Conference Report: Review of Clinical Implementation of Advanced Quantitative Imaging Techniques for Personalized Radiotherapy
Abstract
1. Introduction
2. Conference Sections
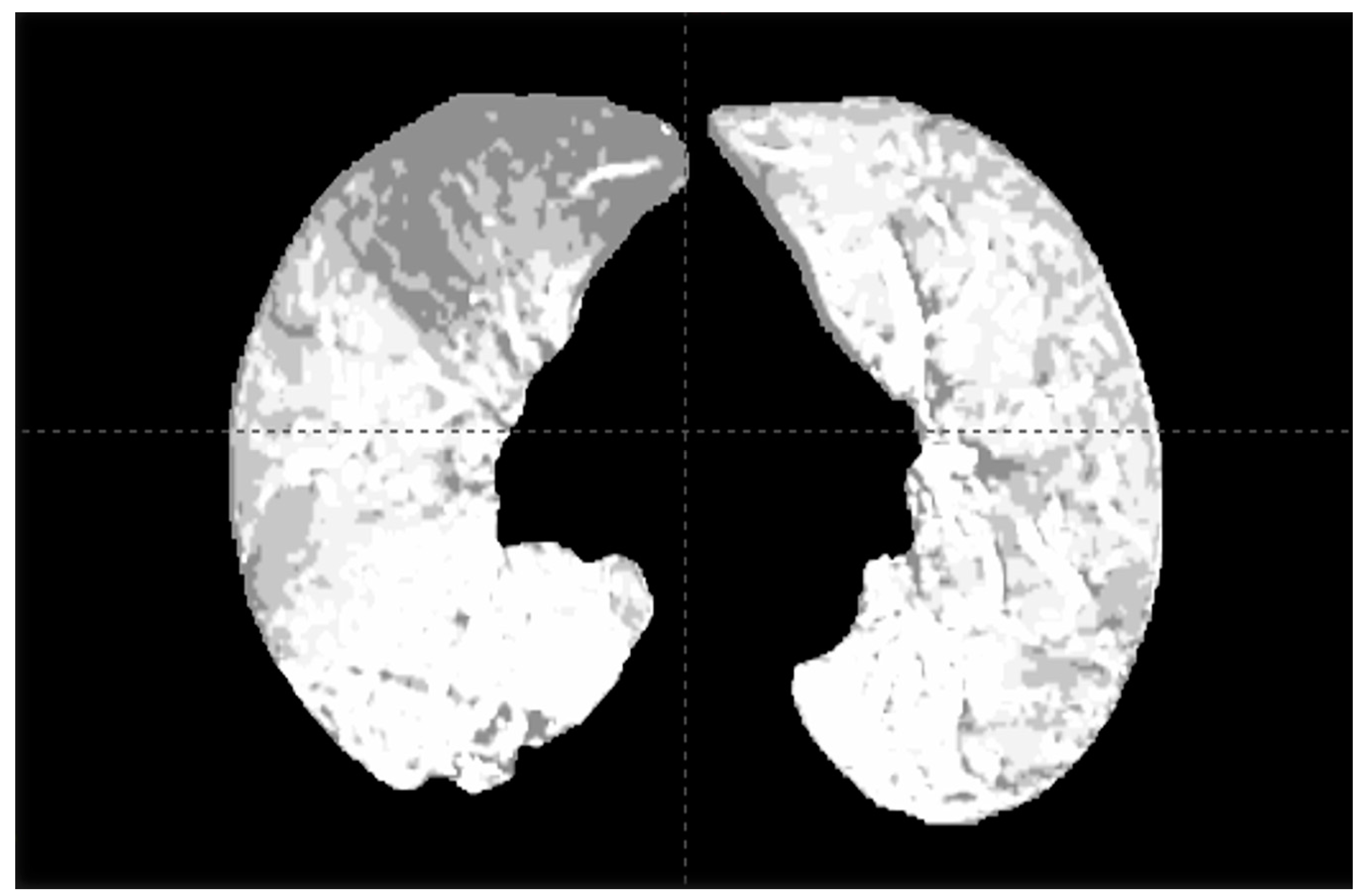
2.1. Dual-Energy CT and SPECT for Functional Lung Assessment
2.2. V/Q PET for Functional Lung Assessment and Avoidance
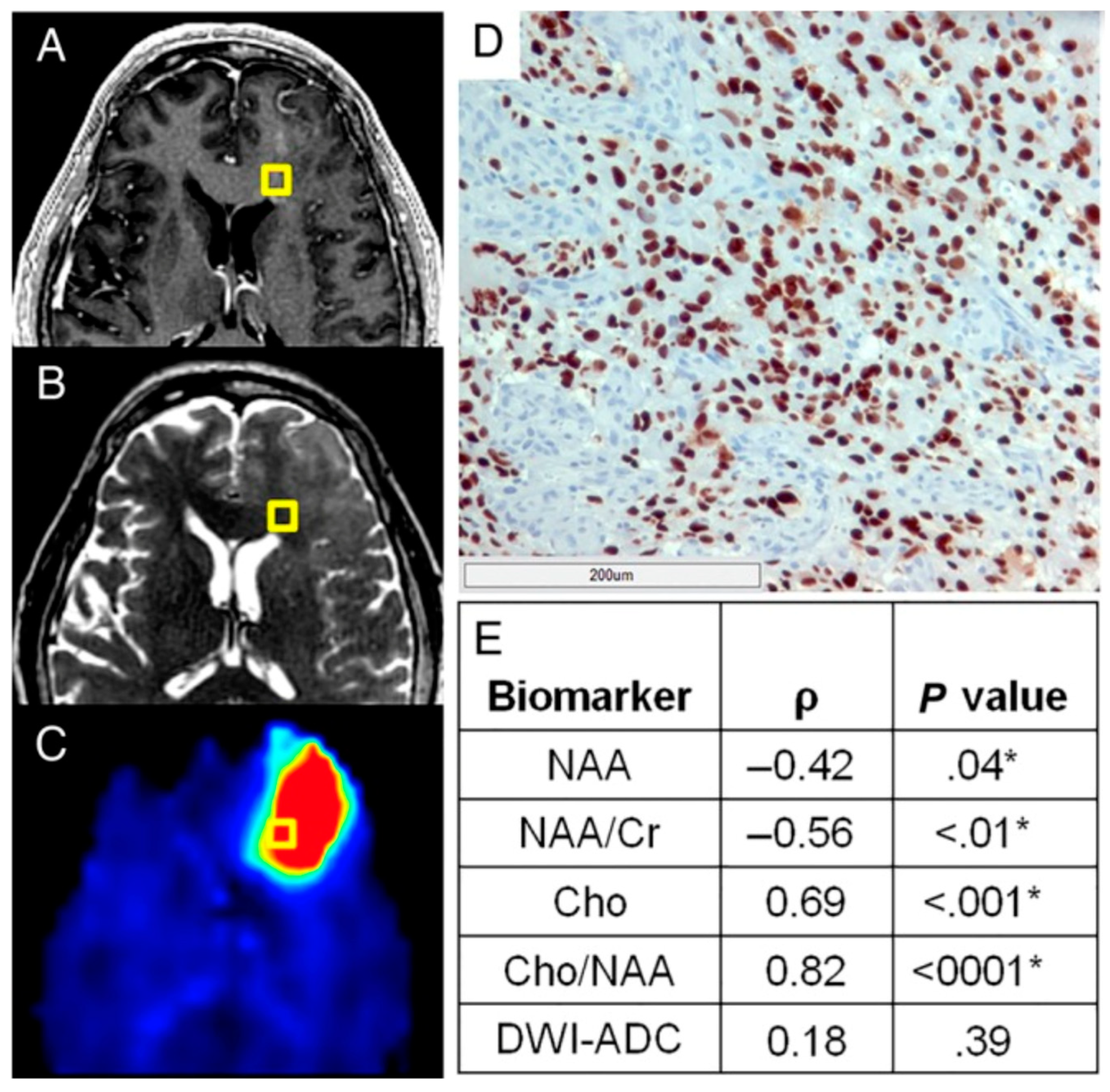
2.3. Implementation of Quantitative Imaging Modalities for Brain Tumors to Guide RT Targeting
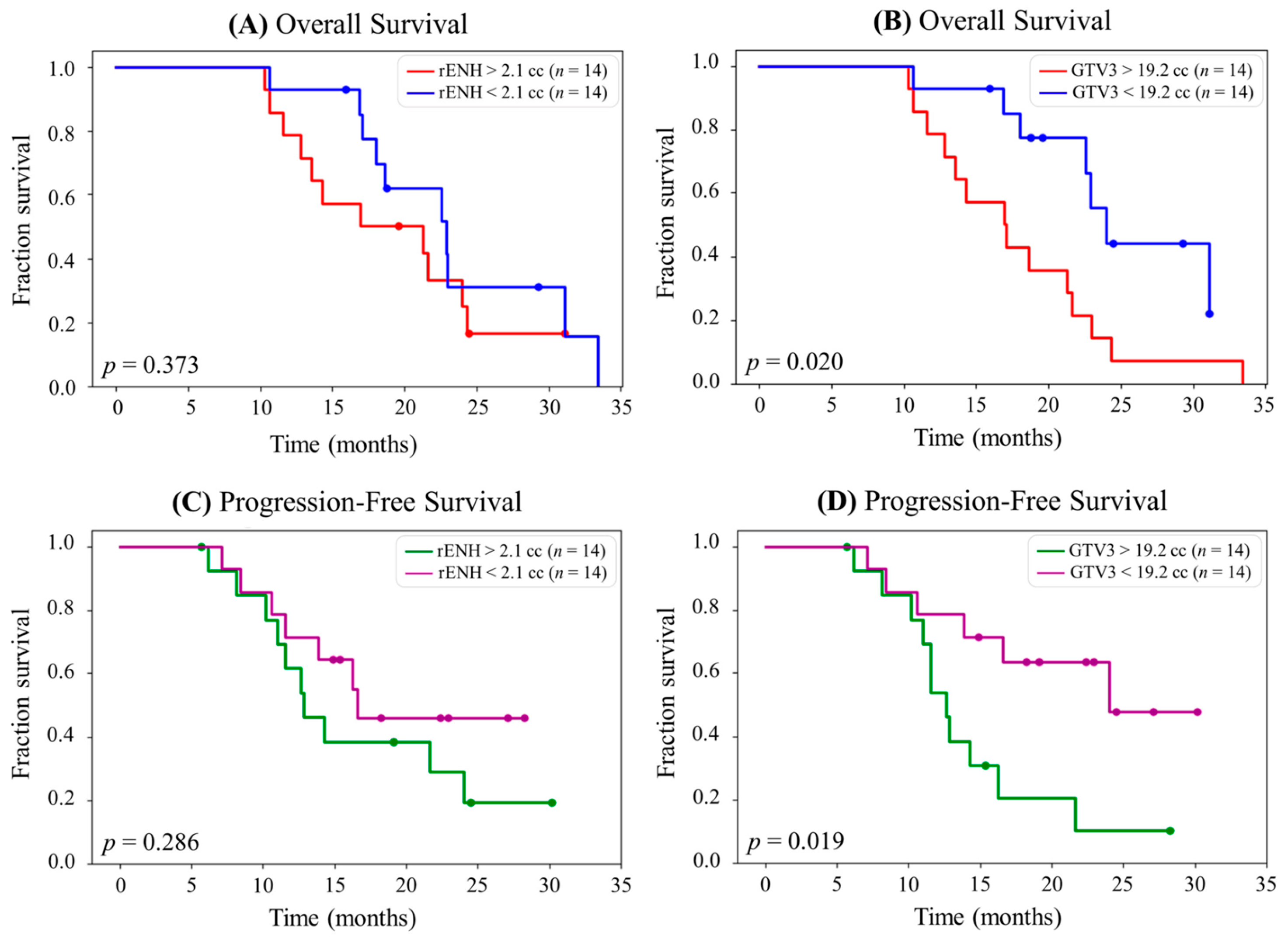
2.4. Development of “Personalized Radiotherapy” Techniques Through the Incorporation of Novel Imaging Methodologies
2.5. QA Issues with Widespread Implementation of Quantitative Imaging Modalities
3. Brief Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohan, R.; Barest, G.; Brewster, L.J.; Chui, C.S.; Kutcher, G.J.; Laughlin, J.S.; Fuks, Z. A comprehensive three-dimensional radiation treatment planning system. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Khoo, V.S.; Dearnaley, D.P.; Finnigan, D.J.; Padhani, A.; Tanner, S.F.; Leach, M.O. Magnetic resonance imaging (MRI): Considerations and applications in radiotherapy treatment planning. Radiother. Oncol. 1997, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ciernik, I.F.; Dizendorf, E.; Baumert, B.G.; Reiner, B.; Burger, C.; Davis, J.B.; Lütolf, U.M.; Steinert, H.C.; Von Schulthess, G.K. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): A feasibility study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 853–863. [Google Scholar] [CrossRef]
- Vinogradskiy, Y.; Castillo, R.; Castillo, E.; Schubert, L.; Jones, B.L.; Faught, A.; Gaspar, L.E.; Kwak, J.; Bowles, D.W.; Waxweiler, T. Results of a multi-institutional phase 2 clinical trial for 4DCT-ventilation functional avoidance thoracic radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Bucknell, N.; Hardcastle, N.; Gunewardena, R.; Nguyen, L.; Callahan, J.; Ball, D.; Selbie, L.; Kron, T.; Turgeon, G.A.; Hofman, M.S.; et al. Mid-treatment adaptive planning during thoracic radiation using 68 Ventilation-Perfusion Positron emission tomography. Clin. Transl. Radiat. Oncol. 2023, 40, 100599. [Google Scholar] [CrossRef]
- Bahig, H.; Campeau, M.P.; Lapointe, A.; Bedwani, S.; Roberge, D.; de Guise, J.; Blais, D.; Vu, T.; Lambert, L.; Chartrand-Lefebvre, C.; et al. Phase 1-2 Study of Dual-Energy Computed Tomography for Assessment of Pulmonary Function in Radiation Therapy Planning. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 334–343. [Google Scholar] [CrossRef]
- Cordova, J.S.; Shu, H.K.; Liang, Z.; Gurbani, S.S.; Cooper, L.A.; Holder, C.A.; Olson, J.J.; Kairdolf, B.; Schreibmann, E.; Neill, S.G.; et al. Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro Oncol. 2016, 18, 1180–1189. [Google Scholar] [CrossRef]
- Cordova, J.S.; Kandula, S.; Gurbani, S.; Zhong, J.; Tejani, M.; Kayode, O.; Patel, K.; Prabhu, R.; Schreibmann, E.; Crocker, I.; et al. Simulating the Effect of Spectroscopic MRI as a Metric for Radiation Therapy Planning in Patients with Glioblastoma. Tomography 2016, 2, 366–373. [Google Scholar] [CrossRef]
- Bowen, S.R.; Saini, J.; Chapman, T.R.; Miyaoka, R.S.; Kinahan, P.E.; Sandison, G.A.; Wong, T.; Vesselle, H.J.; Nyflot, M.J.; Apisarnthanarax, S. Differential hepatic avoidance radiation therapy: Proof of concept in hepatocellular carcinoma patients. Radiother. Oncol. 2015, 115, 203–210. [Google Scholar] [CrossRef]
- Lapointe, A.; Bahig, H.; Blais, D.; Bouchard, H.; Filion, E.; Carrier, J.F.; Bedwani, S. Assessing lung function using contrast-enhanced dual-energy computed tomography for potential applications in radiation therapy. Med. Phys. 2017, 44, 5260–5269. [Google Scholar] [CrossRef]
- Guerrero, T.; Sanders, K.; Castillo, E.; Zhang, Y.; Bidaut, L.; Pan, T.; Komaki, R. Dynamic ventilation imaging from four-dimensional computed tomography. Phys. Med. Biol. 2006, 51, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Kipritidis, J.; Tahir, B.A.; Cazoulat, G.; Hofman, M.S.; Siva, S.; Callahan, J.; Hardcastle, N.; Yamamoto, T.; Christensen, G.E.; Reinhardt, J.M. The VAMPIRE challenge: A multi-institutional validation study of CT ventilation imaging. Med. Phys. 2019, 46, 1198–1217. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Thomas, R.; Callahan, J.; Hardcastle, N.; Pham, D.; Kron, T.; Hicks, R.J.; MacManus, M.P.; Ball, D.L.; Hofman, M.S. High-resolution pulmonary ventilation and perfusion PET/CT allows for functionally adapted intensity modulated radiotherapy in lung cancer. Radiother. Oncol. 2015, 115, 157–162. [Google Scholar] [CrossRef]
- Faught, A.M.; Miyasaka, Y.; Kadoya, N.; Castillo, R.; Castillo, E.; Vinogradskiy, Y.; Yamamoto, T. Evaluating the toxicity reduction with CT-ventilation functional avoidance radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 325–333. [Google Scholar] [CrossRef]
- Yaremko, B.P.; Capaldi, D.P.; Sheikh, K.; Palma, D.A.; Warner, A.; Dar, A.R.; Yu, E.; Rodrigues, G.B.; Louie, A.V.; Landis, M. Functional Lung Avoidance for Individualized Radiotherapy (FLAIR): Results of a Double-Blind, Randomized Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 14, 934. [Google Scholar]
- Ramesh, K.; Mellon, E.A.; Gurbani, S.S.; Weinberg, B.D.; Schreibmann, E.; Sheriff, S.A.; Goryawala, M.; de le Fuente, M.; Eaton, B.R.; Zhong, J.; et al. A multi-institutional pilot clinical trial of spectroscopic MRI-guided radiation dose escalation for newly diagnosed glioblastoma. Neurooncol. Adv. 2022, 4, vdac006. [Google Scholar] [CrossRef] [PubMed]
- Hanania, A.N.; Mainwaring, W.; Ghebre, Y.T.; Hanania, N.A.; Ludwig, M. Radiation-Induced Lung Injury: Assessment and Management. Chest 2019, 156, 150–162. [Google Scholar] [CrossRef]
- Abratt, R.P.; Morgan, G.W.; Silvestri, G.; Willcox, P. Pulmonary complications of radiation therapy. Clin. Chest Med. 2004, 25, 167–177. [Google Scholar] [CrossRef]
- Giuranno, L.; Ient, J.; De Ruysscher, D.; Vooijs, M.A. Radiation-Induced Lung Injury (RILI). Front. Oncol. 2019, 9, 877. [Google Scholar] [CrossRef]
- Palma, D.A.; Senan, S.; Tsujino, K.; Barriger, R.B.; Rengan, R.; Moreno, M.; Bradley, J.D.; Kim, T.H.; Ramella, S.; Marks, L.B.; et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 444–450. [Google Scholar] [CrossRef]
- Zhao, J.; Yorke, E.D.; Li, L.; Kavanagh, B.D.; Li, X.A.; Das, S.; Miften, M.; Rimner, A.; Campbell, J.; Xue, J.; et al. Simple Factors Associated with Radiation-Induced Lung Toxicity After Stereotactic Body Radiation Therapy of the Thorax: A Pooled Analysis of 88 Studies. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.M.; Wang, S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin. Radiat. Oncol. 2015, 25, 100–109. [Google Scholar] [CrossRef]
- Chen, H.; Senan, S.; Nossent, E.J.; Boldt, R.G.; Warner, A.; Palma, D.A.; Louie, A.V. Treatment-Related Toxicity in Patients with Early-Stage Non-Small Cell Lung Cancer and Coexisting Interstitial Lung Disease: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.M.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation dose-volume effects in the lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S70–S76. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Hernandez, M.; Maldonado, F.; Lozano-Ruiz, F.; Munoz-Montano, W.; Nunez-Baez, M.; Arrieta, O. Radiation-induced lung injury: Current evidence. BMC Pulm. Med. 2021, 21, 9. [Google Scholar] [CrossRef]
- Sigel, K.; Wisnivesky, J.P. Comorbidity Profiles of Patients with Lung Cancer: A New Approach to Risk Stratification? Ann Am. Thorac. Soc. 2017, 14, 1512–1513. [Google Scholar] [CrossRef]
- Owen, D.R.; Sun, Y.; Boonstra, P.S.; McFarlane, M.; Viglianti, B.L.; Balter, J.M.; El Naqa, I.; Schipper, M.J.; Schonewolf, C.A.; Ten Haken, R.K.; et al. Investigating the SPECT Dose-Function Metrics Associated with Radiation-Induced Lung Toxicity Risk in Patients with Non-small Cell Lung Cancer Undergoing Radiation Therapy. Adv. Radiat. Oncol. 2021, 6, 100666. [Google Scholar] [CrossRef]
- Roach, P.J.; Schembri, G.P.; Bailey, D.L. V/Q scanning using SPECT and SPECT/CT. J. Nucl. Med. 2013, 54, 1588–1596. [Google Scholar] [CrossRef]
- Elojeimy, S.; Cruite, I.; Bowen, S.; Zeng, J.; Vesselle, H. Overview of the Novel and Improved Pulmonary Ventilation-Perfusion Imaging Applications in the Era of SPECT/CT. AJR Am. J. Roentgenol. 2016, 207, 1307–1315. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, L.; Zhou, Q.; Bi, J.; Li, Y.; Pi, G.; Wei, W.; Hu, D.; Rao, Q.; Li, H.; et al. A pilot study of function-based radiation therapy planning for lung cancer using hyperpolarized xenon-129 ventilation MRI. J. Appl. Clin. Med. Phys. 2022, 23, e13502. [Google Scholar] [CrossRef]
- Rankine, L.J.; Wang, Z.; Kelsey, C.R.; Bier, E.; Driehuys, B.; Marks, L.B.; Das, S.K. Hyperpolarized (129)Xe Magnetic Resonance Imaging for Functional Avoidance Treatment Planning in Thoracic Radiation Therapy: A Comparison of Ventilation- and Gas Exchange-Guided Treatment Plans. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 1044–1057. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, H.; Salem, A.; Tijssen, R.H.N.; Dubec, M.; Wetscherek, A.; Van Es, C.; Belderbos, J.; Faivre-Finn, C.; McDonald, F.; lung tumour site group of the international Atlantic MR-Linac Consortium. Magnetic resonance imaging in precision radiation therapy for lung cancer. Transl. Lung Cancer Res. 2017, 6, 689–707. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, G.M.D.; Zhang, H.; D’Souza, W.; Ha, L.; Rosenberger, J.M. Four-dimensional computed tomography-based ventilation imaging in intensity-modulated radiation therapy treatment planning for pulmonary functional avoidance. J. Appl. Clin. Med. Phys. 2023, 24, e13920. [Google Scholar] [CrossRef]
- Miller, R.; Castillo, R.; Castillo, E.; Jones, B.L.; Miften, M.; Kavanagh, B.; Lu, B.; Werner-Wasik, M.; Ghassemi, N.; Lombardo, J.; et al. Characterizing Pulmonary Function Test Changes for Patients with Lung Cancer Treated on a 2-Institution, 4-Dimensional Computed Tomography-Ventilation Functional Avoidance Prospective Clinical Trial. Adv. Radiat. Oncol. 2023, 8, 101133. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Chen, J.L.; Lan, H.T.; Tai, M.H.; Kuo, S.H.; Shih, J.Y.; Chang, Y.C. Xenon-Enhanced Ventilation Computed Tomography for Functional Lung Avoidance Radiation Therapy in Patients with Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 356–365. [Google Scholar] [CrossRef]
- Kong, X.; Sheng, H.X.; Lu, G.M.; Meinel, F.G.; Dyer, K.T.; Schoepf, U.J.; Zhang, L.J. Xenon-enhanced dual-energy CT lung ventilation imaging: Techniques and clinical applications. AJR Am. J. Roentgenol. 2014, 202, 309–317. [Google Scholar] [CrossRef]
- Lapointe, A.; Lalonde, A.; Bahig, H.; Carrier, J.F.; Bedwani, S.; Bouchard, H. Robust quantitative contrast-enhanced dual-energy CT for radiotherapy applications. Med. Phys. 2018, 45, 3086–3096. [Google Scholar] [CrossRef]
- van Elmpt, W.; Landry, G.; Das, M.; Verhaegen, F. Dual energy CT in radiotherapy: Current applications and future outlook. Radiother. Oncol. 2016, 119, 137–144. [Google Scholar] [CrossRef]
- Zhang, S.; Lapointe, A.; Simard, M.; Filion, M.P.; Campeau, D.; Roberge, H.; Bouchard, J.F.; Carrier, D.; Blais, S.; Bedwani, H. Bahig. (ABSTRACT) Evaluation of Radiation Dose Effect on Lung Function Using Iodine Maps Derived from Serial Dual-Energy Computed Tomograms. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e366–e367. [Google Scholar]
- Zhang, S.; Simard, M.; Lapointe, A.; Filion, É.; Campeau, M.-P.; Vu, T.T.T.; Roberge, D.; Carrier, J.-F.; Blais, D.; Bedwani, S. Evaluation of radiation dose effect on lung function using iodine maps derived from dual-energy computed tomography. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, 894–903. [Google Scholar]
- De Bari, B.; Deantonio, L.; Bourhis, J.; Prior, J.O.; Ozsahin, M. Should we include SPECT lung perfusion in radiotherapy treatment plans of thoracic targets? Evidences from the literature. Crit. Rev. Oncol. Hematol. 2016, 102, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Callahan, J.; Hofman, M.S.; Siva, S.; Kron, T.; Schneider, M.E.; Binns, D.; Eu, P.; Hicks, R.J. High-resolution imaging of pulmonary ventilation and perfusion with 68Ga-VQ respiratory gated (4-D) PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, M.; Bucknell, N.; Woon, B.; Kron, T.; Hofman, M.S.; Siva, S.; Hardcastle, N. Dose-Response Relationship Between Radiation Therapy and Loss of Lung Perfusion Comparing Positron Emission Tomography and Dual-Energy Computed Tomography in Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 118, 1135–1143. [Google Scholar] [CrossRef]
- McIntosh, L.; Jackson, P.; Hardcastle, N.; Bressel, M.; Kron, T.; Callahan, J.W.; Steinfort, D.; Bucknell, N.; Hofman, M.S.; Siva, S. Automated assessment of functional lung imaging with 68Ga-ventilation/perfusion PET/CT using iterative histogram analysis. EJNMMI Phys. 2021, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Hardcastle, N.; Kron, T.; Bressel, M.; Callahan, J.; MacManus, M.P.; Shaw, M.; Plumridge, N.; Hicks, R.J.; Steinfort, D.; et al. Ventilation/Perfusion Positron Emission Tomography--Based Assessment of Radiation Injury to Lung. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 408–417. [Google Scholar] [CrossRef]
- Bucknell, N.W.; Hardcastle, N.; Woon, B.; Selbie, L.; Bressel, M.; Byrne, K.; Callahan, J.; Hanna, G.G.; Hofman, M.S.; Ball, D.; et al. The HI-FIVE Trial: A Prospective Trial Using 4-Dimensional (68)Ga Ventilation-Perfusion Positron Emission Tomography-Computed Tomography for Functional Lung Avoidance in Locally Advanced Non-small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 887–892. [Google Scholar] [CrossRef]
- Go, K.G.; Kamman, R.L.; Mooyaart, E.L.; Heesters, M.A.; Pruim, J.; Vaalburg, W.; Paans, A.M. Localised proton spectroscopy and spectroscopic imaging in cerebral gliomas, with comparison to positron emission tomography. Neuroradiology 1995, 37, 198–206. [Google Scholar] [CrossRef]
- Nelson, S.J.; Vigneron, D.B.; Dillon, W.P. Serial evaluation of patients with brain tumors using volume MRI and 3D 1H MRSI. NMR Biomed. 1999, 12, 123–138. [Google Scholar] [CrossRef]
- Chang, J.; Thakur, S.; Perera, G.; Kowalski, A.; Huang, W.; Karimi, S.; Hunt, M.; Koutcher, J.; Fuks, Z.; Amols, H.; et al. Image-fusion of MR spectroscopic images for treatment planning of gliomas. Med. Phys. 2006, 33, 32–40. [Google Scholar]
- Graves, E.E.; Pirzkall, A.; Nelson, S.J.; Larson, D.; Verhey, L. Registration of magnetic resonance spectroscopic imaging to computed tomography for radiotherapy treatment planning. Med. Phys. 2001, 28, 2489–2496. [Google Scholar]
- Narayana, A.; Chang, J.; Thakur, S.; Huang, W.; Karimi, S.; Hou, B.; Kowalski, A.; Perera, G.; Holodny, A.; Gutin, P.H. Use of MR spectroscopy and functional imaging in the treatment planning of gliomas. Br. J. Radiol. 2007, 80, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Pirzkall, A.; Li, X.; Oh, J.; Chang, S.; Berger, M.S.; Larson, D.A.; Verhey, L.J.; Dillon, W.P.; Nelson, S.J. 3D MRSI for resected high-grade gliomas before RT: Tumor extent according to metabolic activity in relation to MRI. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Maudsley, A.A.; Domenig, C.; Govind, V.; Darkazanli, A.; Studholme, C.; Arheart, K.; Bloomer, C. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn. Reson. Med. 2009, 61, 548–559. [Google Scholar] [CrossRef]
- Cordova, J.S.; Gurbani, S.S.; Olson, J.J.; Liang, Z.; Cooper, L.A.; Shu, H.G.; Schreibmann, E.; Neill, S.G.; Hadjipanayis, C.G.; Holder, C.A.; et al. A systematic pipeline for the objective comparison of whole-brain spectroscopic MRI with histology in biopsy specimens from grade III glioma. Tomography 2016, 2, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, S.; Weinberg, B.; Cooper, L.; Mellon, E.; Schreibmann, E.; Sheriff, S.; Maudsley, A.; Goryawala, M.; Shu, H.K.; Shim, H. The Brain Imaging Collaboration Suite (BrICS): A Cloud Platform for Integrating Whole-Brain Spectroscopic MRI into the Radiation Therapy Planning Workflow. Tomography 2019, 5, 184–191. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.; Tsien, C.; Chenevert, T.; Gilbert, M.R.; Omuro, A.; Mcdonough, J.; Aldape, K.; Srinivasan, A.; Rogers, C.L.; et al. Radiotherapy (RT) Dose-intensification (DI) Using Intensitymodulated RT (IMRT) versus Standard-dose (SD) RT with Temozolomide (TMZ) in Newly Diagnosed Glioblastoma (GBM): Preliminary Results of NRG Oncology BN001. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S22–S23. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Trivedi, A.G.; Ramesh, K.K.; Huang, V.; Mellon, E.A.; Barker, P.B.; Kleinberg, L.R.; Weinberg, B.D.; Shu, H.G.; Shim, H. Spectroscopic MRI-Based Biomarkers Predict Survival for Newly Diagnosed Glioblastoma in a Clinical Trial. Cancers 2023, 15, 3524. [Google Scholar] [CrossRef]
- Laprie, A.; Noel, G.; Chaltiel, L.; Truc, G.; Sunyach, M.P.; Charissoux, M.; Magne, N.; Auberdiac, P.; Biau, J.; Ken, S.; et al. Randomized phase III trial of metabolic imaging-guided dose escalation of radio-chemotherapy in patients with newly diagnosed glioblastoma (SPECTRO GLIO trial). Neuro Oncol. 2024, 26, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.G.; Shim, H. SPECTRO GLIO trial aftermath: Where do we go from here? Neuro Oncol. 2024, 26, 164–165. [Google Scholar] [CrossRef]
- Kim, M.M.; Parmar, H.A.; Aryal, M.P.; Mayo, C.S.; Balter, J.M.; Lawrence, T.S.; Cao, Y. Developing a Pipeline for Multiparametric MRI-Guided Radiation Therapy: Initial Results from a Phase II Clinical Trial in Newly Diagnosed Glioblastoma. Tomography 2019, 5, 118–126. [Google Scholar] [CrossRef]
- Pafundi, D.H.; Laack, N.N.; Youland, R.S.; Parney, I.F.; Lowe, V.J.; Giannini, C.; Kemp, B.J.; Grams, M.P.; Morris, J.M.; Hoover, J.M.; et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: Results of a prospective pilot study. Neuro Oncol. 2013, 15, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Sun, Y.; Aryal, M.P.; Parmar, H.A.; Piert, M.; Rosen, B.; Mayo, C.S.; Balter, J.M.; Schipper, M.; Gabel, N.; et al. A Phase 2 Study of Dose-intensified Chemoradiation Using Biologically Based Target Volume Definition in Patients with Newly Diagnosed Glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Laack, N.N.; Pafundi, D.; Anderson, S.K.; Kaufmann, T.; Lowe, V.; Hunt, C.; Vogen, D.; Yan, E.; Sarkaria, J.; Brown, P.; et al. Initial Results of a Phase 2 Trial of (18)F-DOPA PET-Guided Dose-Escalated Radiation Therapy for Glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1383–1395. [Google Scholar] [CrossRef]
- Schwartz, D.L.; Garden, A.S.; Thomas, J.; Chen, Y.; Zhang, Y.; Lewin, J.; Chambers, M.S.; Dong, L. Adaptive radiotherapy for head-and-neck cancer: Initial clinical outcomes from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 986–993. [Google Scholar] [CrossRef]
- Castelli, J.; Thariat, J.; Benezery, K.; Hasbini, A.; Gery, B.; Berger, A.; Liem, X.; Guihard, S.; Chapet, S.; Thureau, S.; et al. Weekly Adaptive Radiotherapy vs Standard Intensity-Modulated Radiotherapy for Improving Salivary Function in Patients with Head and Neck Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.R.; Bahig, H.; Aristophanous, M.; Blanchard, P.; Kamal, M.; Ding, Y.; Cardenas, C.E.; Brock, K.K.; Lai, S.Y.; Hutcheson, K.A.; et al. Prospective in silico study of the feasibility and dosimetric advantages of MRI-guided dose adaptation for human papillomavirus positive oropharyngeal cancer patients compared with standard IMRT. Clin. Transl. Radiat. Oncol. 2018, 11, 11–18. [Google Scholar] [CrossRef]
- Matoba, M.; Tuji, H.; Shimode, Y.; Toyoda, I.; Kuginuki, Y.; Miwa, K.; Tonami, H. Fractional change in apparent diffusion coefficient as an imaging biomarker for predicting treatment response in head and neck cancer treated with chemoradiotherapy. AJNR Am. J. Neuroradiol. 2014, 35, 379–385. [Google Scholar] [CrossRef]
- Vandecaveye, V.; Dirix, P.; De Keyzer, F.; de Beeck, K.O.; Vander Poorten, V.; Roebben, I.; Nuyts, S.; Hermans, R. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for head and neck squamous cell carcinoma. Eur. Radiol. 2010, 20, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Schakel, T.; Hoogduin, J.M.; Terhaard, C.H.J.; Philippens, M.E.P. Technical Note: Diffusion-weighted MRI with minimal distortion in head-and-neck radiotherapy using a turbo spin echo acquisition method. Med. Phys. 2017, 44, 4188–4193. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.A.; Salzillo, T.; Mulder, S.; Ahmed, S.; Dresner, A.; Preston, K.; He, R.; Christodouleas, J.; Mohamed, A.S.R.; Philippens, M.; et al. Prospective evaluation of in vivo and phantom repeatability and reproducibility of diffusion-weighted MRI sequences on 1.5 T MRI-linear accelerator (MR-Linac) and MR simulator devices for head and neck cancers. Radiother. Oncol. 2023, 185, 109717. [Google Scholar] [CrossRef] [PubMed]
- Joint, H.; Neck Radiation Therapy, M.R.I.D.C.; Head, M.R.-L.C.; Neck Tumor Site, G. Longitudinal diffusion and volumetric kinetics of head and neck cancer magnetic resonance on a 1.5 T MR-linear accelerator hybrid system: A prospective R-IDEAL stage 2a imaging biomarker characterization/pre-qualification study. Clin. Transl. Radiat. Oncol. 2023, 42, 100666. [Google Scholar] [CrossRef]
- Joint, H.; Neck Radiotherapy, M.R.I.D.C.; Mohamed, A.S.R.; Abusaif, A.; He, R.; Wahid, K.A.; Salama, V.; Youssef, S.; McDonald, B.A.; Naser, M.; et al. Prospective validation of diffusion-weighted MRI as a biomarker of tumor response and oncologic outcomes in head and neck cancer: Results from an observational biomarker pre-qualification study. Radiother. Oncol. 2023, 183, 109641. [Google Scholar] [CrossRef]
- Fu, S.; Li, Y.; Han, Y.; Wang, H.; Chen, Y.; Yan, O.; He, Q.; Ma, H.; Liu, L.; Liu, F. Diffusion-Weighted Magnetic Resonance Imaging-Guided Dose Painting in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma Treated With Induction Chemotherapy Plus Concurrent Chemoradiotherapy: A Randomized, Controlled Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 101–113. [Google Scholar] [CrossRef]
- Riaz, N.; Sherman, E.; Pei, X.; Schoder, H.; Grkovski, M.; Paudyal, R.; Katabi, N.; Selenica, P.; Yamaguchi, T.N.; Ma, D.; et al. Precision Radiotherapy: Reduction in Radiation for Oropharyngeal Cancer in the 30 ROC Trial. J. Natl. Cancer. Inst. 2021, 113, 742–751. [Google Scholar] [CrossRef]
- Mierzwa, M.L.; Aryal, M.; Lee, C.; Schipper, M.; VanTil, M.; Morales, K.; Swiecicki, P.L.; Casper, K.A.; Malloy, K.M.; Spector, M.E.; et al. Randomized Phase II Study of Physiologic MRI-Directed Adaptive Radiation Boost in Poor Prognosis Head and Neck Cancer. Clin. Cancer Res. 2022, 28, 5049–5057. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; He, L.; Chen, X.; Pan, D.; Ma, Z.; Liang, C.; Tian, J.; Liang, C. Radiomics signature: A potential biomarker for the prediction of disease-free survival in early-stage (I or II) non—Small cell lung cancer. Radiology 2016, 281, 947–957. [Google Scholar] [CrossRef]
- Lucht, R.E.; Knopp, M.V.; Brix, G. Classification of signal-time curves from dynamic MR mammography by neural networks. Magn. Reson. Imaging 2001, 19, 51–57. [Google Scholar] [CrossRef]
- Chung, C.; Kalpathy-Cramer, J.; Knopp, M.V.; Jaffray, D.A. In the era of deep learning, why reconstruct an image at all? J. Am. Coll. Radiol. 2021, 18, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, T.J.; Bishop-Jodoin, M.; Followill, D.S.; Galvin, J.; Knopp, M.V.; Michalski, J.M.; Rosen, M.A.; Bradley, J.D.; Shankar, L.K.; Laurie, F. Imaging and data acquisition in clinical trials for radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Neupane, T.; Castillo, E.; Chen, Y.; Pahlavian, S.; Castillo, R.; Vinogradskiy, Y.; Choi, W. Predicting Radiation Pneumonitis with Robust 4DCT-Ventilation and 4DCT-Perfusion Imaging Using Prospective Lung Cancer Clinical Trial Data. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, S141. [Google Scholar] [CrossRef]
- Wilson, L.J.; Castillo, R.; Castillo, E.; Jones, B.; Miften, M.; Olsen, L.; Aragam, V.; Meguid, R.; Erickson, C.; Young, A. Results of a Prospective Trial to Evaluate Novel Lung Function Imaging for Lung Cancer Surgery. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, S53. [Google Scholar] [CrossRef]
- Walls, G.; Hugo, G.; Ghadban, R.; Javaheri, A.; Moore, K.; Knutson, N.; Cooper, D.; Rentschler, S.; Samson, P.; Robinson, C. Longitudinal Trends in Myocardial Metabolism following Non-Invasive Cardiac Radioablation for Ventricular Tachycardia Using PET-CT. Int. J. Radiat. Oncol. Biol. Phys. 2024, 120, S140. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Andronesi, O.C.; Barker, P.B.; Bizzi, A.; Bogner, W.; Henning, A.; Nelson, S.J.; Posse, S.; Shungu, D.C.; Soher, B.J. Advanced magnetic resonance spectroscopic neuroimaging: Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4309. [Google Scholar] [CrossRef]
- Castillo, E.; Castillo, R.; Vinogradskiy, Y.; Dougherty, M.; Solis, D.; Myziuk, N.; Thompson, A.; Guerra, R.; Nair, G.; Guerrero, T. Robust CT ventilation from the integral formulation of the Jacobian. Med. Phys. 2019, 46, 2115–2125. [Google Scholar] [CrossRef]
- Long, D.; Tann, M.; Huang, K.; Bartlett, G.; Galle, J.; Furukawa, Y.; Maluccio, M.; Cox, J.; Kong, F. Functional Liver Image-Guided Hepatic Therapy (FLIGHT) with Hepatobiliary Iminodiacetic Acid (HIDA) Scans: Functional Parameters May Predict for Decompensation after Stereotactic Body Radiation Therapy (SBRT). Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, e59. [Google Scholar] [CrossRef]
- Vinogradskiy, Y.; Jackson, M.; Schubert, L.; Jones, B.; Castillo, R.; Castillo, E.; Guerrero, T.; Mitchell, J.; Rusthoven, C.; Miften, M. Assessing the use of 4DCT-ventilation in Pre-operative Surgical Lung Cancer Evaluation. Med. Phys. 2017, 44, 200–208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinogradskiy, Y.; Bahig, H.; Bucknell, N.W.; Buchsbaum, J.; Shu, H.-K.G. Conference Report: Review of Clinical Implementation of Advanced Quantitative Imaging Techniques for Personalized Radiotherapy. Tomography 2024, 10, 1798-1813. https://doi.org/10.3390/tomography10110132
Vinogradskiy Y, Bahig H, Bucknell NW, Buchsbaum J, Shu H-KG. Conference Report: Review of Clinical Implementation of Advanced Quantitative Imaging Techniques for Personalized Radiotherapy. Tomography. 2024; 10(11):1798-1813. https://doi.org/10.3390/tomography10110132
Chicago/Turabian StyleVinogradskiy, Yevgeniy, Houda Bahig, Nicholas W. Bucknell, Jeffrey Buchsbaum, and Hui-Kuo George Shu. 2024. "Conference Report: Review of Clinical Implementation of Advanced Quantitative Imaging Techniques for Personalized Radiotherapy" Tomography 10, no. 11: 1798-1813. https://doi.org/10.3390/tomography10110132
APA StyleVinogradskiy, Y., Bahig, H., Bucknell, N. W., Buchsbaum, J., & Shu, H.-K. G. (2024). Conference Report: Review of Clinical Implementation of Advanced Quantitative Imaging Techniques for Personalized Radiotherapy. Tomography, 10(11), 1798-1813. https://doi.org/10.3390/tomography10110132




