Comparison of 18F-fluorothymidine Positron Emission Tomography/Computed Tomography and 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Patients with Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. PET/CT Protocol
2.2. PET/CT Analysis
2.3. Statistical Analysis
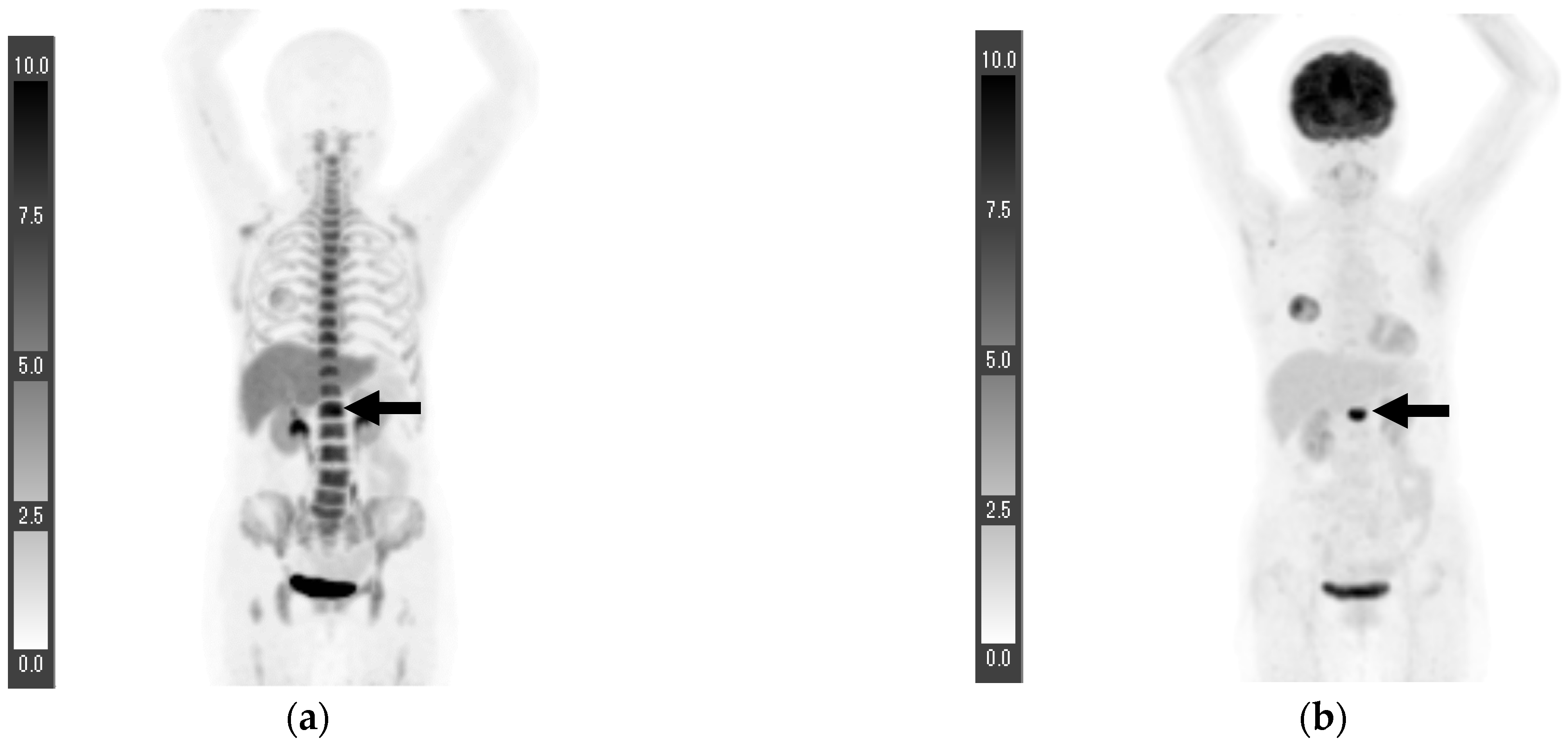
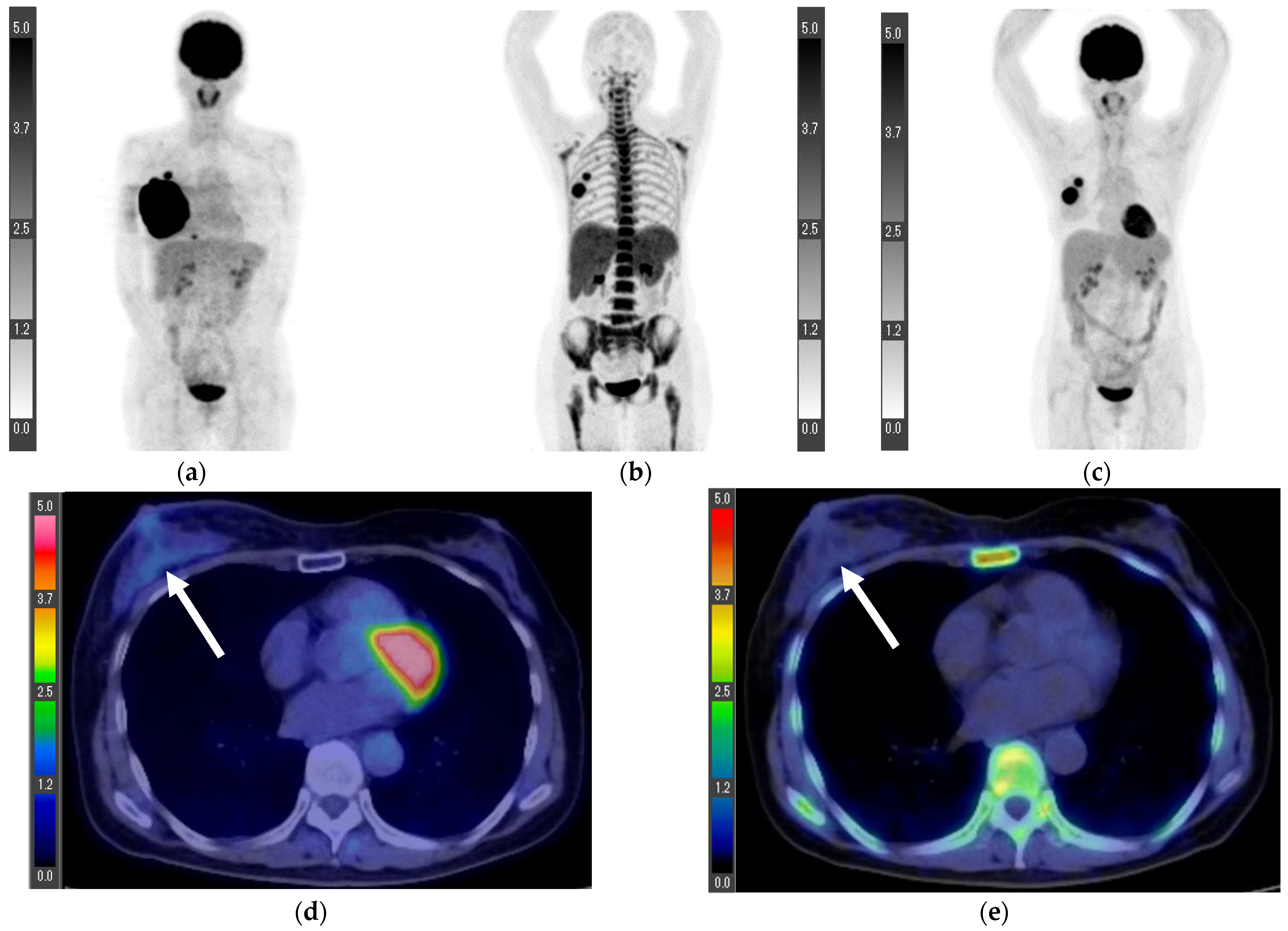
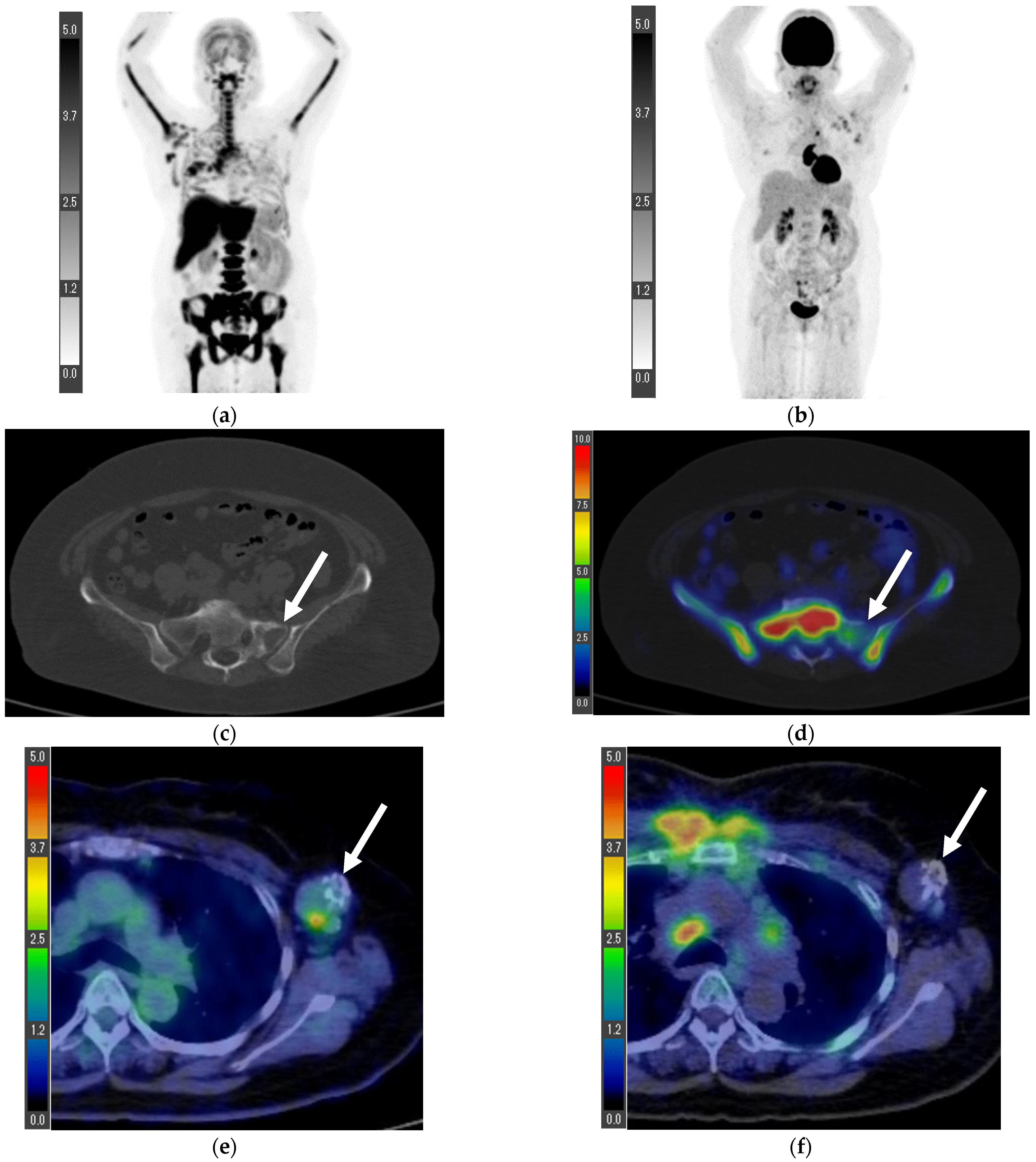
3. Results
3.1. Surgical Cases
3.2. Inoperable Cases
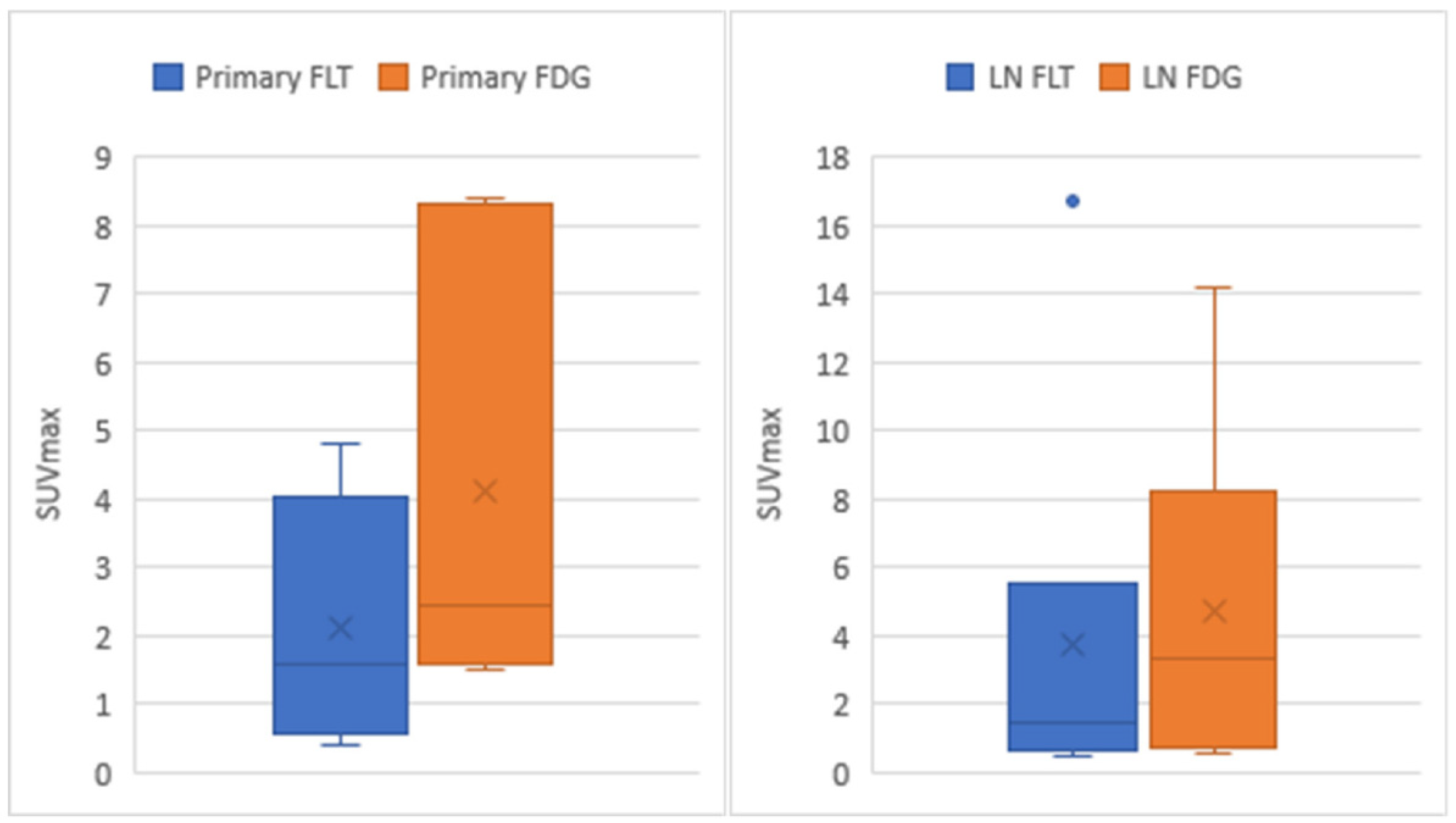
3.3. Statistical Analysis
3.4. Patients with Prior Treatment before PET/CT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, A.; Chavez-Mac Gregor, M.; Lichtensztajn, D.Y.; Yi, M.; Tadros, A.; Hortobagyi, G.N.; Giordano, S.H.; Hunt, K.K.; Mittendorf, E.A. Validation study of the American Joint Committee on Cancer Eighth Edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018, 4, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Fujioka, T.; Katsuta, L.; Tsuchiya, J.; Kubota, K.; Kasahara, M.; Oda, G.; Nakagawa, T.; Onishi, I.; Tateishi, U. Diagnostic performance of time-of-flight PET/CT for evaluating nodal metastasis of the axilla in breast cancer. Nucl. Med. Commun. 2019, 40, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Fujioka, T.; Kubota, K.; Katsuta, L.; Yashima, Y.; Nomura, K.; Yamaga, E.; Tsuchiya, J.; Hosoya, T.; Oda, G.; et al. Relationship between prognostic stage in breast cancer and fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography. J. Clin. Med. 2021, 10, 3173. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, T.; Aogi, K.; Kiyoto, S.; Masumoto, N.; Sugawara, Y.; Okada, M. Role of maximum standardized uptake value in fluorodeoxyglucose positron emission tomography/computed tomography predicts malignancy grade and prognosis of operable breast cancer: A multi-institute study. Breast Cancer Res. Treat. 2013, 141, 269–275. [Google Scholar] [CrossRef]
- Wen, W.; Xuan, D.; Hu, Y.; Li, X.; Liu, L.; Xu, D. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with breast cancer: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0225959. [Google Scholar] [CrossRef]
- Kenny, L.; Coombes, R.C.; Vigushin, D.M.; Al-Nahhas, A.; Shousha, S.; Aboagye, E.O. Imaging early changes in proliferation at 1 week post chemotherapy: A pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1339–1347. [Google Scholar] [CrossRef]
- Shields, A.F.; Grierson, J.R.; Dohmen, B.M.; Machulla, H.J.; Stayanoff, J.C.; Lawhorn-Crews, J.M.; Obradovich, J.E.; Muzik, O.; Mangner, T.J. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998, 4, 1334–1336. [Google Scholar] [CrossRef]
- Been, L.B.; Suurmeijer, A.J.; Cobben, D.C.; Jager, P.L.; Hoekstra, H.J.; Elsinga, P.H. [18F]FLT-PET in oncology: Current status and opportunities. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1659–1672. [Google Scholar] [CrossRef]
- Chen, W.; Cloughesy, T.; Kamdar, N.; Satyamurthy, N.; Bergsneider, M.; Liau, L.; Mischel, P.; Czernin, J.; Phelps, M.E.; Silverman, D.H. Imaging proliferation in brain tumors with 18F-FLT PET: Comparison with 18F-FDG. J. Nucl. Med. 2005, 46, 945–952. [Google Scholar]
- Dittmann, H.; Dohmen, B.M.; Paulsen, F.; Eichhorn, K.; Eschmann, S.M.; Horger, M.; Wehrmann, M.; Machulla, H.J.; Bares, R. [18F]FLT PET for diagnosis and staging of thoracic tumours. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1407–1412. [Google Scholar] [CrossRef]
- Buck, A.K.; Halter, G.; Schirrmeister, H.; Kotzerke, J.; Wurziger, I.; Glatting, G.; Mattfeldt, T.; Neumaier, B.; Reske, S.N.; Hetzel, M. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J. Nucl. Med. 2003, 44, 1426–1431. [Google Scholar] [PubMed]
- Hoshikawa, H.; Nishiyama, Y.; Kishino, T.; Yamamoto, Y.; Haba, R.; Mori, N. Comparison of FLT-PET and FDG-PET for visualization of head and neck squamous cell cancers. Mol. Imaging Biol. 2011, 13, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Nishiyama, Y.; Ishikawa, S.; Nakano, J.; Chang, S.S.; Bandoh, S.; Kanaji, N.; Haba, R.; Kushida, Y.; Ohkawa, M. Correlation of 18F-FLT and 18F-FDG uptake on PET with Ki-67 immunohistochemistry in non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, R.; Yamamoto, Y.; Izuishi, K.; Takebayashi, R.; Hagiike, M.; Murota, M.; Kaji, M.; Haba, R.; Nishiyama, Y. Detection of gastric cancer using 18F-FLT PET: Comparison with 18F-FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 382–388. [Google Scholar] [CrossRef] [PubMed]
- van Westreenen, H.L.; Cobben, D.C.; Jager, P.L.; van Dullemen, H.M.; Wesseling, J.; Elsinga, P.H.; Plukker, J.T. Comparison of 18F-FLT PET and 18F-FDG PET in esophageal cancer. J. Nucl. Med. 2005, 46, 400–404. [Google Scholar] [PubMed]
- Han, D.; Yu, J.; Zhong, X.; Fu, Z.; Mu, D.; Zhang, B.; Xu, G.; Yang, W.; Zhao, S. Comparison of the diagnostic value of 3-deoxy-3-18F-fluorothymidine and 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the assessment of regional lymph node in thoracic esophageal squamous cell carcinoma: A pilot study. Dis. Esophagus 2012, 25, 416–426. [Google Scholar] [CrossRef]
- van Waarde, A.; Cobben, D.C.; Suurmeijer, A.J.; Maas, B.; Vaalburg, W.; de Vries, E.F.; Jager, P.L.; Hoekstra, H.J.; Elsinga, P.H. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J. Nucl. Med. 2004, 45, 695–700. [Google Scholar] [PubMed]
- Wesolowski, R.; Stover, D.G.; Lustberg, M.B.; Shoben, A.; Zhao, M.; Mrozek, E.; Layman, R.M.; Macrae, E.; Duan, W.; Zhang, J.; et al. Phase I study of veliparib on an intermittent and continuous schedule in combination with carboplatin in metastatic breast cancer: A safety and [18F]-fluorothymidine positron emission tomography biomarker study. Oncologist 2020, 25, e1158–e1169. [Google Scholar] [CrossRef]
- Smyczek-Gargya, B.; Fersis, N.; Dittmann, H.; Vogel, U.; Reischl, G.; Machulla, H.J.; Wallwiener, D.; Bares, R.; Dohmen, B.M. PET with [18F]fluorothymidine for imaging of primary breast cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 720–724. [Google Scholar] [CrossRef]
- Contractor, K.B.; Kenny, L.M.; Stebbing, J.; Rosso, L.; Ahmad, R.; Jacob, J.; Challapalli, A.; Turkheimer, F.; Al-Nahhas, A.; Sharma, R.; et al. 3′Deoxy-3′-fluorothymidine positron emission tomography and breast cancer response to docetaxel. Clin. Cancer Res. 2011, 17, 7664–7672. [Google Scholar] [CrossRef]
- Fantini, L.; Belli, M.L.; Azzali, I.; Loi, E.; Bettinelli, A.; Feliciani, G.; Mezzenga, E.; Fedeli, A.; Asioli, S.; Paganelli, G.; et al. Exploratory analysis of 18F-3′-deoxy-3′-fluorothymidine (18F-FLT) PET/CT-based Radiomics for the early evaluation of response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Front. Oncol. 2021, 11, 601053. [Google Scholar] [CrossRef] [PubMed]
- Romine, P.E.; Peterson, L.M.; Kurland, B.F.; Byrd, D.W.; Novakova-Jiresova, A.; Muzi, M.; Specht, J.M.; Doot, R.K.; Link, J.M.; Krohn, K.A.; et al. 18F-fluorodeoxyglucose (FDG) PET or 18F-fluorothymidine (FLT) PET to assess early response to aromatase inhibitors (AI) in women with ER+ operable breast cancer in a window-of-opportunity study. Breast Cancer Res. 2021, 23, 88. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Bollineni, V.R.; Kramer, G.M.; Jansma, E.P.; Liu, Y.; Oyen, W.J. A systematic review on [18F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur. J. Cancer 2016, 55, 81–97. [Google Scholar] [CrossRef]
- Ma, C.; Li, D.; Yin, Y.; Cao, J. Comparison of characteristics of 18F-fluorodeoxyglucose and 18F-fluorothymidine PET during staging of esophageal squamous cell carcinoma. Nucl. Med. Commun. 2015, 36, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Hoshikawa, H.; Kishino, T.; Mori, T.; Nishiyama, Y.; Yamamoto, Y.; Inamoto, R.; Akiyama, K.; Mori, N. Comparison of (18). Acta Oto-Laryngol. 2012, 132, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Ma, H.; Pang, F.; Ren, P.; Kuang, A. Correlations of 18F-FDG and 18F-FLT uptake on PET with Ki-67 expression in patients with lung cancer: A meta-analysis. Acta Radiol. 2018, 59, 188–195. [Google Scholar] [CrossRef]
- Surov, A.; Meyer, H.J.; Wienke, A. Associations between PET parameters and expression of Ki-67 in breast cancer. Transl. Oncol. 2019, 12, 375–380. [Google Scholar] [CrossRef]
- Nakajo, M.; Nakajo, M.; Kajiya, Y.; Jinguji, M.; Nishimata, N.; Shimaoka, S.; Nihara, T.; Aridome, K.; Tanaka, S.; Fukukura, Y.; et al. Diagnostic performance of 18F-fluorothymidine PET/CT for primary colorectal cancer and its lymph node metastasis: Comparison with 18F-fluorodeoxyglucose PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1223–1232. [Google Scholar] [CrossRef]
- Morikawa, A.; Grkovski, M.; Patil, S.; Jhaveri, K.L.; Tang, K.; Humm, J.L.; Holodny, A.; Beal, K.; Schöder, H.; Seidman, A.D. A Phase I trial of sorafenib with whole brain radiotherapy (WBRT) in breast cancer patients with brain metastases and a correlative study of FLT-PET brain imaging. Breast Cancer Res. Treat. 2021, 188, 415–425. [Google Scholar] [CrossRef] [PubMed]
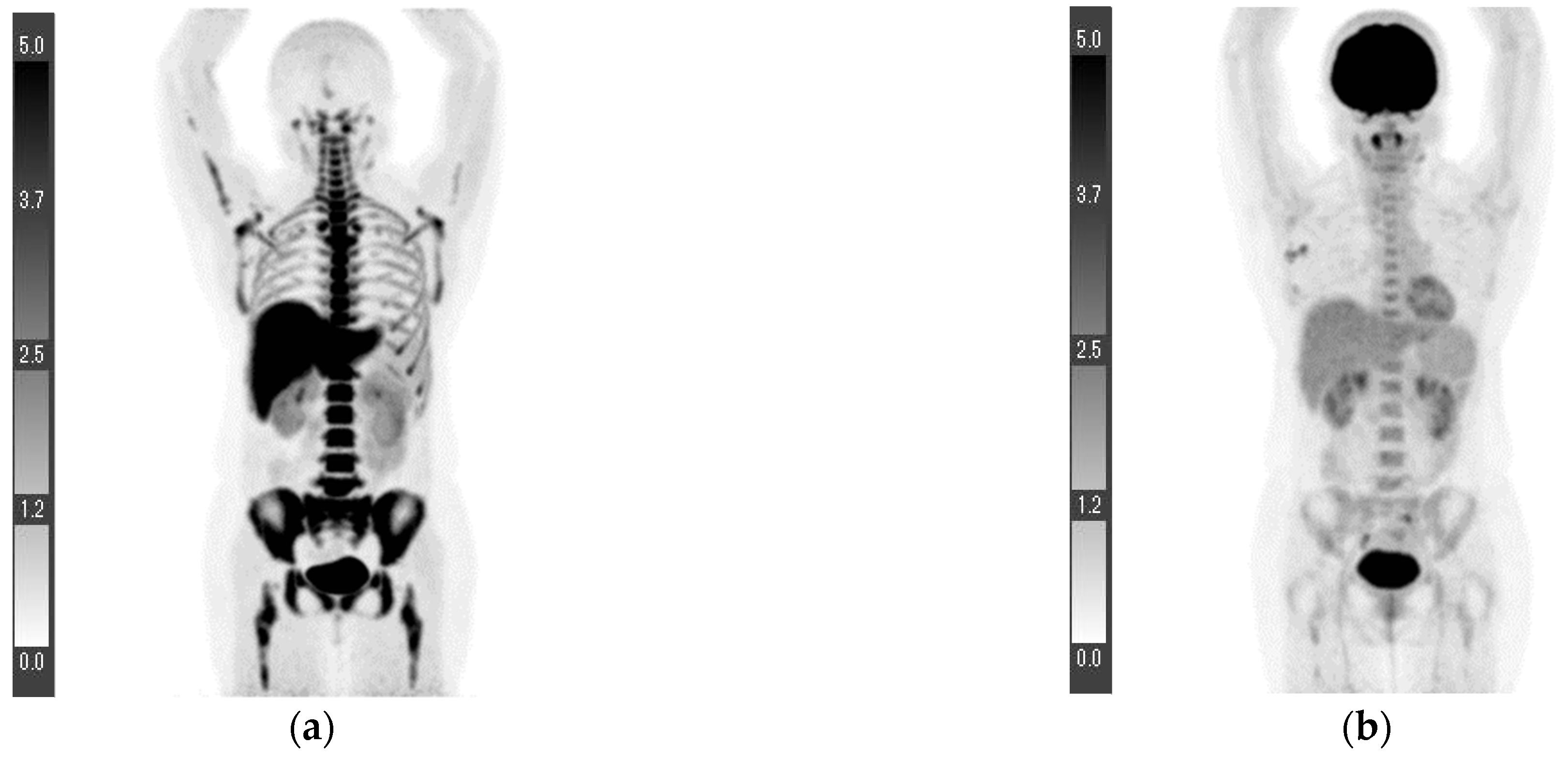
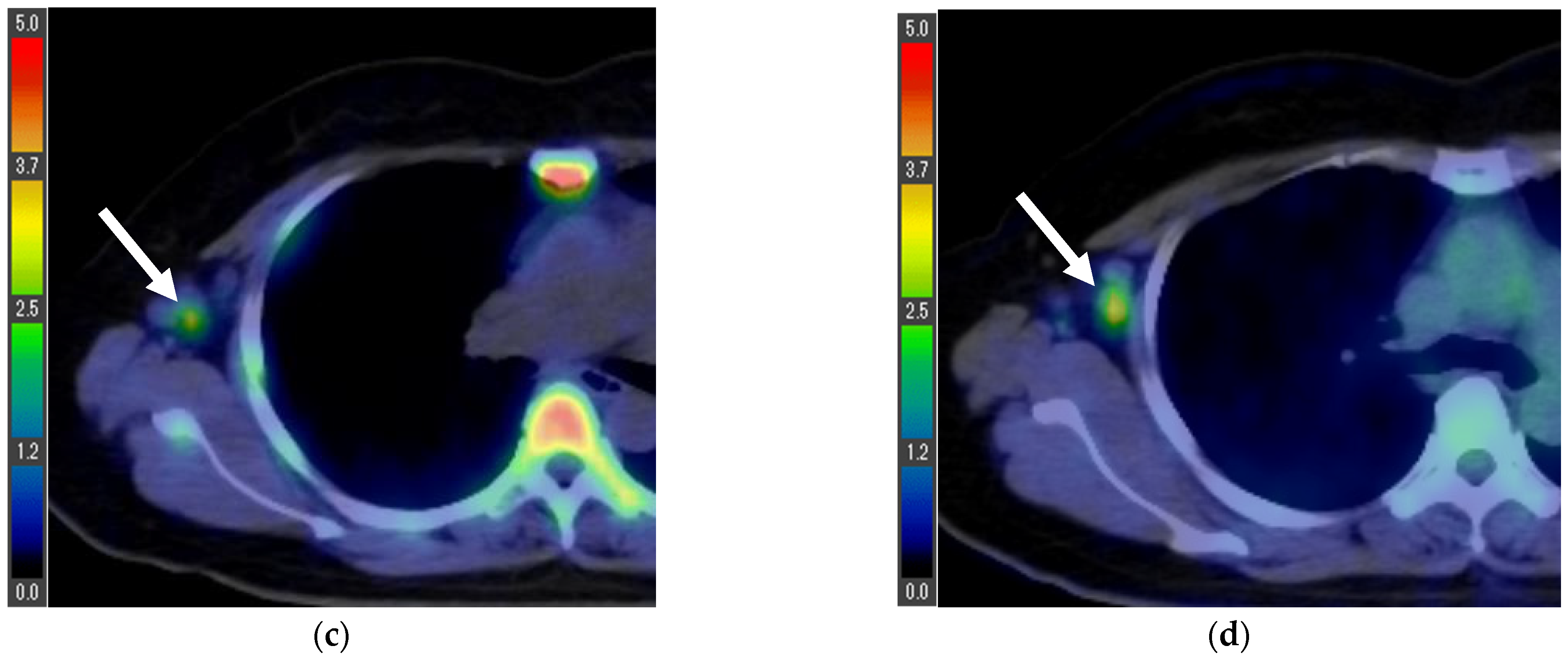
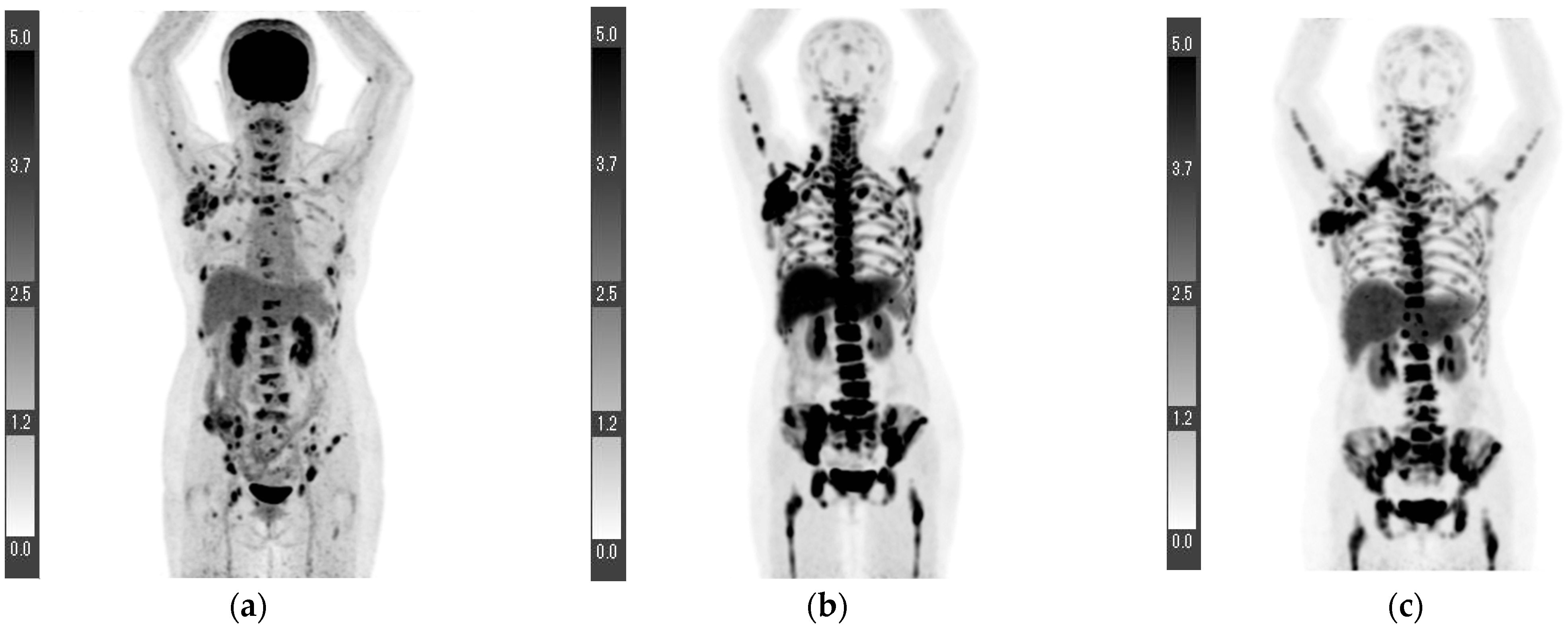
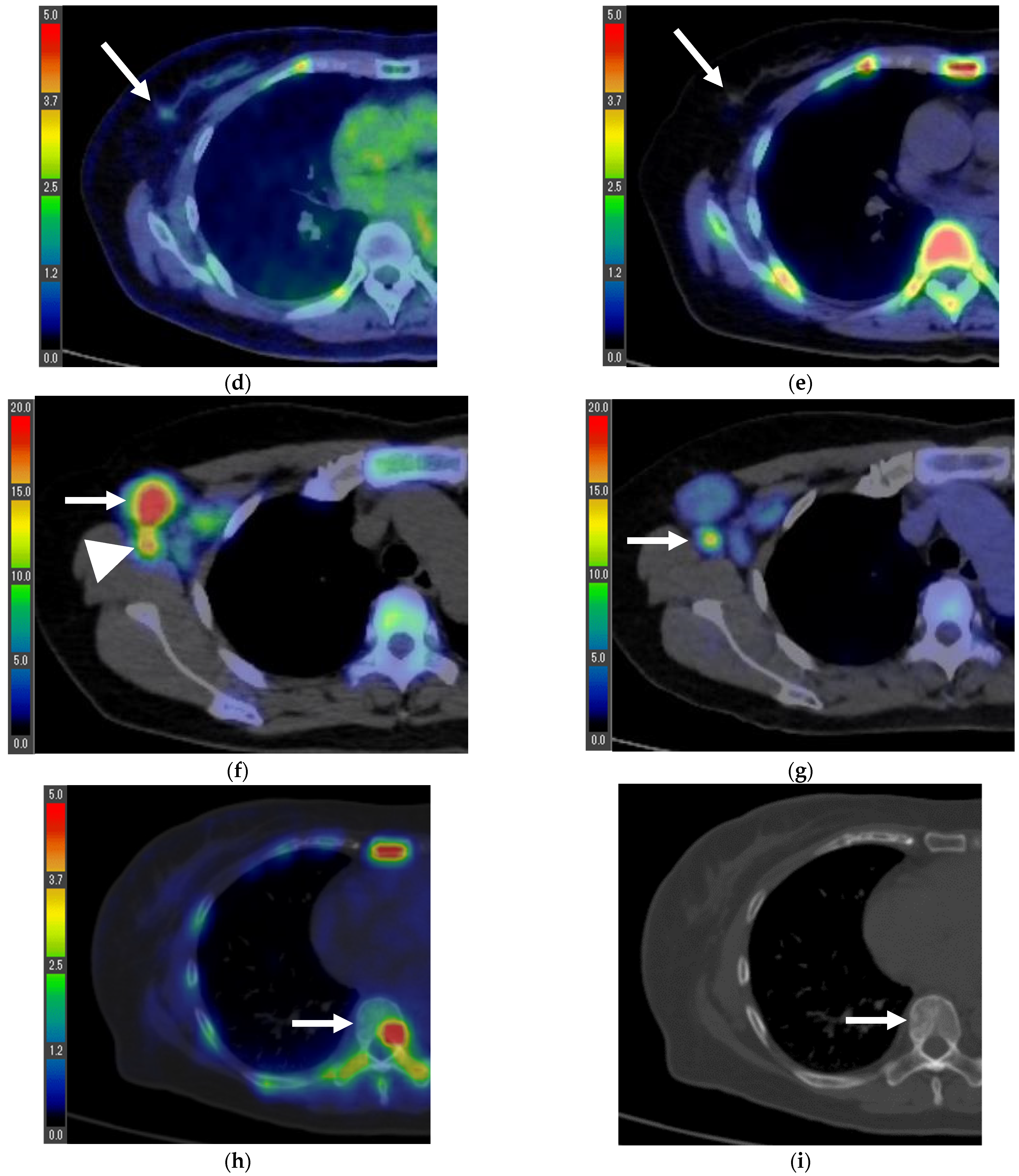
| Age (Years) | 18F-FLT PET/CT (Days) * | 18F-FDG PET/CT (Days) * | Surgery (Days) * | |
|---|---|---|---|---|
| Patient 1 | 49 | 35 | 33 | 56 |
| Patient 2 | 72 | 27 | 20 | 41 |
| Patient 3 | 73 | 21 | 18 | 27 |
| Patient 4 | 68 | 22 | 19 | - |
| Patient 5 | 71 | 7 | 19 | - |
| Patient 6 | 72 | 15 | 40 | - |
| Patient 7 | 63 | - | - | - |
| Patient 8 | 52 | - | - | - |
| Patient | Primary FLT | Primary FDG | LN FLT | LN FDG | Stage | Histological Diagnosis | Nuclear Grade | ER | PR | HER2 | Ki-67 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.0 | 1.8 | 1.6 * | 3.4 * | T1cN2aM0 ** | Invasive ductal carcinoma | 1 | + | + | − | 1.0 |
| 2 | 0.6 | 1.6 | 0.7 | 0.8 | T1bN0M0 ** | Invasive ductal carcinoma | 1 | + | + | − | 6.7 |
| 3 | 2.2 | 3.1 | 0.5 | 0.6 | T1cN0M0 ** | Invasive ductal carcinoma | 2 | + | + | − | 24.6 |
| 4 | 0.4 | 1.5 | 16.7 * | 14.2 * | T1bN3aM1 *** (Lymph node and bone metastases) | Invasive ductal carcinoma | Data loss | + | + | − | Data loss |
| 5 | 3.8 | 8.3 | 1.8 | 6.3 | T2N3bM0 *** | Invasive ductal carcinoma | 1 | + | + | − | 25 |
| 6 | 4.8 | 8.4 | 1.4 | 3.2 | T4bN1M1 *** (Lumbar vertebra metastasis) | Invasive ductal carcinoma | 1 | + | + | − | 8.9 |
| 7 **** | 7.0 | 10.1 | 1.2 | 0.7 | T4bN0M0 | Invasive ductal carcinoma | Data loss | − | − | + | 59 |
| 8 ***** | - | - | 1.1 | 4.1 | N3bM1 (Lymph node, lung, and bone metastases) | Invasive ductal carcinoma | 3 | + | − | + | 29.3 |
| Significant Difference | Correlation Coefficient | |
|---|---|---|
| Primary breast cancer | p = 0.031 * | 0.969 |
| Axillary lymph node | p = 0.246 ** | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, M.; Fujioka, T.; Ichikawa, R.; Inomata, R.; Katsuta, L.; Yashima, Y.; Yamaga, E.; Tsuchiya, J.; Hayashi, K.; Kumaki, Y.; et al. Comparison of 18F-fluorothymidine Positron Emission Tomography/Computed Tomography and 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Patients with Breast Cancer. Tomography 2022, 8, 2533-2546. https://doi.org/10.3390/tomography8050211
Mori M, Fujioka T, Ichikawa R, Inomata R, Katsuta L, Yashima Y, Yamaga E, Tsuchiya J, Hayashi K, Kumaki Y, et al. Comparison of 18F-fluorothymidine Positron Emission Tomography/Computed Tomography and 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Patients with Breast Cancer. Tomography. 2022; 8(5):2533-2546. https://doi.org/10.3390/tomography8050211
Chicago/Turabian StyleMori, Mio, Tomoyuki Fujioka, Ryota Ichikawa, Reina Inomata, Leona Katsuta, Yuka Yashima, Emi Yamaga, Junichi Tsuchiya, Kumiko Hayashi, Yuichi Kumaki, and et al. 2022. "Comparison of 18F-fluorothymidine Positron Emission Tomography/Computed Tomography and 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Patients with Breast Cancer" Tomography 8, no. 5: 2533-2546. https://doi.org/10.3390/tomography8050211
APA StyleMori, M., Fujioka, T., Ichikawa, R., Inomata, R., Katsuta, L., Yashima, Y., Yamaga, E., Tsuchiya, J., Hayashi, K., Kumaki, Y., Oda, G., Nakagawa, T., Onishi, I., Kubota, K., & Tateishi, U. (2022). Comparison of 18F-fluorothymidine Positron Emission Tomography/Computed Tomography and 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Patients with Breast Cancer. Tomography, 8(5), 2533-2546. https://doi.org/10.3390/tomography8050211






