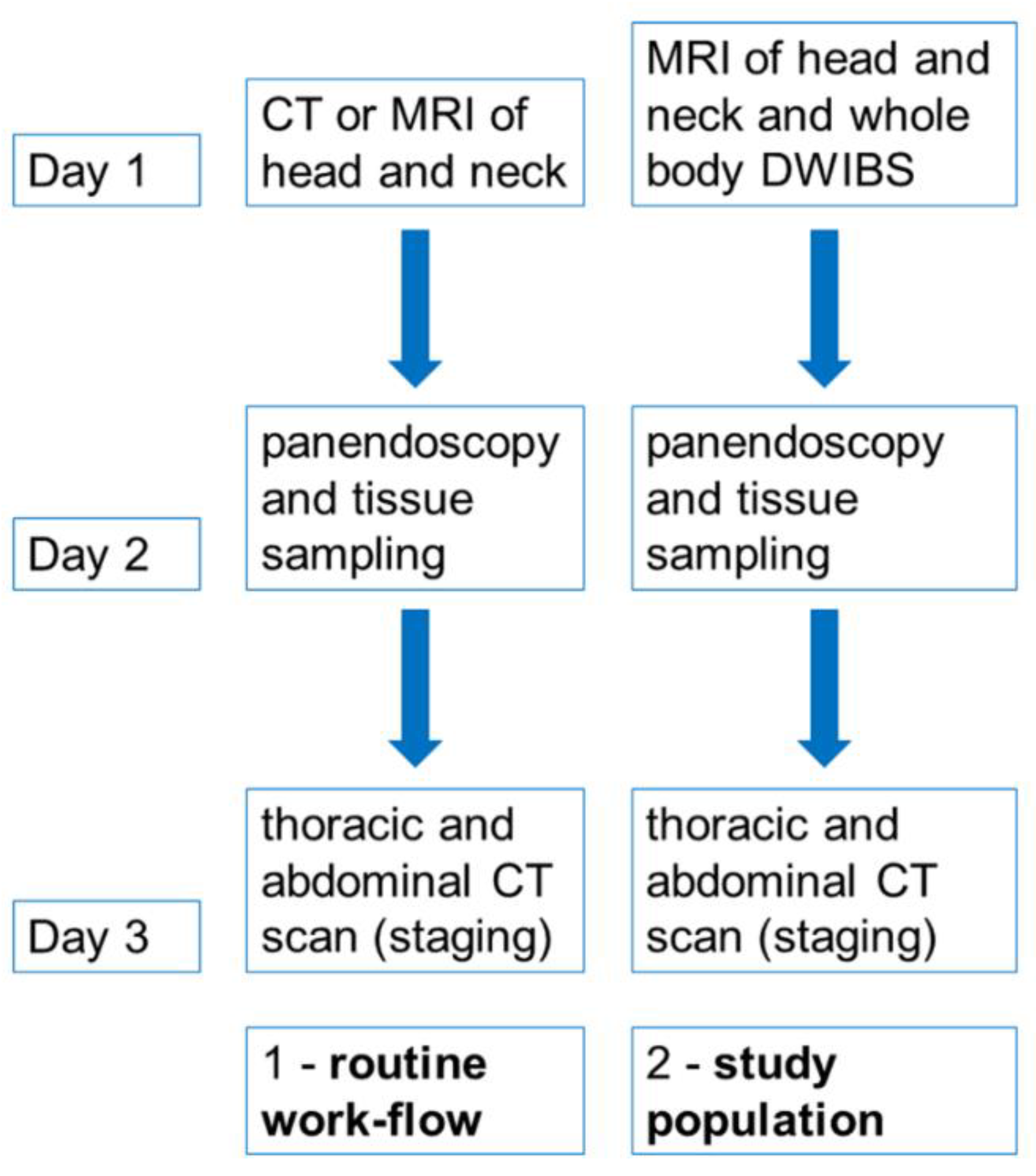
Whole-Body Imaging
2. Materials and Methods
2.1. Patients
2.2. MRI Examination
2.3. CT Examination
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Findings of DWIBS
3.3. Patients’ Further Clinical Course
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CE-CT | contrast-enhanced computed tomography |
| CI95 | 95% Confidence interval |
| CT | computed tomography |
| CUP | cancer of unknown primary |
| DWIBS | diffusion-weighted imaging with background suppression |
| FN | false negative |
| FNR | false negative rate |
| FP | false positive |
| FPR | false positive rate |
| LR+ | positive likelihood ratio |
| LR− | negative likelihood ratio |
| N | negative |
| NPV | negative prediction value |
| P | positive |
| PPV | positive prediction value |
| SD | standard deviation |
| TN | true negative |
| TNR | true negative rate |
| TP | true positive |
| TPR | true positive rate |
References
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik, Therapie und Nachsorge des Larynxkarzinoms, Langversion 1.1, 2019, AWMF-Registernummer: 017/076OL. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/larynxkarzinom/ (accessed on 25 August 2022).
- Noij, P.; Boerhout, E.; Pieters-van den Bos, I.; Comans, E.F.; Oprea-Lager, D.; Reinhard, R.; Hoekstra, O.S.; de Bree, R.; de Graaf, P.; Castelijns, J.A. Whole-body-MR imaging including DWIBS in the work-up of patients with head and neck squamous cell carcinoma: A feasibility study. Eur. J. Radiol. 2014, 83, 1144–1151. [Google Scholar] [CrossRef]
- Cramer, J.D.; Reddy, A.; Ferris, R.L.; Duvvuri, U.; Samant, S. Comparison of the seventh and eighth edition American Joint Committee on Cancer oral cavity staging systems. Laryngoscope 2021, 128, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 8th ed.; AJCC Cancer Staging Form Supplement; Springer: New York, NY, USA, 2017. [Google Scholar]
- Fruehwald-Pallamar, J.; Czerny, C.; Mayerhoefer, M.E.; Halpern, B.S.; Eder-Czembirek, C.; Brunner, M.; Schuetz, M.; Weber, M.; Fruehwald, L.; Herneth, A.M. Functional imaging in head and neck squamous cell carcinoma: Correlation of PET/CT and diffusion-weighted imaging at 3 T. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.C.; Wang, H.M.; Yen, T.C.; Lin, C.Y.; Chin, S.C.; Liao, C.T.; Wai, Y.Y.; Wang, J.J.; Ng, S.H. 18F-FDGPET/CT and 3.0-T whole-body MRI for the detection of distant metastases and second primary tumours in patients with untreated oropharyngeal/hypopharyngeal carcinoma: A comparative study. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1607–1619. [Google Scholar] [CrossRef]
- Ng, S.H.; Chan, S.C.; Yen, T.C.; Chang, J.T.; Liao, C.T.; Ko, S.F.; Wang, H.M.; Wai, Y.Y.; Wang, J.J.; Chen, M.C. Pretreatment evaluation of distant-site status in patients with nasopharyngeal carcinoma: Accuracy of whole-body MRI at 3-T and FDG-PET-CT. Eur. Radiol. 2009, 19, 2965–2976. [Google Scholar] [CrossRef]
- Takahara, T.; Imai, Y.; Yamashita, T.; Yasuda, S.; Nasu, S.; Van Cauteren, M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): Technical improvement using free breathing, STIR and high resolution3D display. Radiat. Med. 2004, 22, 275–282. [Google Scholar] [PubMed]
- Mürtz, P.; Krautmacher, C.; Träber, F.; Gieseke, J.; Schild, H.H.; Willinek, W.A. Diffusion-weighted whole-body MR imaging with background body signal suppression: A feasibility study at 3.0 T. Eur. Radiol. 2007, 17, 3031–3037. [Google Scholar] [CrossRef]
- Tomizawa, M.; Shinozaki, F.; Uchida, Y.; Uchiyama, K.; Tanaka, S.; Sunaoshi, T.; Kano, D.; Sugiyama, E.; Shite, M.; Haga, R.; et al. Comparison of DWIBS/T2 image fusion and PET/CT for the diagnosis of cancer in the abdominal cavity. Exp. Ther. Med. 2017, 14, 3754–3760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, D.; Ye, W.; Long, L.; Lu, Y.; Wei, Y. Diffusion-weighted imaging with background body signal suppression (DWIBS) distinguishes benign lesions from malignant pulmonary solitary lesions. Am. J. Transl. Res. 2021, 13, 88–101. [Google Scholar] [PubMed]
- Usuda, K.; Iwai, S.; Yamagata, A.; Iijima, Y.; Motono, N.; Matoba, M.; Doai, M.; Yamada, S.; Ueda, Y.; Hirata, K.; et al. Diffusion-weighted whole-body imaging with background suppression (DWIBS) is effective and economical for detection of metastasis or recurrence of lung cancer. Thorac. Cancer 2021, 12, 676–684. [Google Scholar] [CrossRef]
- Haughey, B.H.; Gates, G.A.; Arfken, C.L.; Harvey, J. Meta-analysis of second malignant tumors in head and neck cancer: The case for an endoscopic screening protocol. Ann. Otol. Rhinol. Laryngol. 1992, 101 Pt 1, 105–112. [Google Scholar] [CrossRef]
- de Bree, R.; Deurloo, E.E.; Snow, G.B.; Leemans, C.R. Screening for distant metastases in patients with head and neck cancer. Laryngoscope 2000, 110 Pt 1, 397–401. [Google Scholar] [CrossRef]
- Warner, G.C.; Cox, G.J. Evaluation of chest radiography versus chest computed tomography in screening for pulmonary malignancy in advanced head and neck cancer. J. Otolaryngol. 2003, 32, 107–109. [Google Scholar] [CrossRef]
- Kaanders, J.H.; Hordijk, G.J. Dutch Cooperative Head and Neck Oncology Group. Carcinoma of the larynx: The Dutch national guideline for diagnostics, treatment, supportive care and rehabilitation. Radiother. Oncol. 2002, 63, 299–307. [Google Scholar] [CrossRef]
- Rezk, M.; Nasr, I.; Ali, I.; Abdelhamed, H. Comparative Study between 18F FDG-PET/CT and Whole Body MRI DWIBS in Assessment of Recurrent Breast Cancer (Prospective, Comparative, Cross-sectional Study Design). Indian J. Nucl. Med. 2019, 34, 1–9. [Google Scholar]
- Civantos, F.J.; Vermorken, J.B.; Shah, J.P.; Rinaldo, A.; Suárez, C.; Kowalski, L.P.; Rodrigo, J.P.; Olsen, K.; Strojan, P.; Mäkitie, A.A.; et al. Metastatic Squamous Cell Carcinoma to the Cervical Lymph Nodes From an Unknown Primary Cancer: Management in the HPV Era. Front. Oncol. 2020, 10, 593164. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Esser, M.; Brecht, I.; Tsiflikas, I.; Schäfer, J.F. Ganzkörper-MRT bei Tumorprädispositionssyndromen [Whole-body MRI in cancer predisposition syndromes]. Radiologie, 2022; Epub ahead of print. (In German). [Google Scholar] [CrossRef]
| Alcohol Consumption (n/%) | History of Smoking (n/%) | HPV+ (n/%) |
|---|---|---|
| 13/43% | 21/70% | 6/20% |
| Patient Number | Diagnosis | tnm Stage | Histopathology |
|---|---|---|---|
| 1 | tonsil carcinoma | pT1 cN2b cM0 | squamous cell carcinoma |
| 2 | laryngeal carcinoma | cT2 cN1 cM0 | squamous cell carcinoma |
| 3 | tonsil carcinoma | pT1 pN3b cM0 | squamous cell carcinoma |
| 4 | bronchial carcinoma | not applicable | adenocarcinoma |
| 5 | CUP syndrome | pTx pN+ cM0 | squamous cell carcinoma |
| 6 | CUP syndrome | pTx pN+ cM0 | squamous cell carcinoma |
| 7 | parotid cancer | pT3 pN2b cM0 | squamous cell carcinoma |
| 8 | laryngeal carcinoma | cT4a cN2a cM0 | squamous cell carcinoma |
| 9 | tonsil carcinoma | pT1 pN2b cM0 | squamous cell carcinoma |
| 10 | hypopharyngeal carcinoma | cT4a cN2b cM0 | squamous cell carcinoma |
| 11 | tonsil carcinoma | cT4a cN2c cM0 | squamous cell carcinoma |
| 12 | CUP syndrome | pTx pN+ cM0 | squamous cell carcinoma |
| 13 | tongue base carcinoma | pT1 pN2b cM0 | squamous cell carcinoma |
| 14 | CUP syndrome | pTx pN+ cM0 | squamous cell carcinoma |
| 15 | tonsil carcinoma | cT2 cN2c cM0 | squamous cell carcinoma |
| 16 | lymphoma | not applicable | anaplastic large cell lymphoma |
| 17 | tonsil carcinoma | pT1 pN2b cM0 | squamous cell carcinoma |
| 18 | leukemia | not applicable | acute myeloid leukemia |
| 19 | bronchial carcinoma | not applicable | squamous cell carcinoma |
| 20 | hypopharyngeal carcinoma | pT2 pN3b cM1 | squamous cell carcinoma |
| 21 | tongue base carcinoma | pT1 cN2b cM0 | squamous cell carcinoma |
| 22 | metastatic melanoma | not applicable | malignant melanoma |
| 23 | CUP syndrome | pTx pN+ cM0 | squamous cell carcinoma |
| 24 | hypopharyngeal carcinoma | cT3 cN2b cM0 | squamous cell carcinoma |
| 25 | thyroid carcinoma | pT4a pN1b cM1 | follicular thyroid carcinoma |
| 26 | lymphoma | not applicable | follicular b-cell lymphoma |
| 27 | epiglottic carcinoma | cT1 cN2b cM0 | squamous cell carcinoma |
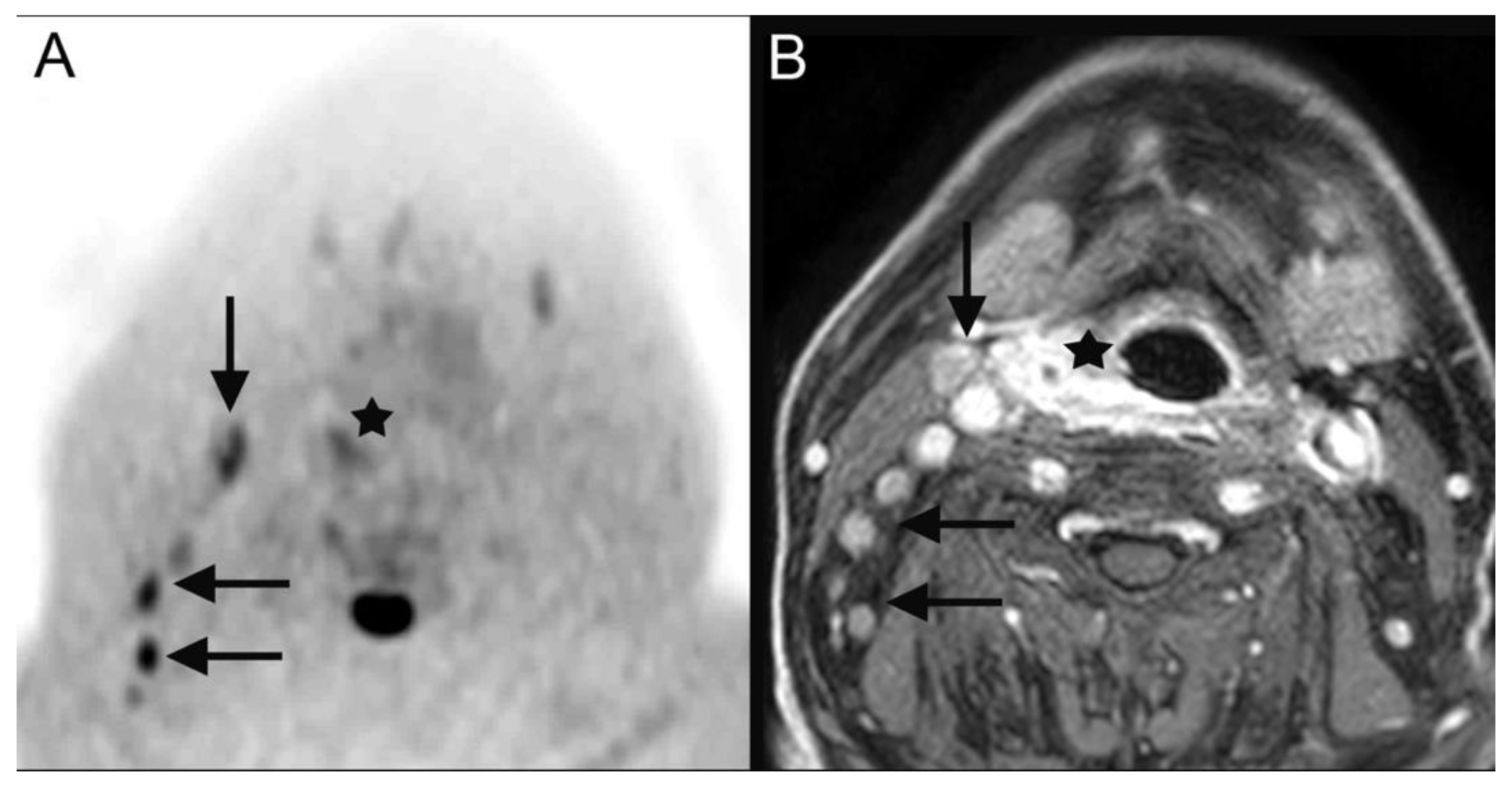
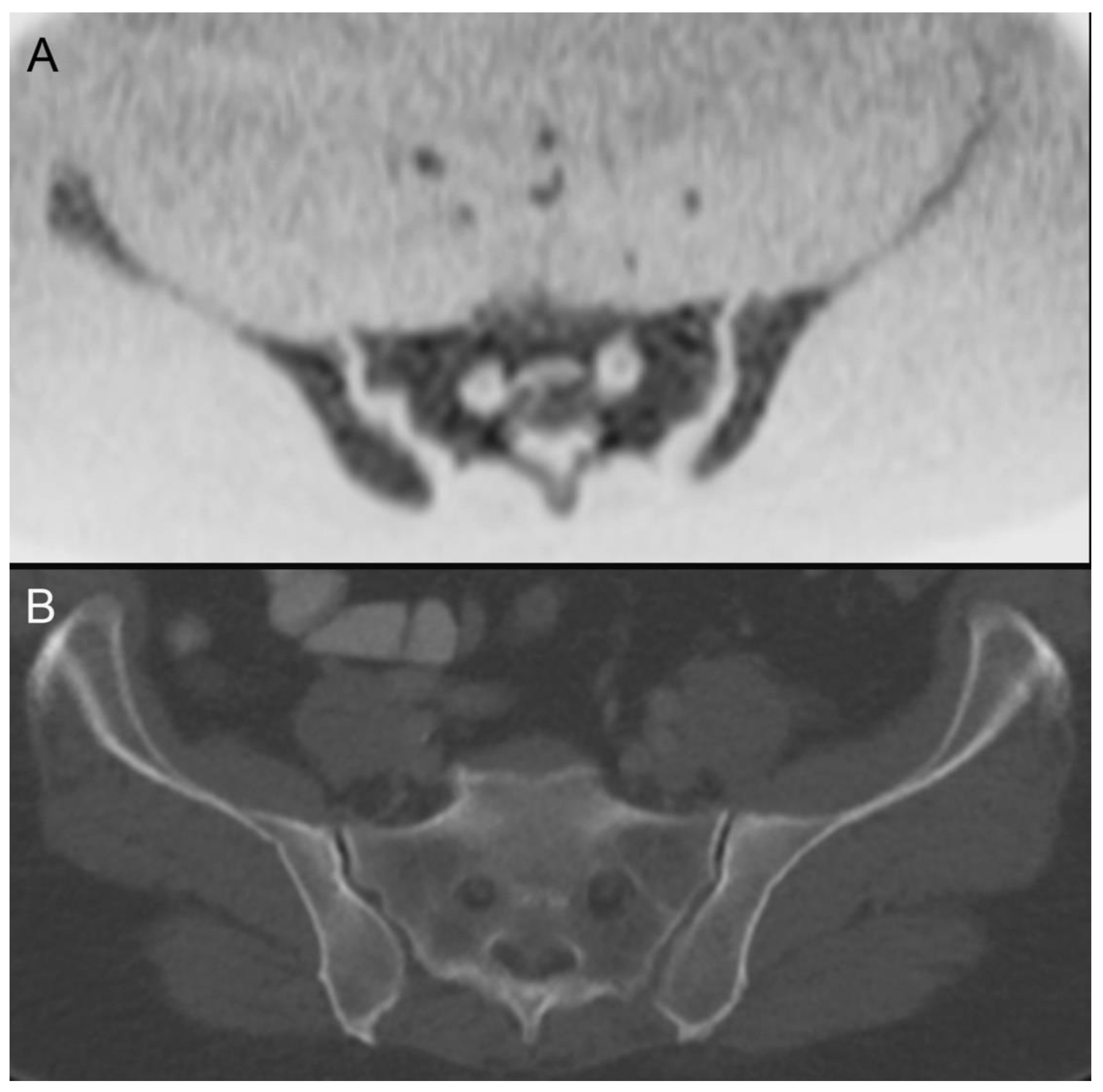
| 28 | hypopharyngeal carcinoma (Figure 2) | pT2 pN2b cM0 | squamous cell carcinoma |
| 29 | hypopharyngeal carcinoma | cT4a cN2c cM0 | squamous cell carcinoma |
| 30 | sinunasal carcinoma | cT2 cN2b cM0 | neuroendocrine carcinoma |
| Patient Number | Additional Diagnosis CT Only | Additional Diagnosis DWIBS Only | Additional Diagnosis in Both Modalities |
|---|---|---|---|
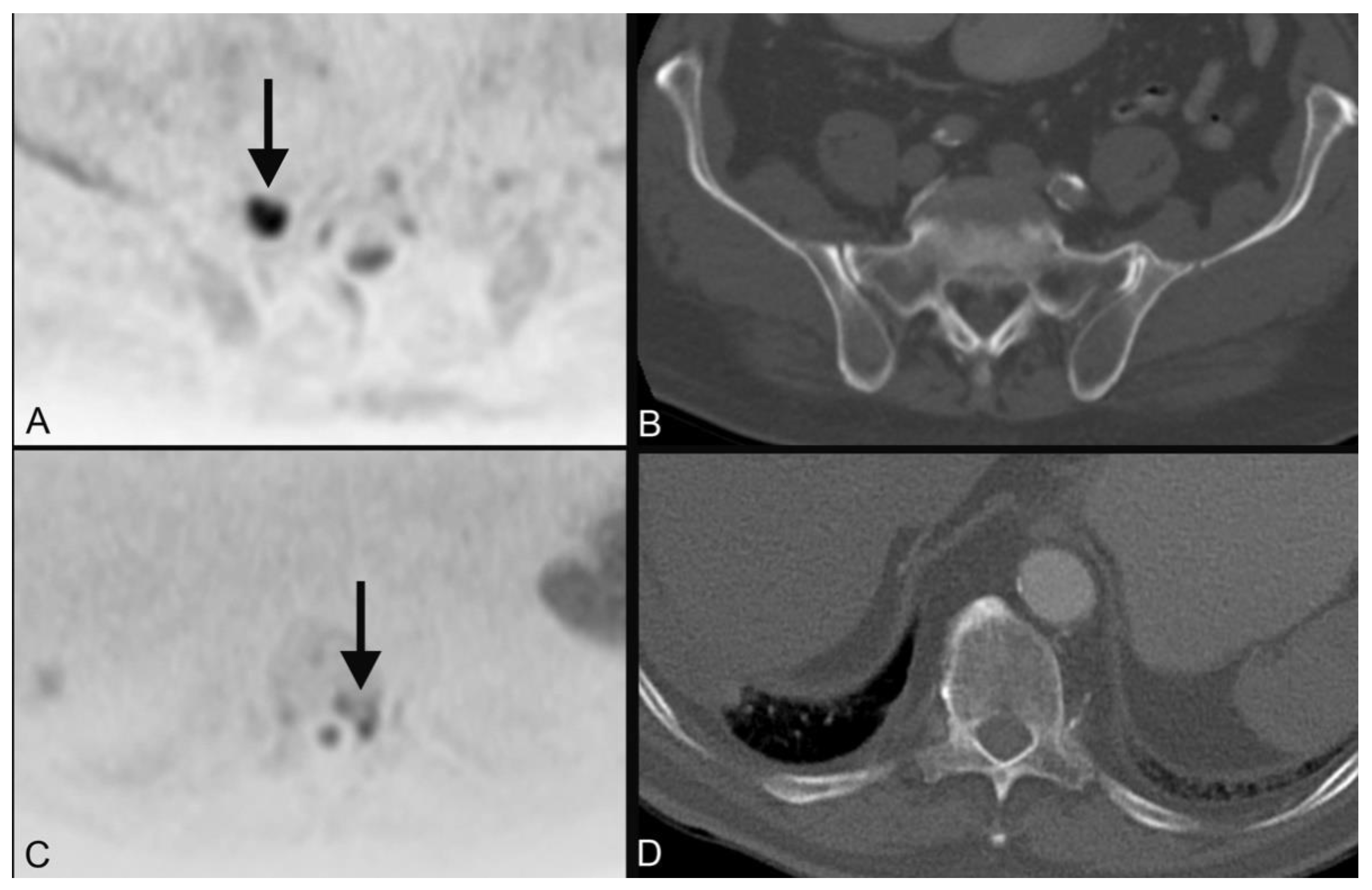
| 1 | plasmocytoma (Figure 3) | ||
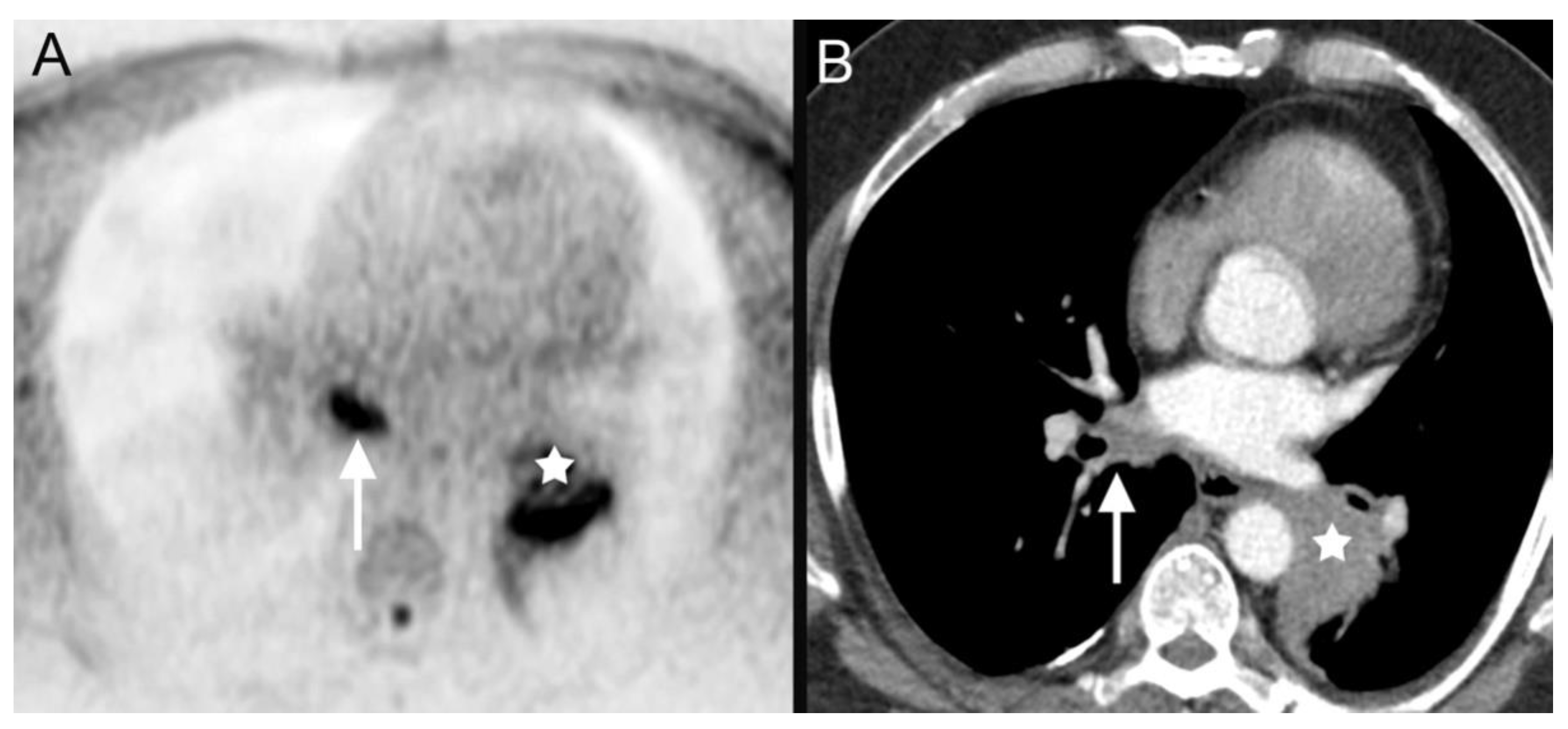
| 4 | bronchial carcinoma (Figure 4) | ||
| 10 | esophageal cancer | ||
| 16 | breast lymphoma | ||
| 18 | leukemia (Figure 5) | ||
| 19 | bronchial carcinoma | ||
| 20 | single liver metastases | ||
| 22 | several metastases of malignant melanoma | ||
| 25 | several metastases of thyroid cancer |
| Patient Number | Diagnosis | Therapeutic Decision (Multidisciplinary Tumor Conference) |
|---|---|---|
| 1 | tonsil carcinoma | surgical resection, adjuvant chemoradiotherapy |
| 2 | laryngeal carcinoma | primary chemoradiotherapy |
| 3 | tonsil carcinoma | surgical resection, adjuvant chemoradiotherapy |
| 4 | bronchial carcinoma | systemic chemotherapy |
| 5 | CUP syndrome | primary chemoradiotherapy |
| 6 | CUP syndrome | primary chemoradiotherapy |
| 7 | parotid cancer | surgical resection only as patient denies chemotherapy as well as radiotherapy |
| 8 | laryngeal carcinoma | primary chemoradiotherapy as patient denies tracheotomy |
| 9 | tonsil carcinoma | surgical resection, adjuvant chemoradiotherapy |
| 10 | hypopharyngeal carcinoma | palliative radiotherapy |
| 11 | tonsil carcinoma | best supportive care |
| 12 | CUP syndrome | primary chemoradiotherapy |
| 13 | tongue base carcinoma | surgical resection, adjuvant chemoradiotherapy |
| 14 | CUP syndrome | primary chemoradiotherapy |
| 15 | tonsil carcinoma | primary chemoradiotherapy |
| 16 | lymphoma | chemoradiotherapy |
| 17 | tonsil carcinoma | surgical resection, adjuvant chemoradiotherapy |
| 18 | leukemia | chemotherapy |
| 19 | bronchial carcinoma | systemic chemotherapy |
| 20 | hypopharyngeal carcinoma | palliative chemotherapy |
| 21 | tongue base carcinoma | surgical resection, adjuvant chemoradiotherapy |
| 22 | metastatic melanoma | immunotherapy |
| 23 | CUP syndrome | primary chemoradiotherapy |
| 24 | hypopharyngeal carcinoma | primary chemoradiotherapy |
| 25 | thyroid carcinoma | surgical resection and radio-iodine treatment |
| 26 | lymphoma | chemoradiotherapy |
| 27 | epiglottic carcinoma | surgical resection, adjuvant chemoradiotherapy |
| 28 | hypopharyngeal carcinoma (Figure 2) | primary chemoradiotherapy |
| 29 | hypopharyngeal carcinoma | best supportive care |
| 30 | sinunasal carcinoma | neoadjuvant chemoradiotherapy, surgical resection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schicho, A.; Habicher, W.; Wendl, C.; Stroszczynski, C.; Strotzer, Q.; Dollinger, M.; Schreyer, A.G.; Schleder, S. Clinical Value of Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS) for Staging of Patients with Suspected Head and Neck Cancer. Tomography 2022, 8, 2522-2532. https://doi.org/10.3390/tomography8050210
Schicho A, Habicher W, Wendl C, Stroszczynski C, Strotzer Q, Dollinger M, Schreyer AG, Schleder S. Clinical Value of Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS) for Staging of Patients with Suspected Head and Neck Cancer. Tomography. 2022; 8(5):2522-2532. https://doi.org/10.3390/tomography8050210
Chicago/Turabian StyleSchicho, Andreas, Werner Habicher, Christina Wendl, Christian Stroszczynski, Quirin Strotzer, Marco Dollinger, Andreas G. Schreyer, and Stephan Schleder. 2022. "Clinical Value of Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS) for Staging of Patients with Suspected Head and Neck Cancer" Tomography 8, no. 5: 2522-2532. https://doi.org/10.3390/tomography8050210
APA StyleSchicho, A., Habicher, W., Wendl, C., Stroszczynski, C., Strotzer, Q., Dollinger, M., Schreyer, A. G., & Schleder, S. (2022). Clinical Value of Diffusion-Weighted Whole-Body Imaging with Background Body Signal Suppression (DWIBS) for Staging of Patients with Suspected Head and Neck Cancer. Tomography, 8(5), 2522-2532. https://doi.org/10.3390/tomography8050210





