Applications of Advanced Imaging for Radiotherapy Planning and Response Assessment in the Central Nervous System
Abstract
1. Introduction
1.1. Overview
1.2. Gliomas and Brain Metastases
1.3. Neuroimaging and Response Assessment
1.4. Clinical Challenges
1.5. Advanced MRI and PET
1.6. Structure of This Review
2. Advanced MRI on MR-Linacs for Adaptive Radiotherapy of Gliomas
2.1. MRI-Linear Accelerators
2.2. Technical Validation: Quantitative Relaxometry
2.3. Technical Validation: Apparent Diffusion Coefficient
2.4. Technical Validation: Perfusion Imaging
2.5. Technical Validation: Saturation Transfer
2.6. Clinical Validation Studies
2.7. Summary and Future Directions
3. FET-PET/MRI in Gliomas
4. Contrast-Enhanced Imaging of Radiation Necrosis for Brain Metastases
4.1. Conventional MRI
4.2. Artificial Intelligence Approaches
4.3. Contrast-Enhanced T2-FLAIR: A New Imaging Biomarker
5. Predicting Radiation Necrosis with Metabolic Imaging
5.1. MRS, Perfusion, and Non-MRI Techniques
5.2. Pilot MT/CEST MRI Studies for Predicting RN vs. TP
5.3. Differentiation of RN and TP in a Clinical Setting
5.4. Pulsed Saturation
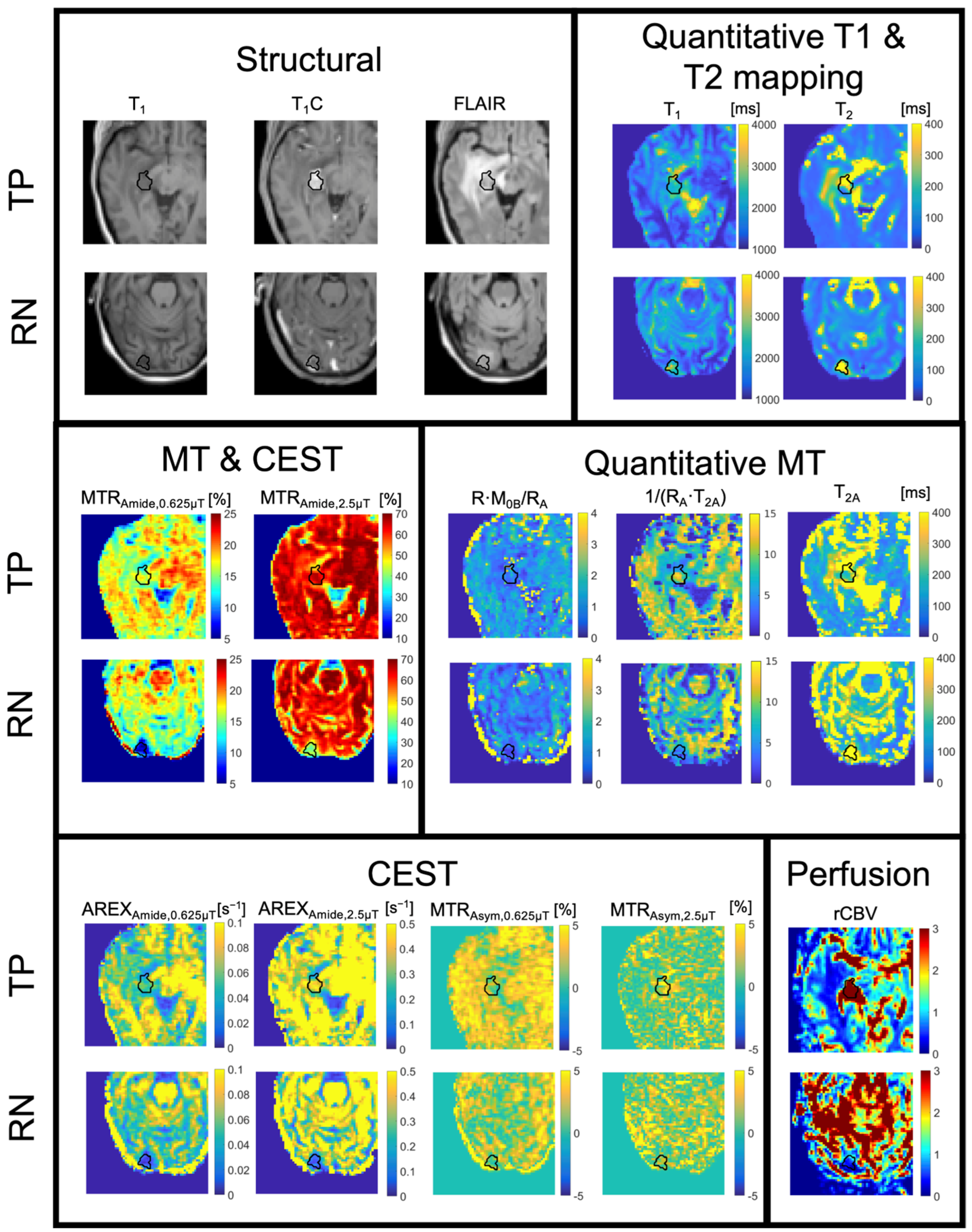
5.5. MT/CEST Maps in a Specific Case of Radiation Necrosis Versus Tumor Progression
5.6. Origins of the MT/CEST Signal
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Wen, P.Y.; Chang, S.M.; Dirven, L.; Lim, M.; Monje, M.; Reifenberger, G. Glioma. Nat. Rev. Dis. Prim. 2024, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Hegi, M.E.; Diserens, A.-C.; Gorlia, T.; Hamou, M.-F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Zhang, Y.; Phillips, J.J.; Morshed, R.A.; Young, J.S.; McCoy, L.; Lafontaine, M.; Luks, T.; Ammanuel, S.; Kakaizada, S.; et al. Interactive Effects of Molecular, Therapeutic, and Patient Factors on Outcome of Diffuse Low-Grade Glioma. J. Clin. Oncol. 2023, 41, 2029–2042. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection with Survival in Glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef]
- Forst, D.A.; Nahed, B.V.; Loeffler, J.S.; Batchelor, T.T. Low-Grade Gliomas. Oncologist 2014, 19, 403–413. [Google Scholar] [CrossRef]
- Rudà, R.; Horbinski, C.; Bent, M.v.D.; Preusser, M.; Soffietti, R. IDH inhibition in gliomas: From preclinical models to clinical trials. Nat. Rev. Neurol. 2024, 20, 395–407. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Bent, M.J.v.D.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N. Engl. J. Med. 2023, 389, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgård, G.; Solheim, O. Comparison of a Strategy Favoring Early Surgical Resection vs a Strategy Favoring Watchful Waiting in Low-Grade Gliomas. JAMA 2012, 308, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Prim. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of Brain Metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Survival in Patients with Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J. Clin. Oncol. 2020, 38, 3773–3784. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef]
- Lin, X.; DeAngelis, L.M. Treatment of Brain Metastases. J. Clin. Oncol. 2015, 33, 3475–3484. [Google Scholar] [CrossRef]
- Redmond, K.J.; Gui, C.; Benedict, S.; Milano, M.T.; Grimm, J.; Vargo, J.A.; Soltys, S.G.; Yorke, E.; Jackson, A.; El Naqa, I.; et al. Tumor Control Probability of Radiosurgery and Fractionated Stereotactic Radiosurgery for Brain Metastases. Int. J. Radiat. Oncol. 2021, 110, 53–67. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients with 1 to 3 Brain Metastases. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef]
- Nagpal, S.; Milano, M.T.; Chiang, V.L.; Soltys, S.G.; Brackett, A.; Halasz, L.M.; Garg, A.K.; Sahgal, A.; Ahluwalia, M.S.; Tom, M.C.; et al. Executive summary of the American Radium Society appropriate use criteria for brain metastases in epidermal growth factor receptor mutated-mutated and ALK-fusion non-small cell lung cancer. Neuro-Oncology 2024, 26, 1195–1212. [Google Scholar] [CrossRef]
- Watanabe, M.; Tanaka, R.; Takeda, N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 1992, 34, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Rajky, O.; Winkler, F.; Bartsch, R.; Furtner, J.; Hainfellner, J.A.; Goodman, S.L.; Weller, M.; Schittenhelm, J.; Preusser, M. Invasion patterns in brain metastases of solid cancers. Neuro-Oncology 2013, 15, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Bent, M.v.D.; Youssef, G.; Cloughesy, T.F.; Ellingson, B.M.; Weller, M.; Galanis, E.; Barboriak, D.P.; de Groot, J.; Gilbert, M.R.; et al. RANO 2.0: Update to the Response Assessment in Neuro-Oncology Criteria for High- and Low-Grade Gliomas in Adults. J. Clin. Oncol. 2023, 41, 5187–5199. [Google Scholar] [CrossRef]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; Dancey, J.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
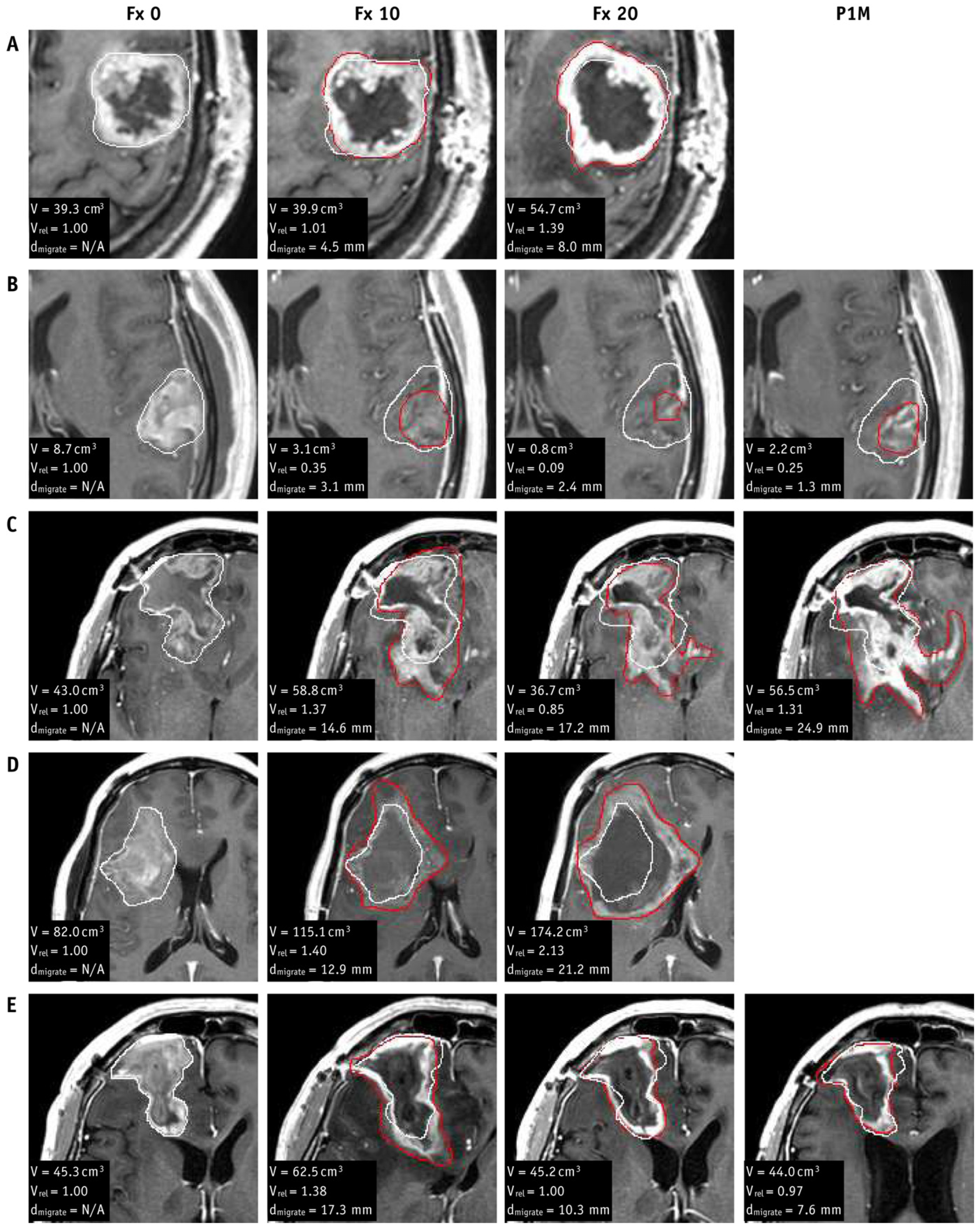
- Stewart, J.; Sahgal, A.; Lee, Y.; Soliman, H.; Tseng, C.-L.; Detsky, J.; Husain, Z.; Ho, L.; Das, S.; Maralani, P.J.; et al. Quantitating Interfraction Target Dynamics During Concurrent Chemoradiation for Glioblastoma: A Prospective Serial Imaging Study. Int. J. Radiat. Oncol. 2021, 109, 736–746. [Google Scholar] [CrossRef]
- Bernchou, U.; Arnold, T.S.T.; Axelsen, B.; Klüver-Kristensen, M.; Mahmood, F.; Harbo, F.S.G.; Asmussen, J.T.; Hansen, O.; Bertelsen, A.S.; Hansen, S.; et al. Evolution of the gross tumour volume extent during radiotherapy for glioblastomas. Radiother. Oncol. 2021, 160, 40–46. [Google Scholar] [CrossRef]
- Azoulay, M.; Chang, S.D.; Gibbs, I.C.; Hancock, S.L.; Pollom, E.L.; Harsh, G.R.; Adler, J.R.; Harraher, C.; Li, G.; Gephart, M.H.; et al. A phase I/II trial of 5-fraction stereotactic radiosurgery with 5-mm margins with concurrent temozolomide in newly diagnosed glioblastoma: Primary outcomes. Neuro-Oncology 2020, 22, 1182–1189. [Google Scholar] [CrossRef]
- Detsky, J.; Chan, A.; Palhares, D.; Hudson, J.; Stewart, J.; Chen, H.; Das, S.; Lipsman, N.; Lim-Fat, M.; Perry, J.; et al. MR-Linac On-Line Weekly Adaptive Radiotherapy for High Grade Glioma (HGG): Results from the UNITED Single Arm Phase II Trial. Int. J. Radiat. Oncol. 2024, 120, S4. [Google Scholar] [CrossRef]
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.M.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavaré, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014. [Google Scholar] [CrossRef]
- Patel, A.P.; Tirosh, I.; Trombetta, J.J.; Shalek, A.K.; Gillespie, S.M.; Wakimoto, H.; Cahill, D.P.; Nahed, B.V.; Curry, W.T.; Martuza, R.L.; et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 2014, 344, 1396–1401. [Google Scholar] [CrossRef]
- Thust, S.C.; Bent, M.J.v.D.; Smits, M. Pseudoprogression of brain tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Brandes, A.A.; Franceschi, E.; Tosoni, A.; Blatt, V.; Pession, A.; Tallini, G.; Bertorelle, R.; Bartolini, S.; Calbucci, F.; Andreoli, A.; et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 2008, 26, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Zikou, A.; Sioka, C.; Alexiou, G.A.; Fotopoulos, A.; Voulgaris, S.; Argyropoulou, M.I. Radiation Necrosis, Pseudoprogression, Pseudoresponse, and Tumor Recurrence: Imaging Challenges for the Evaluation of Treated Gliomas. Contrast Media Mol. Imaging 2018, 2018, 6828396. [Google Scholar] [CrossRef]
- Vellayappan, B.; Tan, C.L.; Yong, C.; Khor, L.K.; Koh, W.Y.; Yeo, T.T.; Detsky, J.; Lo, S.; Sahgal, A. Diagnosis and Management of Radiation Necrosis in Patients with Brain Metastases. Front. Oncol. 2018, 8, 395. [Google Scholar] [CrossRef]
- Sagiyama, K.; Mashimo, T.; Togao, O.; Vemireddy, V.; Hatanpaa, K.J.; Maher, E.A.; Mickey, B.E.; Pan, E.; Sherry, A.D.; Bachoo, R.M.; et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 4542–4547. [Google Scholar] [CrossRef]
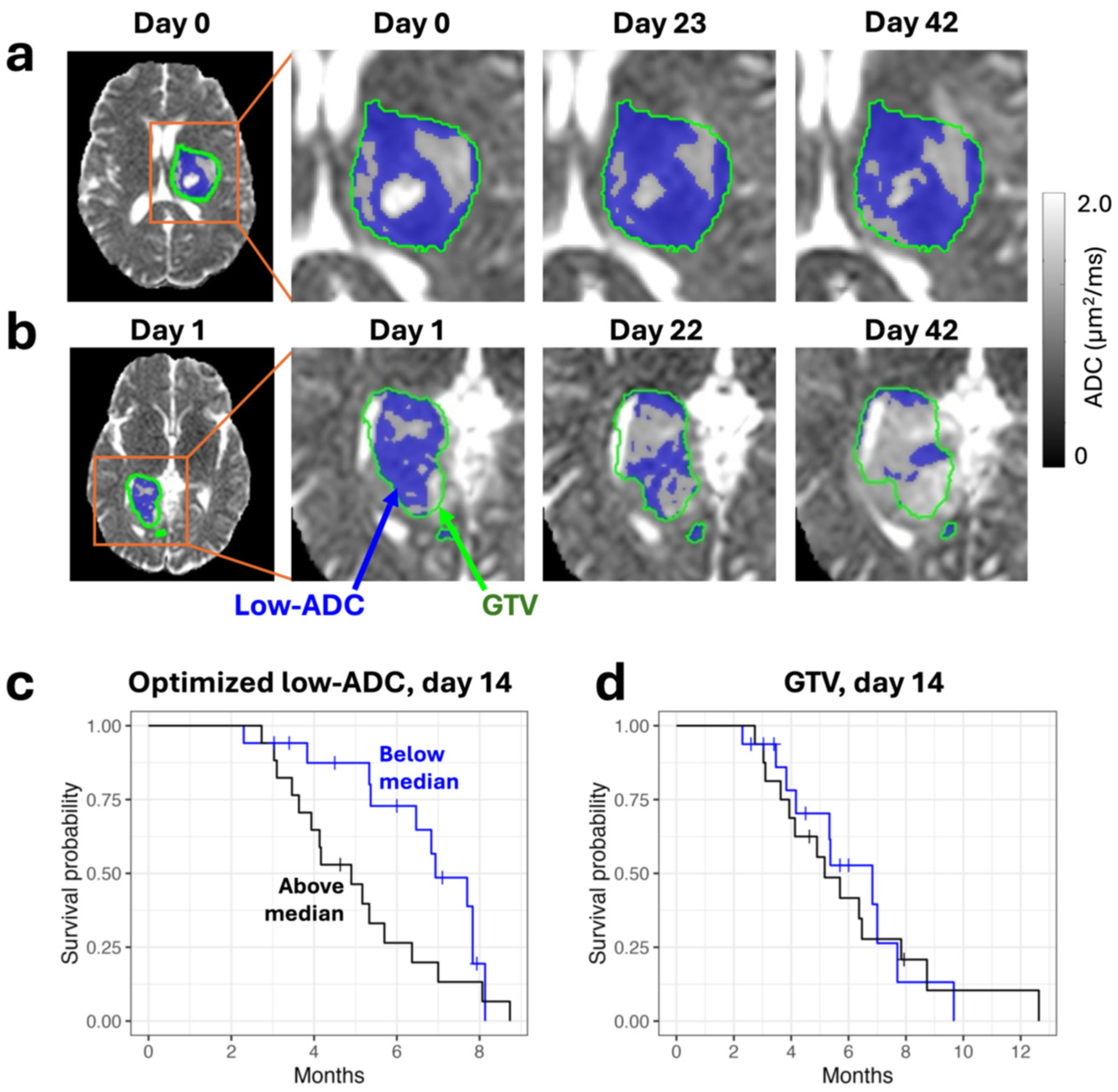
- Hamstra, D.A.; Galbán, C.J.; Meyer, C.R.; Johnson, T.D.; Sundgren, P.C.; Tsien, C.; Lawrence, T.S.; Junck, L.; Ross, D.J.; Rehemtulla, A.; et al. Functional Diffusion Map as an Early Imaging Biomarker for High-Grade Glioma: Correlation with Conventional Radiologic Response and Overall Survival. J. Clin. Oncol. 2008, 26, 3387–3394. [Google Scholar] [CrossRef]
- Galbán, C.J.; Chenevert, T.L.; Meyer, C.R.; Tsien, C.; Lawrence, T.S.; A Hamstra, D.; Junck, L.; Sundgren, P.C.; Johnson, T.D.; Ross, D.J.; et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat. Med. 2009, 15, 572–576. [Google Scholar] [CrossRef]
- Mehrabian, H.; Myrehaug, S.; Soliman, H.; Sahgal, A.; Stanisz, G.J. Evaluation of Glioblastoma Response to Therapy with Chemical Exchange Saturation Transfer. Int. J. Radiat. Oncol. 2018, 101, 713–723. [Google Scholar] [CrossRef]
- Lawrence, L.S.; Chan, R.W.; Chen, H.; Stewart, J.; Ruschin, M.; Theriault, A.; Myrehaug, S.; Detsky, J.; Maralani, P.J.; Tseng, C.-L.; et al. Diffusion-weighted imaging on an MRI-linear accelerator to identify adversely prognostic tumour regions in glioblastoma during chemoradiation. Radiother. Oncol. 2023, 188, 109873. [Google Scholar] [CrossRef]
- Carrete, L.R.; Young, J.S.; Cha, S. Advanced Imaging Techniques for Newly Diagnosed and Recurrent Gliomas. Front. Neurosci. 2022, 16, 787755. [Google Scholar] [CrossRef] [PubMed]
- Mountz, J.M.; Ahmed, R.; Oborski, M.J.; Lieberman, F.S.; Hwang, M. Malignant gliomas: Current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag. Res. 2014, 6, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.J.; Kinahan, P. Positron Emission Tomography: Current Challenges and Opportunities for Technological Advances in Clinical and Preclinical Imaging Systems. Annu. Rev. Biomed. Eng. 2015, 17, 385–414. [Google Scholar] [CrossRef]
- Moreau, A.; Febvey, O.; Mognetti, T.; Frappaz, D.; Kryza, D. Contribution of Different Positron Emission Tomography Tracers in Glioma Management: Focus on Glioblastoma. Front. Oncol. 2019, 9, 1134. [Google Scholar] [CrossRef]
- Boxerman, J.L.; Quarles, C.C.; Hu, L.S.; Erickson, B.J.; Gerstner, E.R.; Smits, M.; Kaufmann, T.J.; Barboriak, D.P.; Huang, R.H.; Wick, W.; et al. Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro-Oncology 2020, 22, 1262–1275. [Google Scholar] [CrossRef]
- Boxerman, J.L.; Shiroishi, M.S.; Ellingson, B.M.; Pope, W.B. Dynamic Susceptibility Contrast MR Imaging in Glioma. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, P.J.; Li, S.; Yang, Y.; van der Heide, U.A. Quantitative MRI on MR-Linacs: Towards Biological Image-Guided Adaptive Radiotherapy. Semin. Radiat. Oncol. 2024, 34, 107–119. [Google Scholar] [CrossRef]
- Singnurkar, A.; Poon, R.; Detsky, J. 18F-FET-PET imaging in high-grade gliomas and brain metastases: A systematic review and meta-analysis. J. Neuro-Oncol. 2023, 161, 1–12. [Google Scholar] [CrossRef]
- Robert, J.A.; Leclerc, A.; Ducloie, M.; Emery, E.; Agostini, D.; Vigne, J. Contribution of [18F]FET PET in the Management of Gliomas, from Diagnosis to Follow-Up: A Review. Pharmaceuticals 2024, 17, 1228. [Google Scholar] [CrossRef]
- Mayo, Z.S.; Halima, A.; Broughman, J.R.; Smile, T.D.; Tom, M.C.; Murphy, E.S.; Suh, J.H.; Lo, S.S.; Barnett, G.H.; Wu, G.; et al. Radiation necrosis or tumor progression? A review of the radiographic modalities used in the diagnosis of cerebral radiation necrosis. J. Neuro-Oncol. 2023, 161, 23–31. [Google Scholar] [CrossRef]
- Mayo, Z.S.; Billena, C.; Suh, J.H.; Lo, S.S.; Chao, S.T. The dilemma of radiation necrosis from diagnosis to treatment in the management of brain metastases. Neuro-Oncology 2024, 26 (Suppl. S1), S56–S65. [Google Scholar] [CrossRef] [PubMed]
- Otazo, R.; Lambin, P.; Pignol, J.-P.; Ladd, M.E.; Schlemmer, H.-P.; Baumann, M.; Hricak, H. MRI-guided Radiation Therapy: An Emerging Paradigm in Adaptive Radiation Oncology. Radiology 2021, 298, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tseng, C.-L.; Balter, J.M.; Teng, F.; Parmar, H.A.; Sahgal, A. MR-guided radiation therapy: Transformative technology and its role in the central nervous system. Neuro-Oncology 2017, 19 (Suppl. S2), ii16–ii29. [Google Scholar] [CrossRef] [PubMed]
- O’COnnor, J.P.B.; Aboagye, E.O.; Adams, J.E.; Aerts, H.J.W.L.; Barrington, S.F.; Beer, A.J.; Boellaard, R.; Bohndiek, S.E.; Brady, M.; Brown, G.; et al. Imaging biomarker roadmap for cancer studies. Nat. Rev. Clin. Oncol. 2017, 14, 169–186. [Google Scholar] [CrossRef]
- Raunig, D.L.; McShane, L.M.; Pennello, G.; Gatsonis, C.; Carson, P.L.; Voyvodic, J.T.; Wahl, R.L.; Kurland, B.F.; Schwarz, A.J.; Gönen, M.; et al. Quantitative imaging biomarkers: A review of statistical methods for technical performance assessment. Stat. Methods Med. Res. 2015, 24, 27–67. [Google Scholar] [CrossRef]
- Lawrence, L.S.P.; Maralani, P.J.; Das, S.; Sahgal, A.; Stanisz, G.J.; Lau, A.Z. Magnetic resonance imaging techniques for monitoring glioma response to chemoradiotherapy. J. Neuro-Oncol. 2024, 171, 255–264. [Google Scholar] [CrossRef]
- Henriksen, O.M.; Álvarez-Torres, M.d.M.; Figueiredo, P.; Hangel, G.; Keil, V.C.; Nechifor, R.E.; Riemer, F.; Schmainda, K.M.; Warnert, E.A.H.; Wiegers, E.C.; et al. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 1: Perfusion and Diffusion Techniques. Front. Oncol. 2022, 12, 810263. [Google Scholar] [CrossRef]
- Booth, T.C.; Wiegers, E.C.; Warnert, E.A.H.; Schmainda, K.M.; Riemer, F.; Nechifor, R.E.; Keil, V.C.; Hangel, G.; Figueiredo, P.; Álvarez-Torres, M.D.M.; et al. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 2: Spectroscopy, Chemical Exchange Saturation, Multiparametric Imaging, and Radiomics. Front. Oncol. 2022, 11, 811425. [Google Scholar] [CrossRef]
- Raaymakers, B.W.; Lagendijk, J.J.W.; Overweg, J.; Kok, J.G.M.; E Raaijmakers, A.J.; Kerkhof, E.M.; van der Put, R.W.; Meijsing, I.; Crijns, S.P.M.; Benedosso, F.; et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: Proof of concept. Phys. Med. Biol. 2009, 54, N229–N237. [Google Scholar] [CrossRef]
- Mutic, S.; Dempsey, J.F. The ViewRay System: Magnetic Resonance–Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef]
- Fallone, B.G. The Rotating Biplanar Linac–Magnetic Resonance Imaging System. Semin. Radiat. Oncol. 2014, 24, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.J.; Barton, M.; Crozier, S. The Australian Magnetic Resonance Imaging–Linac Program. Semin. Radiat. Oncol. 2014, 24, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.C. Quantitative Relaxometry of the Brain. Top. Magn. Reson. Imaging 2010, 21, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Kooreman, E.S.; van Houdt, P.J.; Nowee, M.E.; van Pelt, V.W.; Tijssen, R.H.; Paulson, E.S.; Gurney-Champion, O.J.; Wang, J.; Koetsveld, F.; van Buuren, L.D.; et al. Feasibility and accuracy of quantitative imaging on a 1.5 T MR-linear accelerator. Radiother. Oncol. 2019, 133, 156–162. [Google Scholar] [CrossRef]
- Kooreman, E.S.; Tanaka, M.; ter Beek, L.C.; Peters, F.P.; Marijnen, C.A.M.; van der Heide, U.A.; van Houdt, P.J. T1ρ for Radiotherapy Treatment Response Monitoring in Rectal Cancer Patients: A Pilot Study. J. Clin. Med. 2022, 11, 1998. [Google Scholar] [CrossRef]
- Tran, B.; Lawrence, L.; Binda, S.; Chugh, B.; Lau, A. Real-time radiation beam imaging on an MR linear accelerator using quantitative T1 mapping. In Proceedings of the 2024 ISMRM Annual Meeting, Singapore, 4–9 May 2024. [Google Scholar]
- Chen, Y.; Liu, S.; Wang, Y.; Kang, Y.; Haacke, E.M. STrategically Acquired Gradient Echo (STAGE) imaging, part I: Creating enhanced T1 contrast and standardized susceptibility weighted imaging and quantitative susceptibility mapping. Magn. Reson. Imaging 2018, 46, 130–139. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wu, D.; Wang, Y.; Sethi, S.K.; Yang, G.; Xie, H.; Xia, S.; Haacke, E.M. STrategically Acquired Gradient Echo (STAGE) imaging, part II: Correcting for RF inhomogeneities in esti-mating T1 and proton density. Magn. Reson. Imaging 2018, 46, 140–150. [Google Scholar] [CrossRef]
- Haacke, E.M.; Chen, Y.; Utriainen, D.; Wu, B.; Wang, Y.; Xia, S.; He, N.; Zhang, C.; Wang, X.; Lagana, M.M.; et al. STrategically Acquired Gradient Echo (STAGE) imaging, part III: Technical advances and clinical appli-cations of a rapid multi-contrast multi-parametric brain imaging method. Magn. Reson. Imaging 2020, 65, 15–26. [Google Scholar] [CrossRef]
- Nejad-Davarani, S.P.; Zakariaei, N.; Chen, Y.; Haacke, E.M.; Hurst, N.J.; Siddiqui, M.S.; Schultz, L.R.; Snyder, J.M.; Walbert, T.; Glide-Hurst, C.K. Rapid multicontrast brain imaging on a 0.35T MR-linac. Med. Phys. 2020, 47, 4064–4076. [Google Scholar] [CrossRef]
- Park, C.K.S.; Warner, N.S.; Kaza, E.; Sudhyadhom, A. Optimization and validation of low-field MP2RAGE T1 mapping on 0.35T MR-Linac: Toward adaptive dose painting with hypoxia biomarkers. Med. Phys. 2024, 51, 8124–8140. [Google Scholar] [CrossRef]
- Bruijnen, T.; van der Heide, O.; Intven, M.P.W.; Mook, S.; Lagendijk, J.J.W.; Berg, C.A.T.V.D.; Tijssen, R.H.N. Technical feasibility of magnetic resonance fingerprinting on a 1.5T MRI-linac. Phys. Med. Biol. 2020, 65, 22NT01. [Google Scholar] [CrossRef] [PubMed]
- Mickevicius, N.J.; Kim, J.P.; Zhao, J.; Morris, Z.S.; Hurst, N.J.; Glide-Hurst, C.K. Toward magnetic resonance fingerprinting for low-field MR-guided radiation therapy. Med. Phys. 2021, 48, 6930–6940. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR Imaging of Intravoxel Incoherent Motions: Application to Diffusion and Perfusion in Neurologic Disorders. Radiology 1986, 161, 401–407. Available online: https://mriquestions.com/uploads/3/4/5/7/34572113/dlbihanradiology1986.pdf (accessed on 24 August 2020). [CrossRef]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Aubin, M.L.; Vignaud, J.; Laval-Jeantet, M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988, 168, 497–505. [Google Scholar] [CrossRef]
- Le Bihan, D. Molecular diffusion, tissue microdynamics and microstructure. NMR Biomed. 1995, 8, 375–386. [Google Scholar] [CrossRef]
- Ross, B.D.; Chenevert, T.L.; Kim, B.; Ben-Yoseph, O. Magnetic Resonance Imaging and Spectroscopy: Application to Experimental Neuro-Oncology. Q. Magn. Reason. Biol. Med. 1994, 1, 89–106. [Google Scholar]
- Chenevert, T.L.; Stegman, L.D.; Taylor, J.M.G.; Robertson, P.L.; Greenberg, H.S.; Rehemtulla, A.; Ross, B.D. Diffusion Magnetic Resonance Imaging: An Early Surrogate Marker of Therapeutic Efficacy in Brain Tumors. JNCI J. Natl. Cancer Inst. 2000, 92, 2029–2036. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Cloughesy, T.F.; Zaw, T.; Lai, A.; Nghiemphu, P.L.; Harris, R.; Lalezari, S.; Wagle, N.; Naeini, K.M.; Carrillo, J.; et al. Functional diffusion maps (fDMs) evaluated before and after radiochemotherapy predict progression-free and overall survival in newly diagnosed glioblastoma. Neuro-Oncology 2012, 14, 333–343. [Google Scholar] [CrossRef]
- Lawrence, L.S.; Chan, R.W.; Chen, H.; Keller, B.; Stewart, J.; Ruschin, M.; Chugh, B.; Campbell, M.; Theriault, A.; Stanisz, G.J.; et al. Accuracy and precision of apparent diffusion coefficient measurements on a 1.5 T MR-Linac in central nervous system tumour patients. Radiother. Oncol. 2021, 164, 155–162. [Google Scholar] [CrossRef]
- Jokivuolle, M.; Mahmood, F.; Madsen, K.H.; Harbo, F.S.G.; Johnsen, L.; Lundell, H. Assessing tumor microstructure with time-dependent diffusion imaging: Considerations and feasibility on clinical MRI and MRI-Linac. Med. Phys. 2025, 52, 346–361. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, M.; Sheng, K.; Gao, Y.; Chen, A.; Kamrava, M.; Lee, P.; Agazaryan, N.; Lamb, J.; Thomas, D.; et al. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med. Phys. 2016, 43, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, F.; Zhou, Z.; Cao, M.; Kaprealian, T.; Kamrava, M.; Wang, C.; Neylon, J.; Low, D.A.; Yang, Y.; et al. Distortion-free diffusion MRI using an MRI-guided Tri-Cobalt 60 radiotherapy system: Sequence verification and preliminary clinical experience. Med. Phys. 2017, 44, 5357–5366. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.A.; Salzillo, T.; Mulder, S.; Ahmed, S.; Dresner, A.; Preston, K.; He, R.; Christodouleas, J.; Mohamed, A.S.; Philippens, M.; et al. Prospective evaluation of in vivo and phantom repeatability and reproducibility of diffusion-weighted MRI sequences on 1.5 T MRI-linear accelerator (MR-Linac) and MR simulator devices for head and neck cancers. Radiother. Oncol. 2023, 185, 109717. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, H.; Tong, H.; Wang, S.; Yang, Y.; Liu, G.; Zhang, W. Clinical Applications of Contrast-Enhanced Perfusion MRI Techniques in Gliomas: Recent Advances and Current Challenges. Contrast Media Mol. Imaging 2017, 2017, 1–27. [Google Scholar] [CrossRef]
- Federau, C. Intravoxel incoherent motion MRI as a means to measure in vivo perfusion: A review of the evidence. NMR Biomed. 2017, 30, e3780. [Google Scholar] [CrossRef]
- Haller, S.; Zaharchuk, G.; Thomas, D.L.; Lovblad, K.-O.; Barkhof, F.; Golay, X. Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications. Radiology 2016, 281, 337–356. [Google Scholar] [CrossRef]
- Lawrence, L.S.P.; Chan, R.W.; Chen, H.; Keller, B.; Stewart, J.; Ruschin, M.; Chugh, B.; Campbell, M.; Theriault, A.; Stanisz, G.J.; et al. Technical performance of ADC and IVIM measurements in glioma and normal brain on a 1.5T MR-Linac. In Proceedings of the 2021 ISMRM Annual Meeting, Online, 15–20 May 2021. [Google Scholar]
- Lawrence, L.; Chugh, B.; Stewart, J.; Ruschin, M.; Theriault, A.; Detksy, J.; Myrehaug, S.; Maralani, P.; Tseng, C.-L.; Soliman, H.; et al. First demonstration of arterial spin labeling on a 1.5T MR-Linac for characterizing glioblastoma perfusion dynamics. In Proceedings of the 2024 ISMRM Annual Meeting, Singapore, 4–9 May 2024. [Google Scholar]
- Maziero, D.; Azzam, G.A.; de La Fuente, M.; Stoyanova, R.; Ford, J.C.; Mellon, E.A. Implementation and evaluation of a dynamic contrast-enhanced MR perfusion protocol for glioblastoma using a 0.35 T MRI-Linac system. Phys. Med. 2024, 119, 103316. [Google Scholar] [CrossRef]
- Zhou, J.; Lal, B.; Wilson, D.A.; Laterra, J.; van Zijl, P.C. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn. Reson. Med. 2003, 50, 1120–1126. [Google Scholar] [CrossRef]
- Kucharczyk, W.; Macdonald, P.M.; Stanisz, G.J.; Henkelman, R.M. Relaxivity and magnetization transfer of white matter lipids at MR imaging: Importance of cerebrosides and pH. Radiology 1994, 192, 521–529. [Google Scholar] [CrossRef]
- Chan, R.W.; Lawrence, L.S.; Oglesby, R.T.; Chen, H.; Stewart, J.; Theriault, A.; Campbell, M.; Ruschin, M.; Myrehaug, S.; Atenafu, E.G.; et al. Chemical exchange saturation transfer MRI in central nervous system tumours on a 1.5 T MR-Linac. Radiother. Oncol. 2021, 162, 140–149. [Google Scholar] [CrossRef]
- Tran, B.; Lawrence, L.; Chan, R.; Tseng, C.-L.; Detsky, J.; Soliman, H.; Sahgal, A.; Lau, A. Quantitative Magnetization Transfer Imaging in Glioblastoma Patients using Balanced Steady-state Free Precession on a 1.5 T MR-Linac. In Proceedings of the 2023 ISMRM Annual Meeting, Toronto, ON, Canada, 3–8 June 2023. [Google Scholar]
- Chenevert, T.; Malyarenko, D.; Galbán, C.; Gomez-Hassan, D.; Sundgren, P.; Tsien, C.; Ross, B. Comparison of Voxel-Wise and Histogram Analyses of Glioma ADC Maps for Prediction of Early Therapeutic Change. Tomography 2019, 5, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, L.; Chugh, B.; Stewart, J.; Ruschin, M.; Theriault, A.; Detksy, J.; Myrehaug, S.; Maralani, P.; Tseng, C.-L.; Soliman, H.; et al. Optimizing early response assessment in glioblastoma using diffusion imaging on a 1.5T MR-Linac. In Proceedings of the 2024 ISMRM Annual Meeting, Singapore, 4–9 May 2024. [Google Scholar]
- Chan, R.; Lawrence, L.; Soliman, H.; Ruschin, M.; Stewart, J.; Theriault, A.; Tseng, C.-L.; Myrehaug, S.; Detsky, J.; Maralani, P.; et al. Characterizing Quantitative Magnetization Transfer Maps from an MR-Linac in Regions of Progression in Biopsy-Only Glioblastoma. In Proceedings of the 2023 ISMRM Annual Meeting, Toronto, ON, Canada, 3–8 June 2023. [Google Scholar]
- Kooreman, E.S.; van Houdt, P.J.; Keesman, R.; Pos, F.J.; van Pelt, V.W.; Nowee, M.E.; Wetscherek, A.; Tijssen, R.H.; Philippens, M.E.; Thorwarth, D.; et al. ADC measurements on the Unity MR-linac—A recommendation on behalf of the Elekta Unity MR-linac consortium. Radiother. Oncol. 2020, 153, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, A.L.; Keesman, R.; van Lier, A.L.; Coolens, C.; van Houdt, P.J.; Tree, A.; Wetscherek, A.; Romesser, P.B.; Tyagi, N.; Russo, M.L.; et al. Recommendations for improved reproducibility of ADC derivation on behalf of the Elekta MRI-linac consortium image analysis working group. Radiother. Oncol. 2023, 186, 109803. [Google Scholar] [CrossRef] [PubMed]
- Wetscherek, A.; McDonald, B.; Kooreman, E.; Lau, A.; Paudyal, R.; Shukla-Dave, A.; Lawrence, L.; van Houdt, P.; van der Heide, U. Data-driven optimization of intravoxel incoherent motion imaging for clinical endpoints in radiotherapy on a 1.5 T MR-Linac. In Proceedings of the 2022 ISMRM Annual Meeting, London, UK, 7–12 May 2022. [Google Scholar]
- E Zijlema, S.; Tijssen, R.H.N.; Malkov, V.N.; van Dijk, L.; Hackett, S.L.; Kok, J.G.M.; Lagendijk, J.J.W.; Berg, C.A.T.v.D. Design and feasibility of a flexible, on-body, high impedance coil receive array for a 1.5 T MR-linac. Phys. Med. Biol. 2019, 64, 185004. [Google Scholar] [CrossRef]
- Guerini, A.E.; Nici, S.; Magrini, S.M.; Riga, S.; Toraci, C.; Pegurri, L.; Facheris, G.; Cozzaglio, C.; Farina, D.; Liserre, R.; et al. Adoption of Hybrid MRI-Linac Systems for the Treatment of Brain Tumors: A Systematic Review of the Current Literature Regarding Clinical and Technical Features. Technol. Cancer Res. Treat. 2023, 22, 15330338231199286. [Google Scholar] [CrossRef]
- Straza, M.; Bovi, J.; Puckett, L.; Siker, M.; Saeed, H.; Ahunbay, E.; Chen, X.; Mueller, W.; Pinheiro, F.S.; Connelly, J.; et al. Quantitative Imaging of Patients Undergoing Radiotherapy for Primary Gliomas Using A 1.5 Tesla MR Linac. Int. J. Radiat. Oncol. 2020, 108, e286–e287. [Google Scholar] [CrossRef]
- Law, I.; Albert, N.L.; Arbizu, J.; Boellaard, R.; Drzezga, A.; Galldiks, N.; la Fougère, C.; Langen, K.-J.; Lopci, E.; Lowe, V.; et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: Version 1.0. Eur. J. Nucl. Med. 2019, 46, 540–557. [Google Scholar] [CrossRef]
- Weber, D.C.; Zilli, T.; Buchegger, F.; Casanova, N.; Haller, G.; Rouzaud, M.; Nouet, P.; Dipasquale, G.; Ratib, O.; Zaidi, H.; et al. [(18)F]Fluoroethyltyrosine- positron emission tomography-guided radiotherapy for high-grade glioma. Radiat. Oncol. 2008, 3, 44. [Google Scholar] [CrossRef]
- Niyazi, M.; Geisler, J.; Siefert, A.; Schwarz, S.B.; Ganswindt, U.; Garny, S.; Schnell, O.; Suchorska, B.; Kreth, F.-W.; Tonn, J.-C.; et al. FET–PET for malignant glioma treatment planning. Radiother. Oncol. 2011, 99, 44–48. [Google Scholar] [CrossRef]
- Ehman, E.C.; Johnson, G.B.; Villanueva-Meyer, J.E.; Cha, S.; Leynes, A.P.; Larson, P.E.Z.; Hope, T.A. PET/MRI: Where might it replace PET/CT? J. Magn. Reson. Imaging 2017, 46, 1247–1262. [Google Scholar] [CrossRef]
- Galldiks, N.; Niyazi, M.; Grosu, A.L.; Kocher, M.; Langen, K.-J.; Law, I.; Minniti, G.; Kim, M.M.; Tsien, C.; Dhermain, F.; et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients—A report of the PET/RANO group. Neuro-Oncology 2021, 23, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Pauleit, D.; Floeth, F.; Hamacher, K.; Riemenschneider, M.J.; Reifenberger, G.; Müller, H.-W.; Zilles, K.; Coenen, H.H.; Langen, K.-J. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Harat, M.; Rakowska, J.; Harat, M.; Szylberg, T.; Furtak, J.; Miechowicz, I.; Małkowski, B. Combining amino acid PET and MRI imaging increases accuracy to define malignant areas in adult glioma. Nat. Commun. 2023, 14, 4572. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-S.; Gan, H.K.; Senko, C.; Francis, R.J.; Ebert, M.; Lee, S.T.; Lau, E.; Khasraw, M.; Nowak, A.K.; Bailey, D.L.; et al. [18F]-fluoroethyl-L-tyrosine (FET) in glioblastoma (FIG) TROG 18.06 study: Protocol for a prospective, multicentre PET/CT trial. BMJ Open 2023, 13, e071327. [Google Scholar] [CrossRef]
- Kano, H.; Kondziolka, D.; Lobato-Polo, J.; Zorro, O.; Flickinger, J.C.; Lunsford, L.D. T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 2010, 66, 486–492. [Google Scholar] [CrossRef]
- Dequesada, I.M.; Quisling, R.G.; Yachnis, A.; Friedman, W.A. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 2008, 63, 898–904. [Google Scholar] [CrossRef]
- Stockham, A.L.; Tievsky, A.L.; Koyfman, S.A.; Reddy, C.A.; Suh, J.H.; Vogelbaum, M.A.; Barnett, G.H.; Chao, S.T. Conventional MRI does not reliably distinguish radiation necrosis from tumor recurrence after stereotactic radiosurgery. J. Neuro-Oncol. 2012, 109, 149–158. [Google Scholar] [CrossRef]
- Zach, L.; Guez, D.; Last, D.; Daniels, D.; Grober, Y.; Nissim, O.; Hoffmann, C.; Nass, D.; Talianski, A.; Spiegelmann, R.; et al. Delayed contrast extravasation MRI: A new paradigm in neuro-oncology. Neuro-Oncology 2015, 17, 457–465. [Google Scholar] [CrossRef]
- Bodensohn, R.; Forbrig, R.; Quach, S.; Reis, J.; Boulesteix, A.-L.; Mansmann, U.; Hadi, I.; Fleischmann, D.; Mücke, J.; Holzgreve, A.; et al. MRI-based contrast clearance analysis shows high differentiation accuracy between radiation-induced reactions and progressive disease after cranial radiotherapy. ESMO Open 2022, 7, 100424. [Google Scholar] [CrossRef]
- Larroza, A.; Moratal, D.; Paredes-Sánchez, A.; Soria-Olivas, E.; Chust, M.L.; Arribas, L.A.; Arana, E. Support vector machine classification of brain metastasis and radiation necrosis based on texture analysis in MRI. J. Magn. Reson. Imaging 2015, 42, 1362–1368. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Ho, A.; Jiang, W.; Logan, J.; Wang, X.; Brown, P.D.; McGovern, S.L.; Guha-Thakurta, N.; Ferguson, S.D.; et al. A predictive model for distinguishing radiation necrosis from tumour progression after gamma knife radiosurgery based on radiomic features from MR images. Eur. Radiol. 2018, 28, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Cho, S.J.; Sunwoo, L.; Baik, S.H.; Bae, Y.J.; Choi, B.S.; Jung, C.; Kim, J.H. Classification of true progression after radiotherapy of brain metastasis on MRI using artificial intelligence: A systematic review and meta-analysis. Neuro-Oncology Adv. 2021, 3, vdab080. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, T.J.; Smits, M.; Boxerman, J.; Huang, R.; Barboriak, D.P.; Weller, M.; Chung, C.; Tsien, C.; Brown, P.D.; Shankar, L.; et al. Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro-Oncology 2020, 22, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D.; Coley, S.C.; Romanowski, C.A.J.; Hodgson, T.; Wilkinson, I.D. Contrast-enhanced fluid-attenuated inversion recovery imaging for leptomeningeal disease in children. AJNR Am. J. Neuroradiol. 2003, 24, 719–723. [Google Scholar]
- Heyn, C.; Bishop, J.; Moody, A.R.; Kang, T.; Wong, E.; Howard, P.; Maralani, P.; Symons, S.; MacIntosh, B.J.; Keith, J.; et al. Gadolinium-Enhanced T2 FLAIR Is an Imaging Biomarker of Radiation Necrosis and Tumor Progression in Patients with Brain Metastases. Am. J. Neuroradiol. 2025, 46, 129–135. [Google Scholar] [CrossRef]
- Taylor, J.S.; Langston, J.W.; Reddick, W.E.; Kingsley, P.B.; Ogg, R.J.; Pui, M.H.; Kun, L.E.; Jenkins, J.J.; Chen, G.; Ochs, J.J.; et al. Clinical value of proton magnetic resonance spectroscopy for differentiating recurrent or residual brain tumor from delayed cerebral necrosis. Int. J. Radiat. Oncol. 1996, 36, 1251–1261. [Google Scholar] [CrossRef]
- Schlemmer, H.-P.; Bachert, P.; Henze, M.; Buslei, R.; Herfarth, K.; Debus, J.; van Kaick, G. Differentiation of radiation necrosis from tumor progression using proton magnetic resonance spectroscopy. Neuroradiology 2002, 44, 216–222. [Google Scholar] [CrossRef]
- Kamada, K.; Houkin, K.; Abe, H.; Sawamura, Y.; Kashiwaba, T. Differentiation of Cerebral Radiation Necrosis from Tumor Recurrence by Proton Magnetic Resonance Spectroscopy. Neurol. Med.-Chir. 1997, 37, 250–256. [Google Scholar] [CrossRef]
- Chernov, M.; Hayashi, M.; Izawa, M.; Ochiai, T.; Usukura, M.; Abe, K.; Ono, Y.; Muragaki, Y.; Kubo, O.; Hori, T.; et al. Differentiation of the Radiation-Induced Necrosis and Tumor Recurrence after Gamma Knife Radiosurgery for Brain Metastases: Importance of Multi-Voxel Proton MRS. Min-Minim. Invasive Neurosurg. 2005, 48, 228–234. [Google Scholar] [CrossRef]
- Detsky, J.S.; Keith, J.; Conklin, J.; Symons, S.; Myrehaug, S.; Sahgal, A.; Heyn, C.C.; Soliman, H. Differentiating radiation necrosis from tumor progression in brain metastases treated with stereotactic radiotherapy: Utility of intravoxel incoherent motion perfusion MRI and correlation with histopathology. J. Neuro-Oncol. 2017, 134, 433–441. [Google Scholar] [CrossRef]
- Mehrabian, H.; Desmond, K.L.; Chavez, S.; Bailey, C.; Rola, R.; Sahgal, A.; Czarnota, G.J.; Soliman, H.; Martel, A.L.; Stanisz, G.J. Water Exchange Rate Constant as a Biomarker of Treatment Efficacy in Patients with Brain Metastases Undergoing Stereotactic Radiosurgery. Int. J. Radiat. Oncol. 2017, 98, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, Y.; Tsuyuguchi, N.; Iwai, Y.; Yamanaka, K.; Higashiyama, S.; Takami, T.; Ohata, K. Diagnostic Accuracy of 11C-Methionine PET for Differentiation of Recurrent Brain Tumors from Radiation Necrosis After Radiotherapy. J. Nucl. Med. 2008, 49, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Gunawardana, D.H.; Rosenthal, M.A. Differentiation of tumor recurrence from radiation necrosis in high-grade gliomas using 201Tl-SPECT. J. Clin. Neurosci. 2008, 15, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Travers, S.; Joshi, K.; Miller, D.C.; Singh, A.; Nada, A.; Biedermann, G.; Cousins, J.P.; Litofsky, N.S. Reliability of Magnetic Resonance Spectroscopy and Positron Emission Tomography Computed Tomography in Differentiating Metastatic Brain Tumor Recurrence from Radiation Necrosis. World Neurosurg. 2021, 151, e1059–e1068. [Google Scholar] [CrossRef]
- Henkelman, R.M.; Huang, X.; Xiang, Q.; Stanisz, G.J.; Swanson, S.D.; Bronskill, M.J. Quantitative interpretation of magnetization transfer. Magn. Reson. Med. 1993, 29, 759–766. [Google Scholar] [CrossRef]
- Morrison, C.; Henkelman, R.M. A Model for Magnetization Transfer in Tissues. Magn. Reson. Med. 1995, 33, 475–482. [Google Scholar] [CrossRef]
- Stanisz, G.J.; Odrobina, E.E.; Pun, J.; Escaravage, M.; Graham, S.J.; Bronskill, M.J.; Henkelman, R.M. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn. Reson. Med. 2005, 54, 507–512. [Google Scholar] [CrossRef]
- Maralani, P.J.; Chan, R.W.; Lam, W.W.; Oakden, W.; Oglesby, R.; Lau, A.; Mehrabian, H.; Heyn, C.; Chan, A.K.; Soliman, H.; et al. Chemical Exchange Saturation Transfer MRI: What Neuro-Oncology Clinicians Need to Know. Technol. Cancer Res. Treat. 2023, 22, 15330338231208613. [Google Scholar] [CrossRef]
- Lundbom, N. Determination of magnetization transfer contrast in tissue: An MR imaging study of brain tumors. Am. J. Roentgenol. 1992, 159, 1279–1285. [Google Scholar] [CrossRef]
- Jones, C.K.; Schlosser, M.J.; van Zijl, P.C.; Pomper, M.G.; Golay, X.; Zhou, J. Amide proton transfer imaging of human brain tumors at 3T. Magn. Reson. Med. 2006, 56, 585–592. [Google Scholar] [CrossRef]
- Desmond, K.L.; Moosvi, F.; Stanisz, G.J. Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7 T. Magn. Reson. Med. 2014, 71, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Aletras, A.; Balaban, R. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). J. Magn. Reson. 2000, 143, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Desmond, K.L.; Mehrabian, H.; Chavez, S.; Sahgal, A.; Soliman, H.; Rola, R.; Stanisz, G.J. Chemical exchange saturation transfer for predicting response to stereotactic radiosurgery in human brain metastasis. Magn. Reson. Med. 2017, 78, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
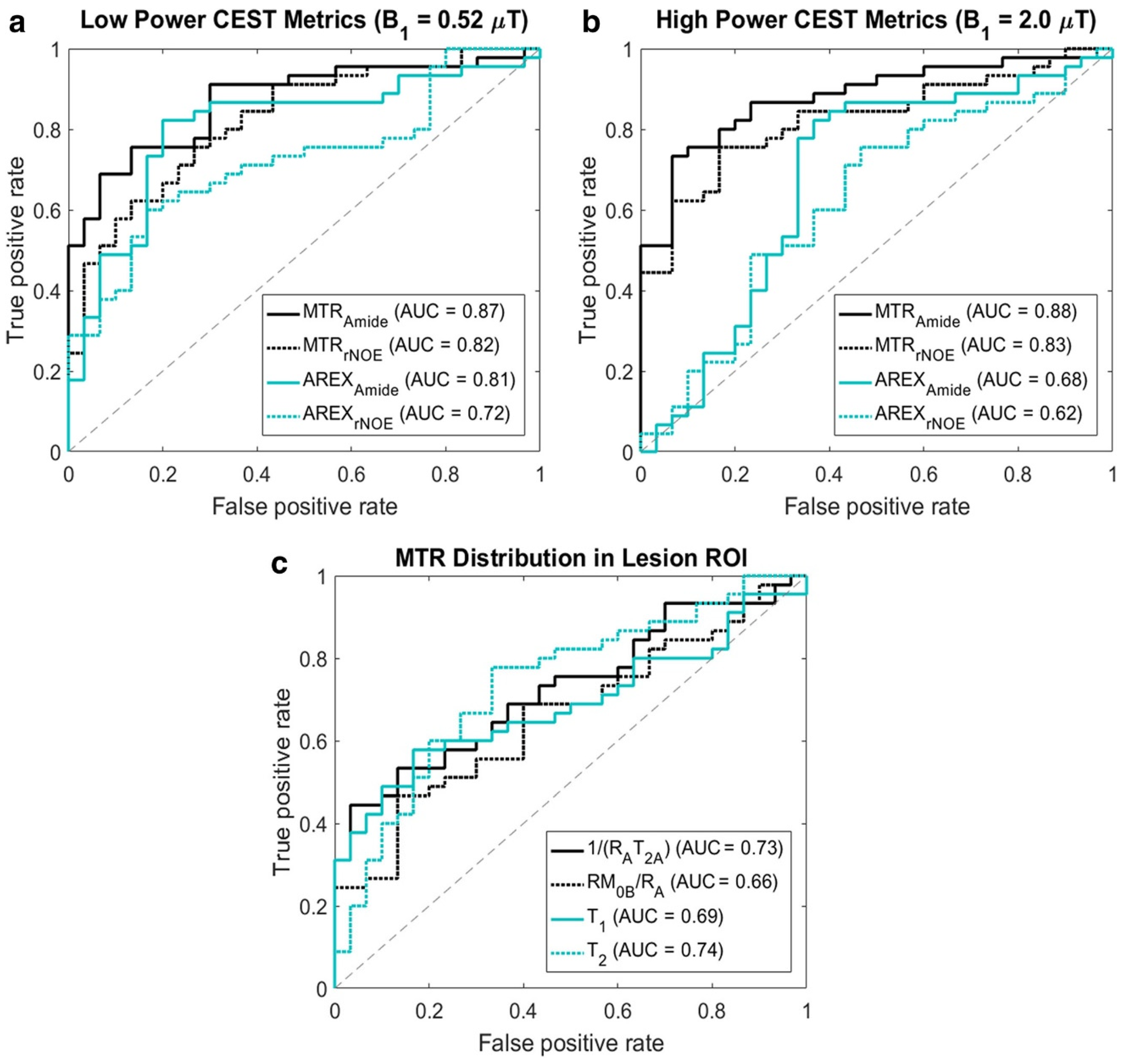
- Mehrabian, H.; Desmond, K.L.; Soliman, H.; Sahgal, A.; Stanisz, G.J. Differentiation between Radiation Necrosis and Tumor Progression Using Chemical Exchange Saturation Transfer. Clin. Cancer Res. 2017, 23, 3667–3675. [Google Scholar] [CrossRef]
- Mehrabian, H.; Chan, R.W.; Sahgal, A.; Chen, H.; Theriault, A.; Lam, W.W.; Myrehaug, S.; Tseng, C.; Husain, Z.; Detsky, J.; et al. Chemical Exchange Saturation Transfer MRI for Differentiating Radiation Necrosis from Tumor Progression in Brain Metastasis—Application in a Clinical Setting. J. Magn. Reson. Imaging 2023, 57, 1713–1725. [Google Scholar] [CrossRef]
- Chan, R.W.; Lam, W.W.; Chen, H.; Murray, L.; Zhang, B.; Theriault, A.; Endre, R.; Moon, S.; Liebig, P.; Maralani, P.J.; et al. Is pulsed saturation transfer sufficient for differentiating radiation necrosis from tumor progression in brain metastases? Neuro-Oncology Adv. 2024, 6, vdae132. [Google Scholar] [CrossRef]
- Portnoy, S.; Stanisz, G.J. Modeling pulsed magnetization transfer. Magn. Reson. Med. 2007, 58, 144–155. [Google Scholar] [CrossRef]
- Zaiss, M.; Xu, J.; Goerke, S.; Khan, I.S.; Singer, R.J.; Gore, J.C.; Gochberg, D.F.; Bachert, P. Inverse Z-spectrum analysis for spillover-, MT-, and T1-corrected steady-state pulsed CEST-MRI—Application to pH-weighted MRI of acute stroke. NMR Biomed. 2014, 27, 240–252. [Google Scholar] [CrossRef]
- Zhou, J.; Tryggestad, E.; Wen, Z.; Lal, B.; Zhou, T.; Grossman, R.; Wang, S.; Yan, K.; Fu, D.-X.; Ford, E.; et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat. Med. 2011, 17, 130–134. [Google Scholar] [CrossRef]
- Zhou, J.; Zaiss, M.; Knutsson, L.; Sun, P.Z.; Ahn, S.S.; Aime, S.; Bachert, P.; Blakeley, J.O.; Cai, K.; Chappell, M.A.; et al. Review and consensus recommendations on clinical APT-weighted imaging approaches at 3T: Application to brain tumors. Magn. Reson. Med. 2022, 88, 546–574. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Shi, G.; Homer, R.; Harsh, G.; Atlas, S.W.; Bednarski, M.D. Magnetic resonance image–guided proteomics of human glioblastoma multiforme. J. Magn. Reson. Imaging 2003, 18, 530–536. [Google Scholar] [CrossRef]


| Section | Method | Disease | Application | Timing |
|---|---|---|---|---|
| 2 | Advanced imaging on MR-Linacs | Gliomas | Adaptive radiotherapy | During |
| 3 | FET-PET | Gliomas | Planning and response assessment | Before and after |
| 4 | Contrast-enhanced imaging | Brain metastases | Tumor progression vs. radiation necrosis | After |
| 5 | MT and CEST | Brain metastases | Tumor progression vs. radiation necrosis | After |
| Study | System | Sequence | Level of Validation |
|---|---|---|---|
| Kooreman et al., 2019 [64] | Unity | Relaxation mapping | Technical |
| Bruijnen et al., 2020 [72] | Unity | Relaxation mapping | Technical |
| Kooreman et al., 2022 [65] | Unity | Relaxation mapping | Technical |
| Tran et al., 2024 [66] | Unity | Relaxation mapping | Technical |
| Park et al., 2024 [71] | Unity | Relaxation mapping | Technical |
| Kooreman et al., 2019 [64] | Unity | ADC | Technical |
| Lawrence et al., 2021 [80] | Unity | ADC | Technical |
| McDonald et al., 2023 [84] | Unity | ADC | Technical |
| Jokivuolle et al., 2025 [81] | Unity | ADC | Technical |
| Lawrence et al., 2023 [40] | Unity | ADC | Clinical |
| Lawrence et al., 2024 [96] | Unity | ADC | Clinical |
| Kooreman et al., 2019 [64] | Unity | DCE | Technical |
| Straza et al., 2020 [103] | Unity | IVIM | Prelim. Technical |
| Lawrence et al., 2021 [88] | Unity | IVIM | Prelim. Technical |
| Chan et al., 2021 [93] | Unity | CEST | Technical & Clinical |
| Tran et al., 2023 [94] | Unity | qMT | Technical |
| Chan et al., 2023 [97] | Unity | qMT | Prelim. Clinical |
| Lawrence et al., 2024 [89] | Unity | ASL | Prelim. Technical |
| Nejad-Devarani, 2020 [70] | MRIdian | Relaxation mapping | Technical |
| Mickevicius et al., 2021 [73] | MRIdian | Relaxation mapping | Technical |
| Yang et al., 2016 [82] | MRIdian | ADC | Technical |
| Gao et al., 2017 [83] | MRIdian | ADC | Technical |
| Maziero et al., 2024 [90] | MRIdian | DCE | Technical & Prelim. Clinical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawrence, L.S.P.; Chan, R.W.; Singnurkar, A.; Detsky, J.; Heyn, C.; Maralani, P.J.; Soliman, H.; Stanisz, G.J.; Sahgal, A.; Lau, A.Z. Applications of Advanced Imaging for Radiotherapy Planning and Response Assessment in the Central Nervous System. Tomography 2025, 11, 68. https://doi.org/10.3390/tomography11060068
Lawrence LSP, Chan RW, Singnurkar A, Detsky J, Heyn C, Maralani PJ, Soliman H, Stanisz GJ, Sahgal A, Lau AZ. Applications of Advanced Imaging for Radiotherapy Planning and Response Assessment in the Central Nervous System. Tomography. 2025; 11(6):68. https://doi.org/10.3390/tomography11060068
Chicago/Turabian StyleLawrence, Liam S. P., Rachel W. Chan, Amit Singnurkar, Jay Detsky, Chris Heyn, Pejman J. Maralani, Hany Soliman, Greg J. Stanisz, Arjun Sahgal, and Angus Z. Lau. 2025. "Applications of Advanced Imaging for Radiotherapy Planning and Response Assessment in the Central Nervous System" Tomography 11, no. 6: 68. https://doi.org/10.3390/tomography11060068
APA StyleLawrence, L. S. P., Chan, R. W., Singnurkar, A., Detsky, J., Heyn, C., Maralani, P. J., Soliman, H., Stanisz, G. J., Sahgal, A., & Lau, A. Z. (2025). Applications of Advanced Imaging for Radiotherapy Planning and Response Assessment in the Central Nervous System. Tomography, 11(6), 68. https://doi.org/10.3390/tomography11060068






