Imaging Neurodegenerative Metabolism in Amyotrophic Lateral Sclerosis with Hyperpolarized [1-13C]pyruvate MRI
Abstract
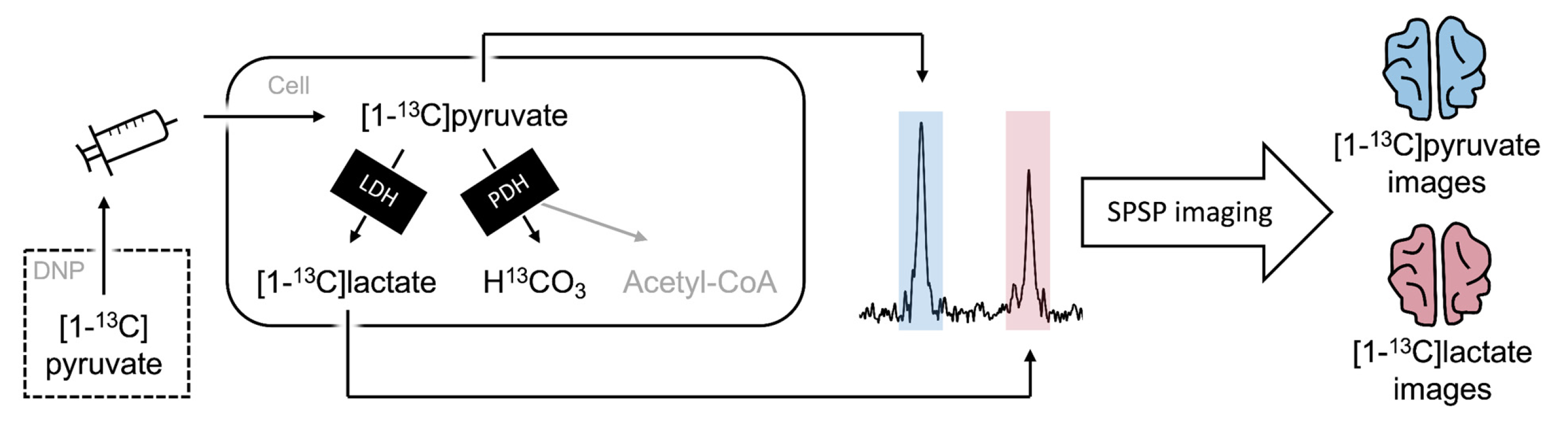
:1. Introduction
2. Case
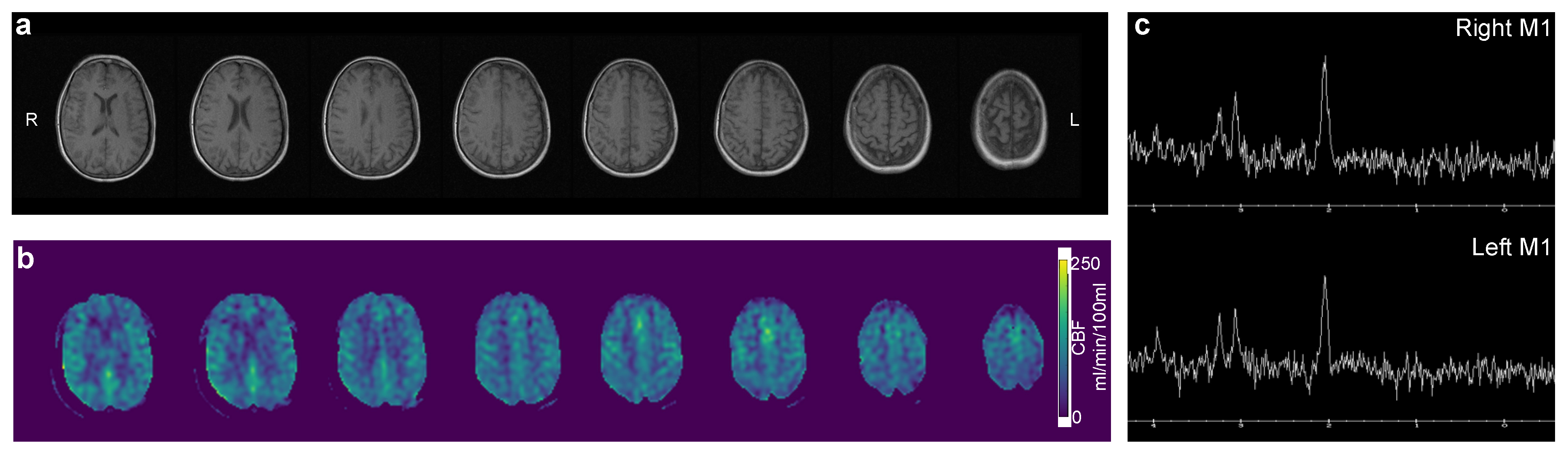
2.1. Clinical Presentation
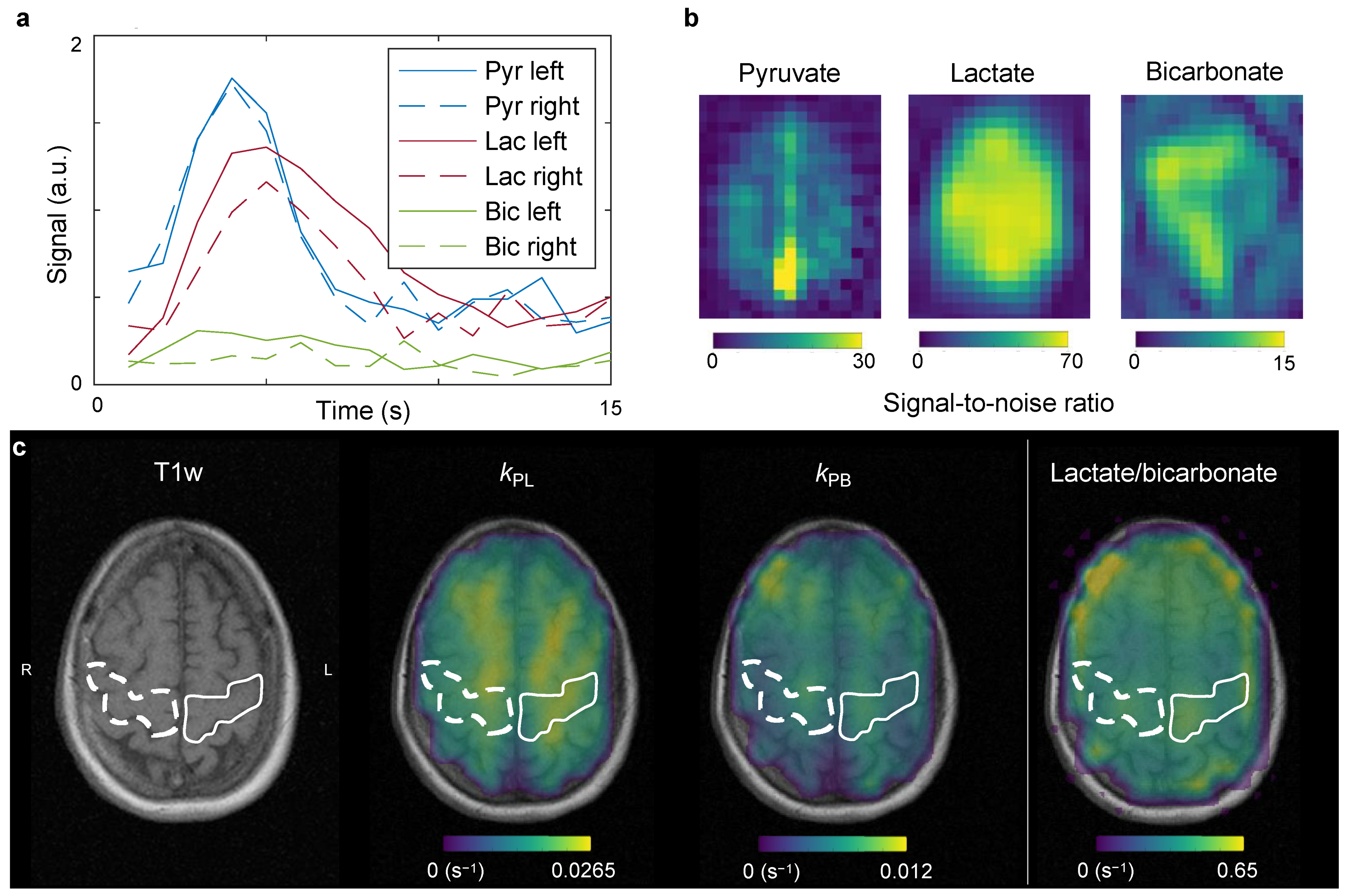
2.2. Hyperpolarized Pyruvate MRI
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.J.; Ohliger, M.A.; Larson, P.E.Z.; Gordon, J.W.; Bok, R.A.; Slater, J.; Villanueva-Meyer, J.E.; Hess, C.P.; Kurhanewicz, J.; Vigneron, D.B. Hyperpolarized 13C MRI: State of the Art and Future Directions. Radiology 2019, 291, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaeggemose, M.; F. Schulte, R.; Laustsen, C. Comprehensive Literature Review of Hyperpolarized Carbon-13 MRI: The Road to Clinical Application. Metabolites 2021, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, A.; Drory, V.; Hardiman, O.; Nakano, I.; Ravits, J.; Robberecht, W.; Shefner, J.; The WFN Research Group On ALS/MND. A revision of the El Escorial criteria. Amyotroph Lateral Scler Front. Degener 2015, 16, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Ju, T.-C.; Chen, H.-M.; Jang, Y.-S.; Lee, L.-M.; Lai, H.-L.; Tai, H.-C.; Fang, J.-M.; Lin, Y.-L.; Tu, P.-H.; et al. Activation of AMP-activated protein kinase α1 mediates mislocalization of TDP-43 in amyotrophic lateral sclerosis. Hum. Mol. Genet. 2015, 24, 787–801. [Google Scholar] [CrossRef]
- Vandoorne, T.; De Bock, K.; Van Den Bosch, L. Energy metabolism in ALS: An underappreciated opportunity? Acta Neuropathol. 2018, 135, 489–509. [Google Scholar] [CrossRef] [Green Version]
- Tefera, T.W.; Steyn, F.J.; Ngo, S.T.; Borges, K. CNS glucose metabolism in Amyotrophic Lateral Sclerosis: A therapeutic target? Cell Biosci. 2021, 11, 14. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.-N. Aerobic glycolysis in amyotrophic lateral sclerosis and Huntington’s disease. Rev. Neurosci. 2018, 29, 547–555. [Google Scholar] [CrossRef]
- Vahsen, B.F.; Gray, E.; Thompson, A.G.; Ansorge, O.; Anthony, D.C.; Cowley, S.A.; Talbot, K.; Turner, M.R. Non-neuronal cells in amyotrophic lateral sclerosis—From pathogenesis to biomarkers. Nat. Rev. Neurol. 2021, 17, 333–348. [Google Scholar] [CrossRef]
- Paganoni, S.; Macklin, E.A.; Hendrix, S.; Berry, J.D.; Elliott, M.A.; Maiser, S.; Karam, C.; Caress, J.B.; Owegi, M.A.; Quick, A.; et al. Trial of Sodium Phenylbutyrate–Taurursodiol for Amyotrophic Lateral Sclerosis. New Engl. J. Med. 2020, 383, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Tankisi, H.; Nielsen, C.S.-Z.; Howells, J.; Cengiz, B.; Samusyte, G.; Koltzenburg, M.; Blicher, J.U.; Møller, A.T.; Pugdahl, K.; Fuglsang-Frederiksen, A.; et al. Early diagnosis of amyotrophic lateral sclerosis by threshold tracking and conventional transcranial magnetic stimulation. Eur. J. Neurol. 2021, 28, 3030–3039. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Wang, S.; Melhem, E.R.; Gee, J.C.; Cucchiara, A.; McCluskey, L.; Elman, L. Linear Associations between Clinically Assessed Upper Motor Neuron Disease and Diffusion Tensor Imaging Metrics in Amyotrophic Lateral Sclerosis. PLoS ONE 2014, 9, e105753. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Goldstein, L.H.; Abrahams, S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: Nature of impairment and implications for assessment. Lancet Neurol. 2013, 12, 368–380. [Google Scholar] [CrossRef]
- Bøgh, N.; Olin, R.B.; Hansen, E.S.; Gordon, J.W.; Bech, S.K.; Bertelsen, L.B.; Sánchez-Heredia, J.D.; Blicher, J.U.; Østergaard, L.; Ardenkjær-Larsen, J.H.; et al. Metabolic MRI with hyperpolarized [1-13C]pyruvate separates benign oligemia from infarcting penumbra in porcine stroke. J. Cereb. Blood Flow Metab. 2021, 41, 2916–2927. [Google Scholar] [CrossRef]
- Schulte, R.F.; Sperl, J.I.; Weidl, E.; Menzel, M.I.; Janich, M.A.; Khegai, O.; Durst, M.; Ardenkjaer-Larsen, J.H.; Glaser, S.J.; Haase, A.; et al. Saturation-recovery metabolic-exchange rate imaging with hyperpolarized [1-13C] pyruvate using spectral-spatial excitation. Magn. Reson. Med. 2013, 69, 1209–1216. [Google Scholar] [CrossRef] [Green Version]
- Larson, P.E.Z.; Chen, H.-Y.; Gordon, J.W.; Korn, N.; Maidens, J.; Arcak, M.; Tang, S.; Criekinge, M.; Carvajal, L.; Mammoli, D.; et al. Investigation of analysis methods for hyperpolarized 13C-pyruvate metabolic MRI in prostate cancer patients. NMR Biomed. 2018, 31, e3997. [Google Scholar] [CrossRef]
- Grist, J.T.; Hansen, E.S.S.; Sánchez-Heredia, J.D.; McLean, M.A.; Tougaard, R.; Riemer, F.; Schulte, R.F.; Kaggie, J.D.; Ardenkjaer-Larsen, J.H.; Laustsen, C.; et al. Creating a clinical platform for carbon-13 studies using the sodium-23 and proton resonances. Magn. Reson. Med. 2020, 84, 1817–1827. [Google Scholar] [CrossRef] [Green Version]
- Grist, J.T.; McLean, M.A.; Riemer, F.; Schulte, R.F.; Deen, S.S.; Zaccagna, F.; Woitek, R.; Daniels, C.J.; Kaggie, J.D.; Matys, T.; et al. Quantifying normal human brain metabolism using hyperpolarized [1-13C]pyruvate and magnetic resonance imaging. Neuroimage 2019, 189, 171–179. [Google Scholar] [CrossRef]
- Guglielmetti, C.; Najac, C.; Didonna, A.; Van der Linden, A.; Ronen, S.M.; Chaumeil, M.M. Hyperpolarized 13C MR metabolic imaging can detect neuroinflammation in vivo in a multiple sclerosis murine model. Proc. Natl. Acad. Sci. USA 2017, 114, E6982–E6991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beers, D.R.; Appel, S.H. Immune dysregulation in amyotrophic lateral sclerosis: Mechanisms and emerging therapies. Lancet Neurol. 2019, 18, 211–220. [Google Scholar] [CrossRef]
- Mason, S. Lactate Shuttles in Neuroenergetics—Homeostasis, Allostasis and Beyond. Front. Neurosci. 2017, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Magistretti, P.J.; Pellerin, L. Cellular Bases of Brain Energy Metabolism and Their Relevance to Functional Brain Imaging: Evidence for a Prominent Role of Astrocytes. Cereb. Cortex 1996, 6, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bøgh, N.; Grist, J.T.; Rasmussen, C.W.; Bertelsen, L.B.; Hansen, E.S.S.; Blicher, J.U.; Tyler, D.J.; Laustsen, C. Lactate saturation limits bicarbonate detection in hyperpolarized 13C-pyruvate MRI of the brain. Magn. Reson. Med. 2022, 1–10. [Google Scholar] [CrossRef]
- Autry, A.W.; Gordon, J.W.; Carvajal, L.; Mareyam, A.; Chen, H.-Y.; Park, I.; Mammoli, D.; Vareth, M.; Chang, S.M.; Wald, L.L.; et al. Comparison between 8- and 32-channel phased-array receive coils for in vivo hyperpolarized 13C imaging of the human brain. Magn. Reson. Med. 2019, 82, 833–841. [Google Scholar] [CrossRef]
- Bøgh, N.; Gordon, J.W.; Hansen, E.S.S.; Bok, R.A.; Blicher, J.U.; Hu, J.Y.; Larson, P.E.Z.; Vigneron, D.B.; Laustsen, C. Initial Experience on Hyperpolarized [1-13C]Pyruvate MRI Multicenter Reproducibility—Are Multicenter Trials Feasible? Tomography 2022, 8, 585–595. [Google Scholar] [CrossRef]
- Capozzi, A.; Cheng, T.; Boero, G.; Roussel, C.; Comment, A. Thermal annihilation of photo-induced radicals following dynamic nuclear polarization to produce transportable frozen hyperpolarized 13C-substrates. Nat. Commun. 2017, 8, 15757. [Google Scholar] [CrossRef] [Green Version]
- Hövener, J.-B.; Pravdivtsev, A.N.; Kidd, B.; Bowers, C.R.; Glöggler, S.; Kovtunov, K.V.; Plaumann, M.; Katz-Brull, R.; Buckenmaier, K.; Jerschow, A.; et al. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew. Chem. Int. Ed. Engl. 2018, 57, 11140–11162. [Google Scholar] [CrossRef]
- Patel, S.; Pinon, A.C.; Lerche, M.H.; Karlsson, M.; Capozzi, A.; Ardenkj, J.H. UV-Irradiated 2-Keto-(1-13C)Isocaproic Acid for High-Performance 13C Hyperpolarized MR. J. Phys. Chem. C 2020, 124, 23859–23866. [Google Scholar] [CrossRef]
- Le Page, L.M.; Guglielmetti, C.; Taglang, C.; Chaumeil, M.M. Imaging Brain Metabolism Using Hyperpolarized 13C Magnetic Resonance Spectroscopy. Trends Neurosci. 2020, 43, 343–354. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bøgh, N.; Laustsen, C.; Hansen, E.S.S.; Tankisi, H.; Bertelsen, L.B.; Blicher, J.U. Imaging Neurodegenerative Metabolism in Amyotrophic Lateral Sclerosis with Hyperpolarized [1-13C]pyruvate MRI. Tomography 2022, 8, 1570-1577. https://doi.org/10.3390/tomography8030129
Bøgh N, Laustsen C, Hansen ESS, Tankisi H, Bertelsen LB, Blicher JU. Imaging Neurodegenerative Metabolism in Amyotrophic Lateral Sclerosis with Hyperpolarized [1-13C]pyruvate MRI. Tomography. 2022; 8(3):1570-1577. https://doi.org/10.3390/tomography8030129
Chicago/Turabian StyleBøgh, Nikolaj, Christoffer Laustsen, Esben S. S. Hansen, Hatice Tankisi, Lotte B. Bertelsen, and Jakob U. Blicher. 2022. "Imaging Neurodegenerative Metabolism in Amyotrophic Lateral Sclerosis with Hyperpolarized [1-13C]pyruvate MRI" Tomography 8, no. 3: 1570-1577. https://doi.org/10.3390/tomography8030129
APA StyleBøgh, N., Laustsen, C., Hansen, E. S. S., Tankisi, H., Bertelsen, L. B., & Blicher, J. U. (2022). Imaging Neurodegenerative Metabolism in Amyotrophic Lateral Sclerosis with Hyperpolarized [1-13C]pyruvate MRI. Tomography, 8(3), 1570-1577. https://doi.org/10.3390/tomography8030129





