1. Introduction
Computed tomography (CT) imaging is a cornerstone of modern diagnostic medicine, providing critical insights into conditions affecting the chest, abdomen, and pelvis. However, its widespread use raises significant concerns about radiation exposure, particularly for vulnerable populations such as children and patients undergoing repeated scans. Optimizing radiation doses is essential to balancing diagnostic efficacy with patient safety, and this goal hinges on establishing Diagnostic Reference Levels (DRLs), personalized dose strategies, and technological advancements [
1,
2,
3].
DRLs are benchmarks for safe imaging practices, allowing facilities to compare their dose levels against established standards to ensure patient safety and diagnostic quality. International bodies such as the International Commission on Radiological Protection (ICRP) and the International Atomic Energy Agency (IAEA) underscore the importance of DRLs in optimizing doses [
4,
5]. However, establishing universally accepted DRLs is complex, given the variability in scanner models, imaging protocols, and patient demographics. Significant variations in DRLs exist globally and between facilities, driven by differences in clinical practices and technological capabilities. These discrepancies highlight the need for research to harmonize DRLs while maintaining flexibility for regional and institutional adaptations [
1,
6].
Personalized dose strategies have emerged as a vital approach to address these challenges. By tailoring radiation doses to individual patient characteristics such as age, gender, and body composition, personalized protocols ensure diagnostic-quality images while minimizing exposure. For instance, McCollough et al. demonstrated that children and smaller patients require lower radiation doses than adults [
7]. Quantifying radiation risks also plays a pivotal role in dose optimization. Laurier D et al. introduced the linear no-threshold (LNT) model to estimate cancer risks from ionizing radiation, emphasizing the need to minimize unnecessary exposure in line with ALARA (As Low As Reasonably Achievable) principles [
8].
CT scans of the chest, abdomen, and pelvis are among the most common diagnostic imaging procedures but account for significant radiation exposure. Establishing DRLs for these scans is crucial in optimizing doses and ensuring patient safety. DRLs act as a reference point, enabling facilities to identify outliers and adjust practices accordingly. They align with the ALARA principle, aiming to minimize exposure while maintaining diagnostic accuracy [
9,
10].
The ICRP and IAEA provide comprehensive guidelines for defining DRLs, emphasizing their role in standardizing practices across facilities. The IAEA advocates for the regular update of DRLs and the sharing of international data to improve radiation safety worldwide. ICRP integrates DRLs into a broader framework of radiation protection, focusing on optimization, justification, and dose limitation. Both organizations stress the need to account for variations in patient demographics, scanner technologies, and imaging protocols when establishing DRLs, ensuring that benchmarks are locally relevant and globally consistent [
5,
9,
11,
12].
Optimizing radiation doses in CT imaging of the chest, abdomen, and pelvis is essential to balancing diagnostic accuracy with patient safety. The establishment of DRLs is a cornerstone of this optimization process, offering a standardized approach to dose management while accommodating regional and institutional variations. Personalized dose strategies, informed by patient-specific factors and supported by technological advancements, further refine this approach. Efforts to harmonize DRLs globally, as advocated by the ICRP and IAEA, and initiatives like those in Saudi Arabia demonstrate the potential for improved safety and efficacy in CT imaging practices. By prioritizing these strategies, healthcare providers can enhance patient care while mitigating the risks associated with ionizing radiation [
4,
13,
14].
This study focused on CAP CT scans as they are widely used in clinical practice, particularly for oncological evaluation (the sample of this study comprised cancer patients being screened for staging and follow-up). Subsequent CAP examinations cover multiple anatomical regions in a single scan. They contribute significantly to cumulative radiation exposure, making it crucial to establish appropriate DRLs for optimizing radiation dose while maintaining diagnostic accuracy.
2. Materials and Methods
This retrospective cross-sectional study assessed radiation dosage associated with CT chest, abdomen, and pelvis (CAP). In total, 106 adult patients were included in the study with the following indications: cancer characterization, staging, and follow-up. Data were collected over three months in 2023 from three scanners manufactured by the same company. Patient demographic data, including age, gender, weight, height, BMI, and clinical indications, were recorded. Scanning parameters included mA per rotation, number of slices, pitch, kVp, gantry rotation time, slice thickness, and field of view (FOV).
2.1. CT Machines
The scans were performed using a Toshiba Aquilion Prime 160-slice CT scanner (TSX-303A) (Toshiba Medical Systems, Otawara, Japan, 2016). This system employed adaptive iterative dose reduction techniques for optimized low-dose imaging through iterative reconstruction. Routine quality control measures were performed on the CT machine to ensure consistency in radiation output, dose delivery, and protocol parameters.
2.2. Radiation Dosimetry
The effective dose (ED) was estimated using region-specific conversion factors (k) applied to the dose-length product (DLP), following ICRP 103 guidelines. For chest–abdomen–pelvis CT scans, a k-value of approximately 0.015 mSv/mGy·cm was used. This method provides an approximate estimation of stochastic radiation risk when direct organ dose measurements are not available.
Radiation dose parameters, including CTDIvol and DLP, were directly extracted from the CT scanner’s dose report for each examination. These values were automatically generated by the Toshiba Aquilion Prime CT system during the scanning process, ensuring accurate and reliable measurement of radiation exposure. The effective dose was calculated using the following formula:
where
HT is the equivalent dose in organ or tissue
T, and
wT is the weighting factor for tissue
T [
15].
According to ICRP publication 119 [
15], the equivalent dose, H
T,R, in tissue or organ T due to radiation R, is given by H
T,R = w
RD
T,R, where D
T,R is the average absorbed dose from radiation R in tissue T and w
R is the radiation weighting factor. Since w
R is dimensionless, the units are the same as for absorbed dose (J/kg), and its special name is sievert (Sv). The total equivalent dose H
T is the sum of H
T,R over all radiation types: H
T = ∑
R H
T;R.
According to ICRP 60 (ICRP 1991), the effective dose is defined as the weighted average of organ dose values HT for several specified organs. How much a particular organ contributes to calculating effective dose depends on its relative sensitivity to radiation-induced effects, as represented by the tissue-weighting factor w
i attributed to the organ [
16].
2.3. CT CAP Examination Protocols
The scan range extended from the lung apices to the lesser trochanter, with patients positioned supine, feet first, and arms raised above their heads. Scout images in anteroposterior and lateral projections were acquired to determine the area of interest and ensure precise positioning. Tube current modulation was applied to distribute the dose evenly across the trunk, with Auto mA adjusted based on patient size and scan requirements. The kVp was set at 120 for standard scans, with 140 kVp used selectively. Patients were instructed to hold their breath to minimize motion artifacts. Scan phases were tailored to clinical indications, with scout, plain, and portal venous phases commonly used. Additional phases, such as arterial or delayed, were performed for specific cases, such as liver metastases or renal issues, with the scan area limited to the upper abdomen or as required.
2.4. Data Analysis
Patient demographics, scanning parameters, CTDIvol, DLP, and effective dose data were extracted in Microsoft Excel for window 10; then, the data were analyzed using SPSS version 27 (IBM, Armonk, NY, USA) and the DATAtab Online Statistics Calculator (DATAtab e.U. Graz, Austria) and Jamovi software 2.4.11. Inferential statistics were used with minimum, mean, and standard deviation values, which were calculated for continuous data; then, regression analysis, Pearson’s correlation, independent sample t-test, and one-way ANOVA test were used to assess the correlations between dose indices, patient demographic data, and scan parameters, with a p value of <0.01 and <0.05 considered statistically significant.
2.5. Ethical Approval
The study was approved by the institutional review board at Princess Nourah bint Abdulrahman University. IRB Log Number: 23-0206.
3. Results
Table 1 provides an outline of the demographic characteristics of the study participants. In terms of gender, more than half were females at 55.7%, and 44.3% were males. When classified by age, the majority (72.6%) were in the age range of 19–59 years. Regarding height, 20.8% were in the height range between 140 and 155 cm, 56.6% were between 156 and 170 cm, and 22.6% were between 171 and 185 cm. Lastly, for weight distribution, the majority (58.5%) were within 61–85 kg.
The findings revealed that the average weight, height, and BMI were 73.39 kg ± 15.16, 1.61 m ± 0.095, and 26.2 kg/m
2 ± 0.31, respectively. The values for KVP (kilovolt peak), mA per R (milliampere per rotation), duration, slice thickness, and the number of slices were 120.57 ± 0.33, 472.42 ± 158.32, 0.61 ± 0.11, 3.38 mm ± 0.37, and 189.09 ± 27.28, respectively. The pitch parameter ranged from 0.50 to 1.37, with a mean of 0.74 ± 0.27. The field of view (FOV) remained constant at 330 cm. The CTDI
vol ranged from 9.42 to 39.54, with an average value of 25.96 ± 6.96. The dose-length product (DLP) varied from 587.00 to 2752.48, with a mean of 1719.63 ± 488.45, whereas the effective dosage ranged from 9.39 to 44.04, with a mean of 27.51 ± 0.81 (
Table 2).
Table 3 presents the correlation of demographic and exposure parameters in both sexes. For weight, males had a higher mean weight of 74.04 ± 15.73 kg, while females were 72.86 ± 14.80 kg, with no significant difference (
p = 0.695; 95% CI: −4.770 to 7.126). Height and BMI were significantly higher in males compared to females, at 1.69 ± 0.07 m compared to 1.56 ± 0.067 m for females (
p < 0.001; 95% CI: 0.10514 to 0.15867) and 2.86 ± 0.24 kg/m
2 compared to 2.43 ± 0.21 kg/m
2 (
p < 0.001 and a 95% CI of 0.34085 to 0.51651), respectively. Concerning the differences in exposure parameters used in both genders, the results demonstrated that they insignificantly differed between males and females; the mean KVP values were similar between males (120.43 ± 2.92) and females (120.68 ± 3.65), with no significant difference (
p = 0.693; 95% CI: −1.517 to 1.013). The mA parameter showed slightly higher values for females (486.25 ± 169.29) compared to males (455.06 ± 143.36,
p = 0.307; 95% CI: −91.429 to 29.048). Rotation time was nearly identical between males (0.60 ± 0.10) and females (0.61 ± 0.11,
p = 0.503; 95% CI: −0.0553 to 0.0273). The mean slice thickness was 3.37 ± 0.38 mm for males and 3.39 ± 0.38 mm for females (
p = 0.802; 95% CI: −0.16496 to 0.12781). However, the number of slices differed significantly, with males averaging 199.64 ± 29.08 compared to 180.69 ± 22.71 for females (
p < 0.001; 95% CI: 8.665 to 29.222). The pitch parameter was nearly symmetrical: 0.75 ± 0.29 for males and 0.74 ± 0.26 for females (
p = 0.850; 95% CI: −0.09845 to 0.11931). The field of view (FOV) was constant at 330.00 ± 0.00 cm for both sexes.
The CTDI
vol parameter illustrated a significant difference between males and females, while the other dose parameters, including DLP, effective dose, and cancer risk, are statistically similar between sexes. The mean CTDIvol for males was 24.30 ± 6.81, which was significantly lower than that for females (27.30 ± 6.85), with a
p-value of 0.027 and a 95% CI of −5.64991 to −0.35088. However, the DLP (dose-length product) values insignificantly differed, with males averaging 1695.18 ± 493.61 compared to 1739.12 ± 487.66 for females (
p = 0.648; 95% CI: −234.43353 to 146.54707). The effective dose was also comparable between sexes, with the average for males being 27.121 ± 7.90 and for females being 27.83 ± 7.80 (
p-value =0.648, 95% CI ranged from −3.75094 to 2.34475), indicating no significant difference. Similarly, the cancer risk insignificantly differed, with slightly higher values in females (0.54 ± 0.34) than in males (0.46 ± 0.28) (
p = 0.184; 95% CI: −0.20208 to 0.03940) (
Table 4).
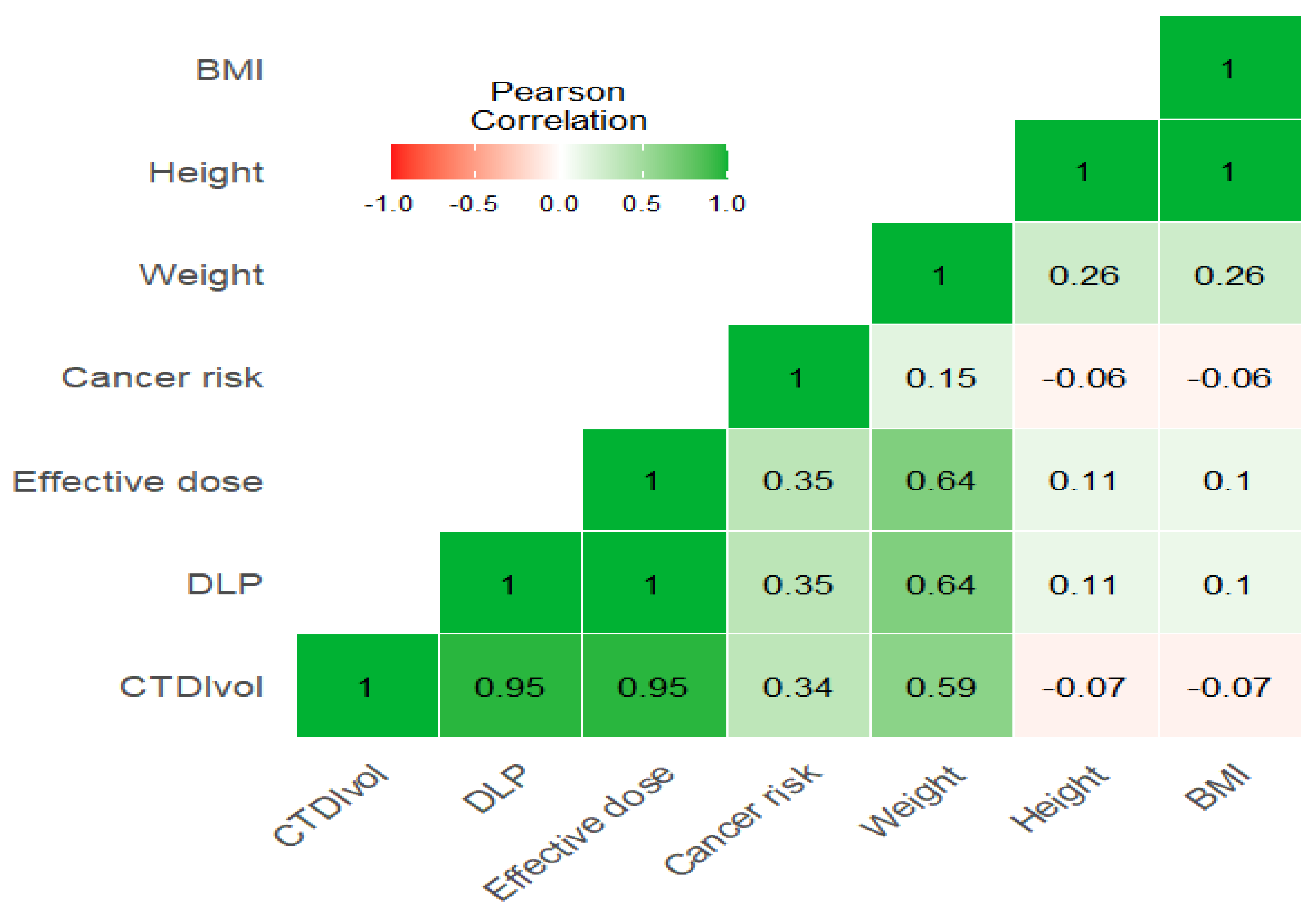
The correlation heatmap in
Figure 1 shows a moderate significant correlation between weight and CTDIvol, and a strong correlation between weight and DLP and effective dose (r = 0.59, 0.64, 0.64, respectively), while there is an insignificant impact of height and BMI with these exposure parameters (r = −0.07, 0.1, 0.1 for both height and weight with parameters). Furthermore, there is a strong significant correlation between CTDIvol with DLP and effective dose (r = 0.95) (
Figure 1 and
Figure 2)
.It was found that there were weak positive correlations of KVp with CTDIvol, DLP, and effective dose (r = 0.27, 0.25, 0.25), while there was no significant correlation between KVp and cancer risk (r = 0.04). Furthermore, MA is strongly correlated with time and pitch (r = 0.78, 0.67, respectively) and moderately correlated with CTDIvol, DLP, and effective dose (r = 0.58). Time demonstrates a strong correlation with pitch (r = 0.84), a moderate correlation with CTDIvol, DLP, and effective dose (r = 0.48, 0.5, 0.5, respectively), and a weak correlation with cancer risk (r = 0.31). Similarly, pitch shows weak correlations with CTDIvol, DLP, and effective dose (r = 0.26, 0.31, 0.31, respectively), while cancer risk is moderately correlated with CTDIvol, DLP, and effective dose (0.34, 0.35, 0.35, respectively) (
Figure 3).
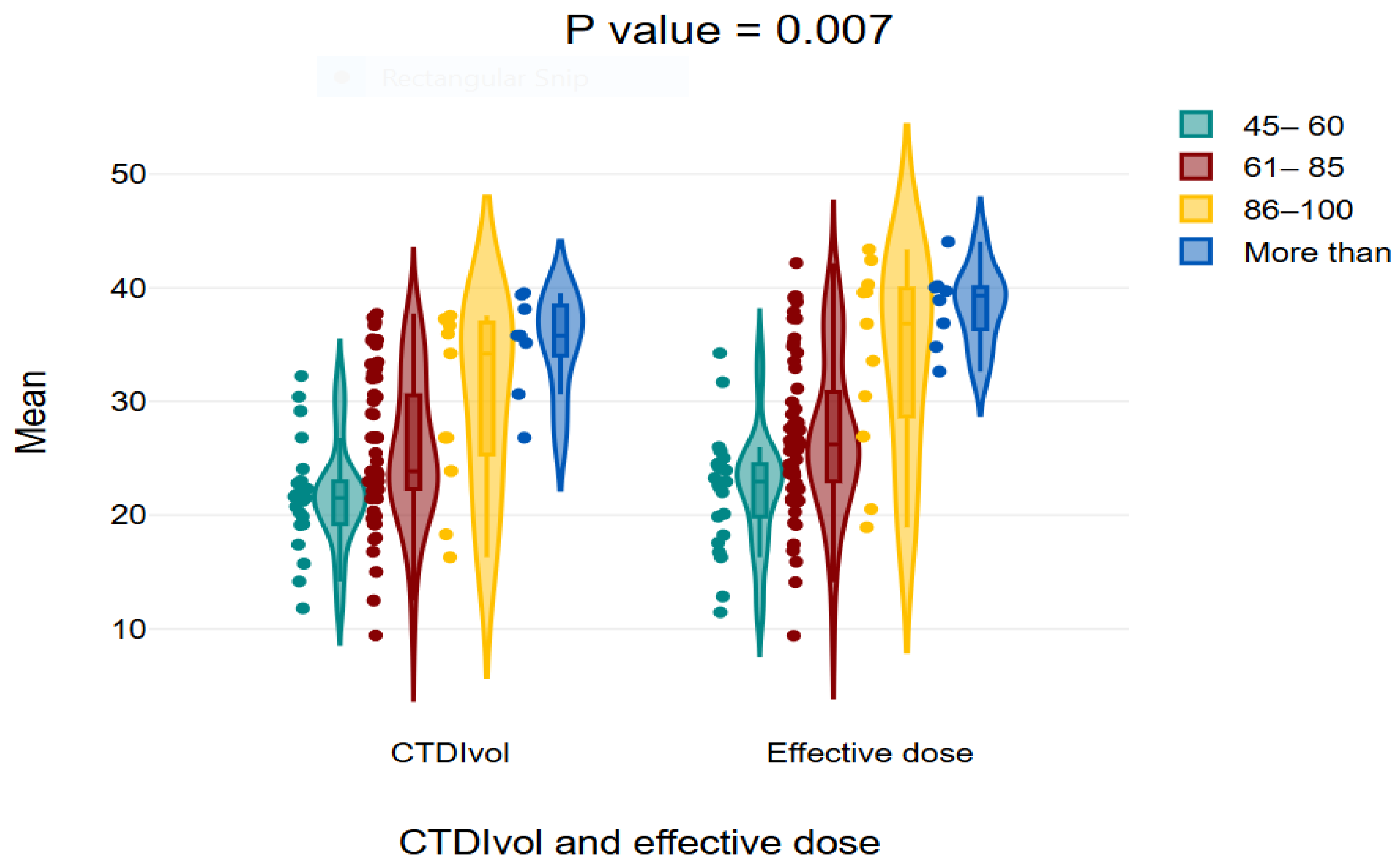
The findings indicate that the middle age group of 40–59 years received the highest mean values for CTDIvol, DLP, and effective dose, whereas the elderly group of 80–99 years received lower values, with statistically significant differences seen across the age groups. The mean CTDIvol values were 23.26 ± 6.16 for the age range of 19–39 years, 28.78 ± 7.34 for 40–59 years, 25.82 ± 5.86 for 60–79 years, and 21.00 ± 5.05 for 80–99 years (
p-value = 0.002). The mean dose-length product (DLP) was 1548.76 ± 442.81 for 19–39 years, 1886.86 ± 516.37 for 40–59 years, 1716.49 ± 427.46 for 60–79 years, and 1748.5 ± 461.05 for 80–99 years (
p = 0.015). The average effective values were 24.78 ± 7.08 for ages 19–39, 30.18 ± 8.26 for ages 40–59, 27.46 ± 6.83 for ages 60–79, and 23.65 ± 7.37 for ages 80–99, (
Table 5).
The results of this study show that the dose parameters have no statistically significant differences between the two groups of BMI (
p > 0.05) (
Table 6).
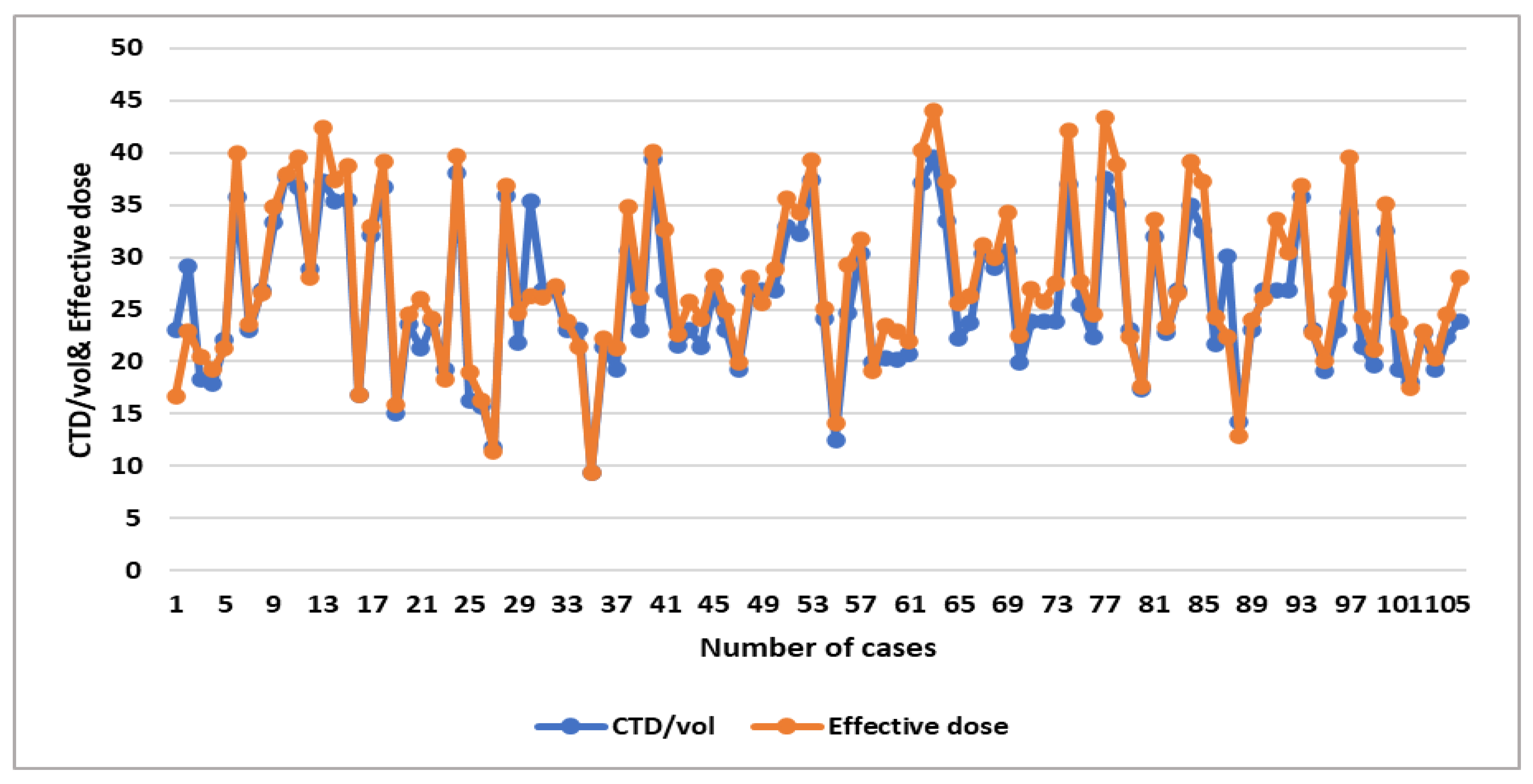
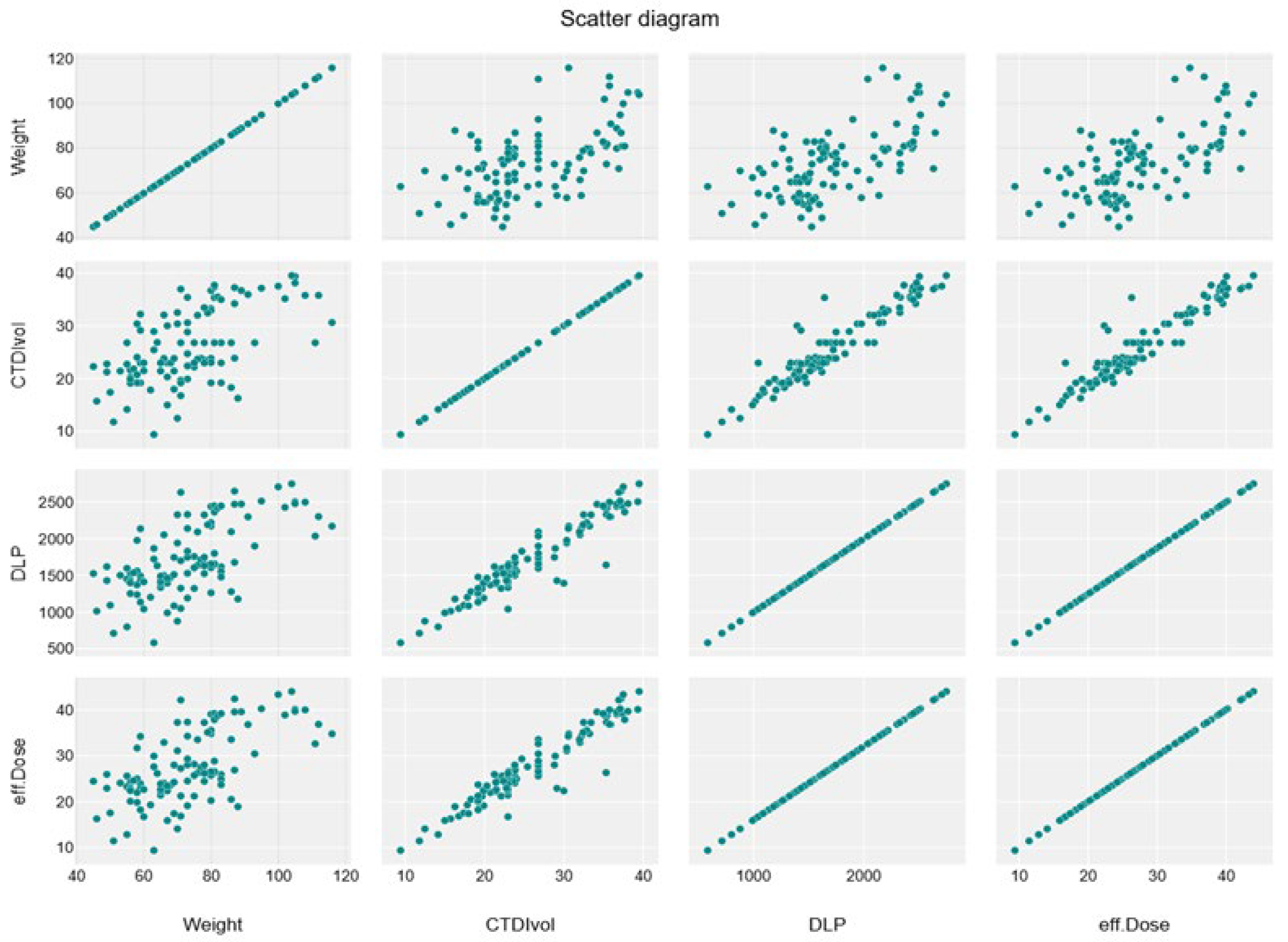
A significant moderate positive linear association was noticed between weight and CTDIvol, DLP, and effective dose (R
2 = 0.3454, 0.4056, and 0.4056, respectively) (
Table 7), which shows the relationship between dose parameters, as shown in the linear regression scatterplot and the line graph (
Figure 3,
Figure 4,
Figure 5 and
Figure 6).
In
Table 8, it is demonstrated that the CT dose parameters in CAP in this study were higher than in the literature.
4. Discussion
Diagnostic reference levels (DRLs) are important methods for optimizing radiation dose in computed tomography (CT) examinations. Multiple studies have established DRLs for common CT procedures, including head, chest, and abdomen–pelvis scans, using CTDIvol and DLP as primary metrics [
30,
31,
32,
33]. This study assessed the radiation doses and associated cancer risks in CT chest, abdomen, and pelvis (CAP) procedures, focusing on the impact of scan parameters and patient demographics. The mean CTDIvol of 25.97 ± 6.96 mGy was higher than reported in similar studies, such as 22.94 mGy in Mansoor et al. and 10–12 mGy in Foley et al., likely due to increased scan length and a higher mean mA per rotation (472.42 mA). Tube current modulation effectively adjusted mA based on tissue thickness, but DLP values (587–2752.48 mGy·cm, mean 1719.64 ± 488.45 mGy·cm) remained relatively high, emphasizing the need to optimize the number of scanning phases based on clinical indications [
2,
3].
Cancer risk for trunk CT scans ranged from 0.048% to 1.581% (mean: 0.5%), with younger age groups (29–39 years) experiencing higher risks due to increased scan lengths and slice counts. In this study, it was found that as the weight of patients increased, the DLP and CTDIvol and effective dose significantly increased, while height and BMI produced an insignificant effect on these parameters. In contrast to this study, Sebelego et al. mention that patients with higher BMI also showed elevated DRL dose parameters [
33].
Technical parameters such as rotation time, pitch, kVp, and slice thickness significantly influenced dose measures. Longer rotation times increased DLP, CTDIvol, and effective dose linearly. Higher kVp (140 vs. 120 kVp) and increased slice thickness (3.75 mm vs. 3.0 mm) also led to substantial dose increases, consistent with findings by Wang et al. Adjusting pitch values showed an expected reduction in radiation dose, with higher doses observed at 0.9 pitch than other values, aligning with previous studies [
13].
In this study, it was found that the DLP (dose-length product) values insignificantly differed among both genders, with males averaging 1695.18 ± 493.61 compared to 1739.12 ± 487.66 for females (
p = 0.648; 95% CI: −234.43353 to 146.54707). Alashban and Shubayr et al.’s results were consistent with this study; they found that the mean DLP values for CT were higher for females compared to male patients, with no statistically significant differences [
17].
The study identified weak positive relationships between KVp and mA with CTDIvol, DLP, and effective dose, although no significant association was seen between KVp and cancer risk, suggesting that although KVp affects radiation dosage, its effects on cancer risk are negligible. This aligns with prior studies indicating that KVp exerts a relatively minimal impact on radiation dose and cancer risk, as it predominantly affects the energy of the X-ray beam rather than the dose itself. Time had a substantial link with pitch (r = 0.84) and a moderate correlation with radiation dose indices. Pitch demonstrated poor relationships with dose indices, suggesting that modifications to pitch have a limited impact on radiation exposure. The risk of cancer exhibited moderate relationships with CTDIvol (r = 0.34), DLP (r = 0.35), and effective dose (r = 0.35), hence affirming the anticipated association between elevated radiation exposures and heightened cancer risk. A previous study indicates that the radiation dose escalates linearly with rising mAs and exponentially with increasing kVp, irrespective of phantom size. Image noise diminished with reduced radiation doses but stabilized at elevated levels. Another study indicates that when the pitch value increases, the computed tomography dose index (CTDI) diminishes. Augmenting the pitch consequently leads to an extended scan duration. The radiation dosage observed in the phantom was consistent across all pitch options on the evaluated multislice helical CT equipment. The unforeseen outcome resulted from an automated proportional escalation in the tube current following an increase in pitch selection. The radiation dosage (CTDIvol and SSDE) escalated with phantom size at standard pitch; however, for elevated pitch, the dose increased up to a threshold and then declined linearly with increasing pitch [
34,
35,
36,
37].
Gender differences were evident, with females experiencing a higher mean cancer risk (0.539%) than males (0.457%), reinforcing findings from prior studies. Additionally, the number of scanning phases emerged as a critical factor, with triple-phase procedures nearly doubling cancer risk (0.37% for single-phase to 0.69% for triple-phase scans). These multiphase studies were often necessary for tumor staging and follow-up, particularly for organ-specific lesions [
15].
The parameters of CT dose (CTDI
vol, DLP, and effective dose) in this study were more than in other studies in different regions [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29], which underscores the urgent need for optimized CT protocols to minimize radiation exposure while maintaining diagnostic accuracy. Implementing justification criteria for scan phases, employing advanced dose reduction technologies, and adhering to the ALARA principle (As Low as Reasonably Achievable) are essential. Future research should focus on developing diagnostic reference levels (DRLs) tailored to specific populations and clinical scenarios to enhance patient safety.