Quantitative Chest CT Analysis to Measure Short-Term Sequelae of COVID-19 Pneumonia: A Monocentric Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Design
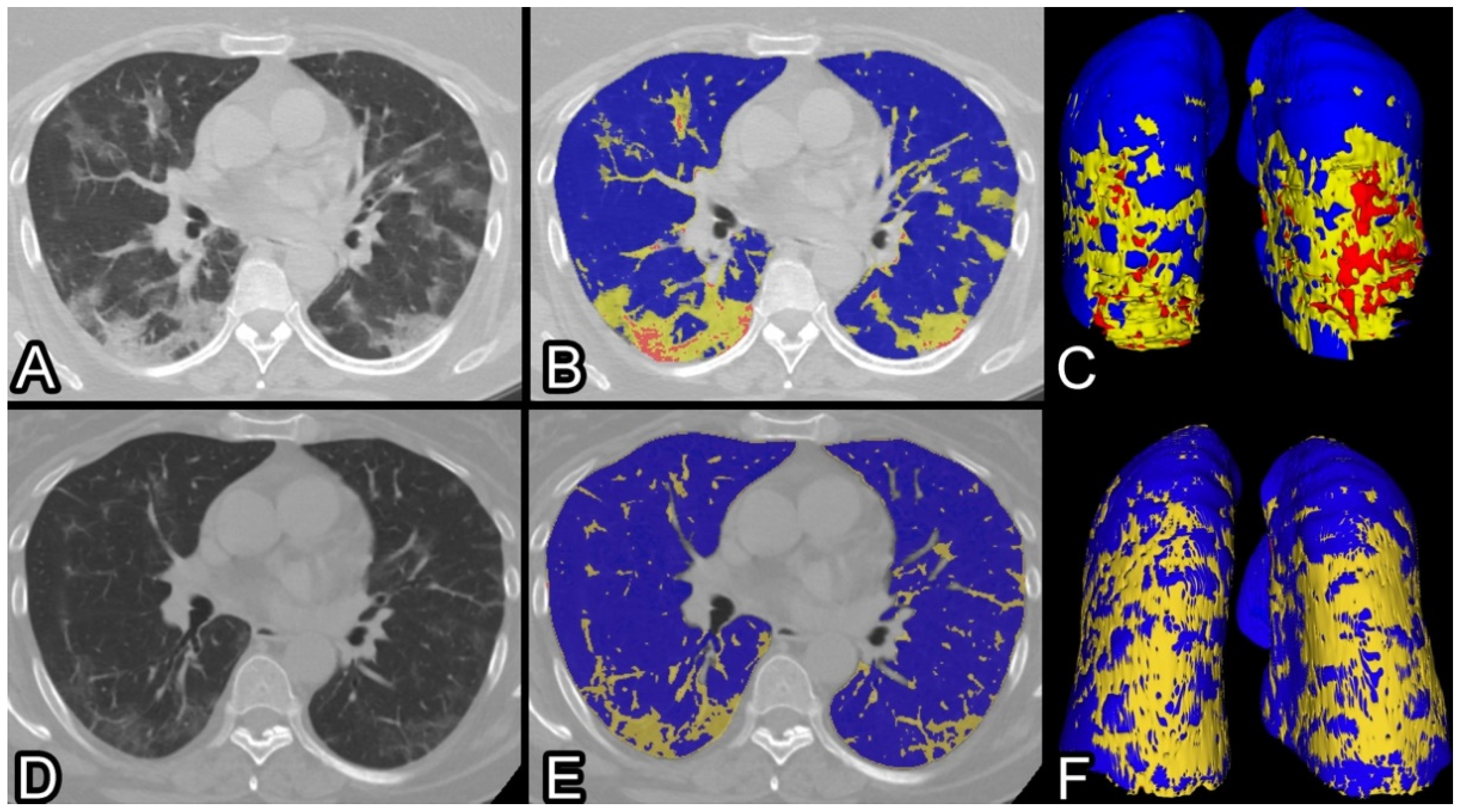
2.2. CT Protocol and Image Analysis
2.3. Outcome
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in china. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Blanco, J.-R.; Cobos-Ceballos, M.-J.; Navarro, F.; Sanjoaquin, I.; Arnaiz de Las Revillas, F.; Bernal, E.; Buzon-Martin, L.; Viribay, M.; Romero, L.; Espejo-Perez, S.; et al. Pulmonary long-term consequences of COVID-19 infections after hospital discharge. Clin. Microbiol. Infect. 2021, 27, 892–896. [Google Scholar] [CrossRef]
- Meije, Y.; Duarte-Borges, A.; Sanz, X.; Clemente, M.; Ribera, A.; Ortega, L.; González-Pérez, R.; Cid, R.; Pareja, J.; Cantero, I.; et al. Hospital de Barcelona COVID-19 team Long-term outcomes of patients following hospitalization for coronavirus disease 2019: A prospective observational study. Clin. Microbiol. Infect. 2021, 27, 1151–1157. [Google Scholar] [CrossRef]
- Gautam, N.; Madathil, S.; Tahani, N.; Bolton, S.; Parekh, D.; Stockley, J.; Goyal, S.; Qureshi, H.; Yasmin, S.; Cooper, B.G.; et al. Medium-term outcome of severe to critically ill patients with SARS-CoV-2 infection. Clin. Infect. Dis. 2021, 74, 301–308. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef] [Green Version]
- Ng, M.-Y.; Lee, E.Y.P.; Yang, J.; Yang, F.; Li, X.; Wang, H.; Lui, M.M.-S.; Lo, C.S.-Y.; Leung, B.; Khong, P.-L.; et al. Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiol. Cardiothorac. Imaging 2020, 2, e200034. [Google Scholar] [CrossRef] [Green Version]
- Kanne, J.P.; Little, B.P.; Chung, J.H.; Elicker, B.M.; Ketai, L.H. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology 2020, 296, E113–E114. [Google Scholar] [CrossRef] [Green Version]
- Cressoni, M.; Gallazzi, E.; Chiurazzi, C.; Marino, A.; Brioni, M.; Menga, F.; Cigada, I.; Amini, M.; Lemos, A.; Lazzerini, M.; et al. Limits of normality of quantitative thoracic CT analysis. Crit. Care 2013, 17, R93. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, Y.; Wei, Y.; Li, M.; Zhang, Y.; Zhang, N.; Zhao, S.; Zeng, H.; Deng, W.; Huang, Z.; et al. Quantitative analysis of chest CT imaging findings with the risk of ARDS in COVID-19 patients: A preliminary study. Ann. Transl. Med. 2020, 8, 594. [Google Scholar] [CrossRef]
- Noll, E.; Soler, L.; Ohana, M.; Ludes, P.-O.; Pottecher, J.; Bennett-Guerrero, E.; Veillon, F.; Goichot, B.; Schneider, F.; Meyer, N.; et al. A novel, automated, quantification of abnormal lung parenchyma in patients with COVID-19 infection: Initial description of feasibility and association with clinical outcome. Anaesth. Crit. Care Pain Med. 2020, 40, 100780. [Google Scholar] [CrossRef] [PubMed]
- Durhan, G.; Ardalı Düzgün, S.; Başaran Demirkazık, F.; Irmak, İ.; İdilman, İ.; Gülsün Akpınar, M.; Akpınar, E.; Öcal, S.; Telli, G.; Topeli, A.; et al. Visual and software-based quantitative chest CT assessment of COVID-19: Correlation with clinical findings. Diagn. Interv. Radiol. 2020, 26, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Muglia, R.; Bolengo, I.; Santonocito, O.G.; Lisi, C.; Angelotti, G.; Morandini, P.; Savevski, V.; Politi, L.S.; Balzarini, L. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Eur. Radiol. 2020, 30, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.; Liu, X.; Zhang, H.; Li, Y.; Liu, J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J. Am. Coll. Radiol. 2020, 17, 701–709. [Google Scholar] [CrossRef]
- Zhu, J.; Zhong, Z.; Li, H.; Ji, P.; Pang, J.; Li, B.; Zhang, J. CT imaging features of 4121 patients with COVID-19: A meta-analysis. J. Med. Virol. 2020, 92, 891–902. [Google Scholar] [CrossRef] [Green Version]
- Frija-Masson, J.; Debray, M.-P.; Boussouar, S.; Khalil, A.; Bancal, C.; Motiejunaite, J.; Galarza-Jimenez, M.A.; Benzaquen, H.; Penaud, D.; Laveneziana, P.; et al. Residual ground glass opacities three months after Covid-19 pneumonia correlate to alteration of respiratory function: The post Covid M3 study. Respir. Med. 2021, 184, 106435. [Google Scholar] [CrossRef]
- So, M.; Kabata, H.; Fukunaga, K.; Takagi, H.; Kuno, T. Radiological and functional lung sequelae of COVID-19: A systematic review and meta-analysis. BMC Pulm. Med. 2021, 21, 97. [Google Scholar] [CrossRef]
- Francone, M.; Iafrate, F.; Masci, G.M.; Coco, S.; Cilia, F.; Manganaro, L.; Panebianco, V.; Andreoli, C.; Colaiacomo, M.C.; Zingaropoli, M.A.; et al. Chest CT score in COVID-19 patients: Correlation with disease severity and short-term prognosis. Eur. Radiol. 2020, 30, 6808–6817. [Google Scholar] [CrossRef]
- Lei, Q.; Li, G.; Ma, X.; Tian, J.; Wu, Y.F.; Chen, H.; Xu, W.; Li, C.; Jiang, G. Correlation between CT findings and outcomes in 46 patients with coronavirus disease 2019. Sci. Rep. 2021, 11, 1103. [Google Scholar] [CrossRef]
- Zhao, W.; Zhong, Z.; Xie, X.; Yu, Q.; Liu, J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am. J. Roentgenol. 2020, 214, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Min, X.; Nan, Y.; Feng, Z.; Li, B.; Cai, W.; Xi, X.; Wang, L. Assessment of the Severity of Coronavirus Disease: Quantitative Computed Tomography Parameters versus Semiquantitative Visual Score. Korean J. Radiol. 2020, 21, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Colombi, D.; Bodini, F.C.; Petrini, M.; Maffi, G.; Morelli, N.; Milanese, G.; Silva, M.; Sverzellati, N.; Michieletti, E. Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia. Radiology 2020, 296, E86–E96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burian, E.; Jungmann, F.; Kaissis, G.A.; Lohöfer, F.K.; Spinner, C.D.; Lahmer, T.; Treiber, M.; Dommasch, M.; Schneider, G.; Geisler, F.; et al. Intensive Care Risk Estimation in COVID-19 Pneumonia Based on Clinical and Imaging Parameters: Experiences from the Munich Cohort. J. Clin. Med. 2020, 9, 1514. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Li, Y.; Zhang, T.; Gao, L.; Jin, D.; Sun, Y.; Ye, X.; Yu, L.; Hu, Z.; et al. From community-acquired pneumonia to COVID-19: A deep learning-based method for quantitative analysis of COVID-19 on thick-section CT scans. Eur. Radiol. 2020, 30, 6828–6837. [Google Scholar] [CrossRef]
- Laino, M.E.; Ammirabile, A.; Lofino, L.; Lundon, D.J.; Chiti, A.; Francone, M.; Savevski, V. Prognostic findings for ICU admission in patients with COVID-19 pneumonia: Baseline and follow-up chest CT and the added value of artificial intelligence. Emerg. Radiol. 2022, 29, 243–262. [Google Scholar] [CrossRef]
- Dai, M.; Liu, X.; Zhu, X.; Liu, T.; Xu, C.; Ye, F.; Yang, L.; Zhang, Y. Temporal changes of CT findings between non-severe and severe cases of COVID-19 pneumonia: A multi-center, retrospective, longitudinal Study. Int. J. Med. Sci. 2020, 17, 2653–2662. [Google Scholar] [CrossRef]
- Huang, L.; Han, R.; Ai, T.; Yu, P.; Kang, H.; Tao, Q.; Xia, L. Serial Quantitative Chest CT Assessment of COVID-19: A Deep Learning Approach. Radiol. Cardiothorac. Imaging 2020, 2, e200075. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Li, L.; Liu, B.; Ye, T.; Li, L.; Liu, D.; Ding, Z.; Chen, G.; Liang, B.; Yang, L.; et al. A novel deep learning-based quantification of serial chest computed tomography in Coronavirus Disease 2019 (COVID-19). Sci. Rep. 2021, 11, 417. [Google Scholar] [CrossRef]
- Yun, Y.; Wang, Y.; Hao, Y.; Xu, L.; Cai, Q. The time course of chest CT lung changes in COVID-19 patients from onset to discharge. European J. Radiol. Open 2021, 8, 100305. [Google Scholar] [CrossRef]
- Li, M.; Lei, P.; Zeng, B.; Li, Z.; Yu, P.; Fan, B.; Wang, C.; Li, Z.; Zhou, J.; Hu, S.; et al. Coronavirus Disease (COVID-19): Spectrum of CT Findings and Temporal Progression of the Disease. Acad. Radiol. 2020, 27, 603–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Ding, X.; Zhang, F. Dynamic evolution of lung abnormalities evaluated by quantitative CT techniques in patients with COVID-19 infection. Epidemiol. Infect. 2020, 148, e136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Gong, H.; Wu, L. Quantitative lung lesion features and temporal changes on chest CT in patients with common and severe SARS-CoV-2 pneumonia. PLoS ONE 2020, 15, e0236858. [Google Scholar] [CrossRef]
- Pavli, A.; Theodoridou, M.; Maltezou, H.C. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Gemelli Against COVID-19 Post-Acute Care Study Group Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef]
- Froidure, A.; Mahsouli, A.; Liistro, G.; De Greef, J.; Belkhir, L.; Gérard, L.; Bertrand, A.; Koenig, S.; Pothen, L.; Yildiz, H.; et al. Integrative respiratory follow-up of severe COVID-19 reveals common functional and lung imaging sequelae. Respir. Med. 2021, 181, 106383. [Google Scholar] [CrossRef]
- Shah, A.S.; Wong, A.W.; Hague, C.J.; Murphy, D.T.; Johnston, J.C.; Ryerson, C.J.; Carlsten, C. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax 2021, 76, 402–404. [Google Scholar] [CrossRef]
- Smet, J.; Stylemans, D.; Hanon, S.; Ilsen, B.; Verbanck, S.; Vanderhelst, E. Clinical status and lung function 10 weeks after severe SARS-CoV-2 infection. Respir. Med. 2020, 176, 106276. [Google Scholar] [CrossRef]
- Bellan, M.; Soddu, D.; Balbo, P.E.; Baricich, A.; Zeppegno, P.; Avanzi, G.C.; Baldon, G.; Bartolomei, G.; Battaglia, M.; Battistini, S.; et al. Respiratory and Psychophysical Sequelae among Patients With COVID-19 Four Months After Hospital Discharge. JAMA Netw. Open 2021, 4, e2036142. [Google Scholar] [CrossRef]
| Data | Median (IQR) or Number of Observations (%) |
|---|---|
| Age | 60 (51, 69) |
| Females | 95 (34%) |
| Males | 187 (66%) |
| symptoms onset (days) | 7 (4, 10) |
| %CL admission | 11% (6, 18) |
| %PAL admission | 9% (5, 14) |
| %CL follow-up | 5% (4, 6) |
| %PAL follow-up | 4% (3, 5) |
| %deltaCL | −4% (−11, −1) |
| %deltaPAL | −3% (−9, −1) |
| length of stay | 9 (7, 15) |
| lung disease | 39 (13%) |
| Therapy | |
| corticosteroid | 21 (7%) |
| heparin | 107 (38%) |
| oxygen | 172 (61%) |
| intubation | 15 (5%) |
| Follow-up | |
| thoracalgia | 14 (5%) |
| dyspnea | 6 (2%) |
| coughing | 3 (1%) |
| days between scans | 48 (42, 59) |
| days to negative swab | 33 (25, 43) |
| OR | SE | p-Value | 95% C.I. | ||
|---|---|---|---|---|---|
| Dyspnea | |||||
| %deltaCL | 0.82 | 0.07 | 0.03 * | 0.69 | 0.98 |
| Corticosteroid | 8.50 | 8.38 | 0.03 * | 1.23 | 58.63 |
| Lung disease | 3.60 | 3.54 | 0.19 | 0.52 | 24.77 |
| Oxygen therapy | 5.49 | 6.35 | 0.14 | 0.57 | 53.01 |
| LR chi2(4) = 12.54 | |||||
| %deltaPAL | 0.81 | 0.07 | 0.02 * | 0.67 | 0.97 |
| Corticosteroid | 7.47 | 7.06 | 0.03 * | 1.17 | 47.63 |
| Oxygen therapy | 6.38 | 7.26 | 0.10 | 0.69 | 59.38 |
| LR chi2(3) = 11.25 | |||||
| Thoracalgia | |||||
| %deltaCL | 1.05 | 0.03 | 0.08 | 0.99 | 1.11 |
| Length of stay | 0.85 | 0.07 | 0.04 * | 0.73 | 0.99 |
| Intubation | 10.47 | 17.42 | 0.16 | 0.40 | 273.05 |
| Age | 0.95 | 0.02 | 0.06 | 0.90 | 1.00 |
| Diabetes | 2.83 | 2.13 | 0.17 | 0.65 | 12.34 |
| LR chi2(5) = 15.87 | |||||
| %deltaPAL | 1.11 | 0.05 | 0.02 * | 1.02 | 1.20 |
| Age | 0.95 | 0.02 | 0.05 * | 0.90 | 1.00 |
| Intubation | 10.63 | 16.75 | 0.13 | 0.48 | 233.21 |
| Length of stay | 0.84 | 0.07 | 0.03 * | 0.72 | 0.98 |
| Diabetes | 2.92 | 2.20 | 0.16 | 0.67 | 12.81 |
| LR chi2(5) = 18.64 | |||||
| Coughing | |||||
| %deltaCL | 0.82 | 0.11 | 0.12 | 0.63 | 1.05 |
| Age | 1.10 | 0.06 | 0.06 | 1.00 | 1.22 |
| LR chi2(2) = 9.25 | |||||
| %deltaPAL | 0.77 | 0.11 | 0.08 | 0.58 | 1.04 |
| Age | 1.11 | 0.06 | 0.04 * | 1.00 | 1.22 |
| LR chi2(2) = 9.56 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanza, E.; Ammirabile, A.; Casana, M.; Pocaterra, D.; Tordato, F.M.P.; Varisco, B.; Lisi, C.; Messana, G.; Balzarini, L.; Morelli, P. Quantitative Chest CT Analysis to Measure Short-Term Sequelae of COVID-19 Pneumonia: A Monocentric Prospective Study. Tomography 2022, 8, 1578-1585. https://doi.org/10.3390/tomography8030130
Lanza E, Ammirabile A, Casana M, Pocaterra D, Tordato FMP, Varisco B, Lisi C, Messana G, Balzarini L, Morelli P. Quantitative Chest CT Analysis to Measure Short-Term Sequelae of COVID-19 Pneumonia: A Monocentric Prospective Study. Tomography. 2022; 8(3):1578-1585. https://doi.org/10.3390/tomography8030130
Chicago/Turabian StyleLanza, Ezio, Angela Ammirabile, Maddalena Casana, Daria Pocaterra, Federica Maria Pilar Tordato, Benedetta Varisco, Costanza Lisi, Gaia Messana, Luca Balzarini, and Paola Morelli. 2022. "Quantitative Chest CT Analysis to Measure Short-Term Sequelae of COVID-19 Pneumonia: A Monocentric Prospective Study" Tomography 8, no. 3: 1578-1585. https://doi.org/10.3390/tomography8030130
APA StyleLanza, E., Ammirabile, A., Casana, M., Pocaterra, D., Tordato, F. M. P., Varisco, B., Lisi, C., Messana, G., Balzarini, L., & Morelli, P. (2022). Quantitative Chest CT Analysis to Measure Short-Term Sequelae of COVID-19 Pneumonia: A Monocentric Prospective Study. Tomography, 8(3), 1578-1585. https://doi.org/10.3390/tomography8030130







