Abstract
The very-low-calorie KD (VLCKD) is characterized by a caloric intake of under 800 kcal/day divided into less than 50 g/day of carbohydrate (13%) and 1 to 1.5 g of protein/kg of body weight (44%) and 43% of fat. This low carbohydrate intake changes the energy source from glucose to ketone bodies. Moreover, clinical trials have consistently shown a beneficial effect of VLCKD in several diseases, such as heart failure, schizophrenia, multiple sclerosis, Parkinson’s, and obesity, among others. The gut microbiota has been associated with the metabolic conditions of a person and is regulated by diet interactions; furthermore, it has been shown that the microbiota has a role in body weight homeostasis by regulating metabolism, appetite, and energy. Currently, there is increasing evidence of an association between gut microbiota dysbiosis and the pathophysiology of obesity. In addition, the molecular pathways, the role of metabolites, and how microbiota modulation could be beneficial remain unclear, and more research is needed. The objective of the present article is to contribute with an overview of the impact that VLCKD has on the intestinal microbiota composition of individuals with obesity through a literature review describing the latest research regarding the topic and highlighting which bacteria phyla are associated with obesity and VLCKD.
1. Introduction
The ketogenic diet (KD) is a nutritional protocol characterized by a high fat and protein intake and low carbohydrate consumption. There are mainly four types of ketogenic diets: (1) classical KD, which is usually based on 90% fat, 4% carbohydrate, and 6% of proteins [1]. (2) medium-chain triglyceride, based on 10% long-chain triglycerides fat, 60% medium-chain triglycerides fat, 20% carbohydrate, and 10% protein [1]. (3) modified Atkins based on 65% fat, 10% carbohydrate, and 25% protein [1]. (4) low glycemic index diet based on 60% fat, 10% carbohydrate, and 30% protein [1].
The classical KD had suffered some variations; for instance, the low-calorie KD (LCKD) with a calorie intake of 800 to 1200 kcal/day based on 58% fat, 13% carbohydrate, and 29% of proteins, and the very-low-calorie KD (VLCKD) characterized by a caloric intake of under 800 kcal/day divided into less than 50 g/day of carbohydrate (13%) and 1 to 1.5 g of protein/kg of body weight (44%), and 43% of fat [2]. In these cases, the carbohydrate intake is reduced, forcing the body to switch to fatty acid oxidation, which induces ketogenesis. Ketogenesis is a metabolic pathway in which the triglycerides are hydrolyzed into fatty acids, and these into ketone bodies that could be used as alternative mitochondrial energy [3,4,5]. VLCKD, fasting, and exercise promote glucose consumption, lowering the insulin level and converting fatty acids into ketone bodies [3,6]. During ketogenesis, there are three types of ketone bodies, acetone, acetoacetate (AcAc), and the mainly produced 3-hydroxybutyrate (BHB) [3]. The ketone bodies are broken down in the mitochondria of metabolically active cells into acetyl-CoA, then to ATP [3,4,6].
There are reports with clinical trials of the metabolic effect of ketone bodies from the VLCKD implicated in the prevention and treatment of human diseases. Clinical trials have consistently shown a beneficial effect of VLCKD in improving heart failure [7,8], neuroprotective properties in schizophrenia, multiple sclerosis, Parkinson’s, and Alzheimer’s diseases [9,10,11,12], improving inflammation [13], reducing obesity [2,14,15,16,17], and recovering muscle force after critical illness [18,19], among others.
Obesity has been described as the accumulation of fat in an individual, causing a direct impact on a person’s health and daily life [20]. Moreover, the World Health Organization (WHO) declared obesity a global epidemic when the individual’s body mass index (BMI) is equal to or greater than 30 kg/m2 [20,21]. Approximately 13% of the adult population was obese in 2016, and it is expected that almost the majority of the population will be obese in 2030 [21,22]. There are reports of the adverse health impact or co-morbidities promoted by obesity, such as heart diseases, hypercholesterolemia, hypertension, and depression [23,24,25,26,27,28]. Additionally, obesity may cause endothelial, inflammatory, and hormonal alterations, which may lead to a hypertensive state, increasing cardiovascular disease (CVD) predisposition [29]. There is increased concern regarding CVD and its correlation with obesity due to epidemiological data, which have shown a direct linear relationship between both; hence if the prevalence of obesity increases, the CVD prevalence will also increase [30]. If there is no adequate follow-up and management of people with obesity, the adverse effects can lead to serious complications and could cause the person’s death [20]. Therefore, it is important to elucidate the etiology of obesity to develop effective interventions to diminish the burden of the disease.
In the last few years, the keto diet has gained popularity as an alternative to reducing weight by lowering appetite through the production of ketone bodies [31]. However, within the diets, a study by Bezerra Bueno N. et al. (2013) [32] compared the conventional low-fat diet in the long term and the VLCKD; the authors found that VLCKD led to a more substantial decrease in weight, making it an excellent alternative for weight loss [32].
Furthermore, the gut microbiota is regulated by diet interactions and has been correlated with the metabolic conditions of a person [33]. Hence, the microbiota composition is variable depending on the environment and dietary patterns of the subjects. For instance, research has shown that the human virome could be correlated to disease development, principally due to its influence on the microbiota [34]. This gut microbiota variability between individuals may make it difficult to identify differences in the composition when comparing diets in people with obesity [35,36]. However, the essential role of the microbiome in the body weight homeostasis maintenance, regulating metabolism, appetite, and energy, has been demonstrated by producing compounds derived from bacteria and influencing the metabolic pathways of the individual [37,38].
The objective of the present article is to provide an overview of the impact that VLCKD has on the intestinal microbiota composition of individuals with obesity through a literature review describing the latest research regarding the topic and highlighting which bacteria are associated with obesity and VLCKD.
2. Gut Microbiota in Obesity
There are reports that changes in the gut microbiota composition of individuals with obesity compared to healthy individuals are not a consequence of obesity. For instance, Turnbaugh P. et al. (2008) [39] showed that colonization of germ-free mice with microbiota associated with obesity led to an increase in fat deposition and total body fat. Hence, the gut microbiota is a contributing factor to obesity pathophysiology [39,40].
The Human Microbiome Project has reported that healthy gut microbiota is mainly composed of Firmicutes, Bacteroidetes, Actinomycetes, Proteus, Fusobacteria, and Verrucomicrobia [41]. Based on these findings, several studies have compared the differences in the microbial community in healthy and individuals with obesity. The first reports are from observational studies performed on animal models. For instance, Ley R.E. et al. (2006) [41] showed fewer Bacteroidetes and more Firmicutes abundance compared to their not obese mice controls. Moreover, the coexistence of both microorganisms minimized the competition for resources, but uncharacterized properties increased the Firmicutes proportion [42]. Similarly, another study by Turnbaugh P. et al. (2006) [40] demonstrated in animal models that the microbiota associated with obesity increases the ability to obtain energy from the diet, and the increase in the Firmicutes/Bacteroidetes ratio in the microbiota was associated with an obese phenotype [39].
Bifidobacterium species are an important and beneficial compound of gut microbiota; they may be used as probiotics due to their role in producing acetate and lactate after glucose fermentation. For instance, Waldram A. et al. (2009) [43] performed a study using spectroscopy, fluorescence in situ hybridization (FISH), and electrophoresis to identify the structural differences between obese and lean mice. The authors identified a reduction in the abundance of Bifidobacterium compared to healthy individuals, suggesting a possible inverse association between Bifidobacterium and obesity [43].
Similar dysbiosis has been described to occur in humans, Duan M. et al. (2021) [44] showed significant differences in the gut microbiota between a control group and adults with obesity. The authors found a clear reduction in the gut microbiota diversity of the latter. The results in the obesity group, at the phylum level, exhibited a decrease in Firmicutes, an increase in Bacteroidetes, and a reduction in the Firmicutes/Bacteroidetes ratio. Similarly, Actinobacteria and Fusobacteria had significantly different proportions. At the species level, nine species, including Fusobacterium mortiferum, Faecalibacterium prausnitzii, Bacteroides uniformis, and Barnesiella intestinihominis, were markedly distinct. Moreover, the lipid and carbohydrate metabolism pathways were abnormal. According to the authors, the changes in Firmicutes and Bacteroidetes could be related to the environmental conditions of the individuals, as the diet of the obese group under study preferred food made of flour [44].
Likewise, Schwiertz A. et al. (2010) [45] performed a study to evaluate the differences in gut microbiota and short-chain fatty acid (SCFA) concentration between adults with obesity and lean subjects in Germany. Regarding the SCFA concentration, the authors found a higher concentration in the obesity group in comparison with the control group. They also reported smaller proportions of Firmicutes and an increased abundance of Bacteroidetes in adults with obesity in contrast with other reports, for instance, the previously mentioned study by Duan M. et al. (2021) [44]. Furthermore, bacteria from the Euryarchaeota phylum (Methanobrevibacter) and Actinobacteria (Bifidobacterium) were detected in lower concentration proportions in the obesity group. Bacteria from Firmicutes and Bacteroidetes produce SCFA, mainly butyrate and propionate, which may perform an important role in obesity. Additionally, SCFA can avoid digestion in the small intestine and be an additional energy source [45].
On the other hand, Koliada A. et al. (2017) [46] carried out a study to understand the association between the gut microbiota, with a special focus on the Firmicutes/Bacteroidetes ratio and BMI in a sample from the Ukranian population. The authors compared the fecal concentrations of the Actinobacteria, Firmicutes, and Bacteroidetes phyla. The results showed differences in the proportion of each bacterial group between the groups. The abundance of Firmicutes increased with a higher BMI, whereas the proportion of Bacteroidetes decreased with an increasing BMI. The Firmicutes/Bacteroidetes ratio also grew with a higher BMI. These results may be explained by the fact that Firmicutes are better energy sources than Bacteroidetes, leading to higher calorie absorption and subsequent weight gain [46].
Similarly, Zhang H. et al. (2009) [47] studied the gut microbiota in people with obesity, after a gastric bypass, and in normal-weight adults. The phylogenetic analyses showed that the Firmicutes phylum was highly abundant in the normal-weight and obese groups; however, after a gastric bypass, the abundance markedly decreased. Moreover, there was a significant increase in the Prevotellaceae family, mainly associated with H2 production, in adults with obesity. The findings led to the hypothesis that the interspecies H2 transfer is an important mechanism for increasing energy uptake in individuals with obesity. Furthermore, the results also indicate the impact that a surgical procedure may have on microbiota [47].
A study by Bervoets L. et al. (2013) [48] identified the differences in the gut microbiota composition between obese and lean children. The authors found that children with obesity had a higher Firmicutes/Bacteroidetes ratio and a higher concentration of Lactobacillus spp. (Firmicutes phylum) [48]. Interestingly, the Lactobacillus genus has been positively and negatively associated with obesity. For instance, Million M. et al. (2013) [49] showed that Lactobacillus reuteri was correlated with a higher BMI [49], whereas Karlsson F.H. et al. (2013) [50] found that Lactobacillus casei was negatively related to obesity [50]. These results suggest a possible strain-specific role of the Lactobacillus genus in obesity [48,49,51].
Xu Z. et al. (2022) [52] performed a systematic review correlating microbiota, obesity, and metabolic disorders, analyzing 2390 reports and including 60 studies. The authors found that Proteobacteria was the most associated phylum with obesity, followed by Firmicutes. Furthermore, the authors also described Bacteroidetes and Actinobacteria as lean-associated phyla [52]; for instance, Bai J. Hu Y. Bruner DW. (2019) found that an increase in the Proteobacteria phylum was correlated with a higher BMI in a cohort of 7–18 years old children [53].
Finally, Moreno-Navarrete J.M. et al. (2018) [54] analyzed the correlation between gut microbiota, insulin sensitivity, and gene expression in subcutaneous and visceral tissue in subjects with obesity. The authors found that the individuals with insulin resistance had an increased abundance of Bacteroidetes and Proteobacteria; and a decrease in the Firmicutes proportion. They also identified that the relative abundance of Firmicutes was associated with markers of brown adipocytes in subcutaneous obesity but not visceral obesity [54].
Obesity has not been associated with a specific bacteria or pathogen, but it may be directly related to dysbiosis in the ecosystem, which is directly influenced by diet and the environment in which the individual develops. For this reason, differences in the abundance of several bacterial strains have been identified in models and patients with obesity.
3. Impact of a Very-Low-Calorie Ketogenic Diet (VLCKD) on the Microbiota of Subjects with Obesity
VLCKD involves carbohydrate deprivation, which is associated with a decrease in glycolysis and an increase in lipolysis, glycogenolysis, and gluconeogenesis for energy generation [55]. During lipolysis, ketone bodies (acetone, 3-β-hydroxybutyrate, acetoacetate) are generated and used as an energy source [55]. Interestingly, ketone bodies can produce more energy than glucose because of the ketosis metabolic effects [36]. KD has also been related to a decrease in the synthesis of reactive oxygen species and the upregulation of energy metabolism genes, mitochondrial biogenesis, and KATP channels [55]. Research has analyzed the impact that VLCKD has on obesity. For instance, Barrea L. et al. (2023) [30] performed a study on 137 women that agreed to participate in the clinical trial. The authors found that after 45 days of VLCKD, all the women in the project experienced a significant weight reduction and improvement in body composition parameters. Furthermore, research regarding VLCKD, and microbiota modulation is lacking [30].
Gutiérrez-Repiso C. et al. (2019) [56] performed a nutritional intervention clinical trial to determine the effects of a VLCKD and symbiotic on the microbiota of thirty-three adults with obesity [56]. The authors found an association between VLCKD and weight loss. They also reported a significant difference in the gut microbiota diversity by analyzing the Shannon Index, which is a measure of species diversity at distinct levels [56,57]. At the genus level, Butyricimonas and Oscillospira abundance increased. Oscillospira, belonging to the Firmicutes phylum, has been positively associated with high-density lipoprotein, butyrate, leanness, human health, and microbial diversity [58,59], whereas the Butyricimonas genus (Bacteroidetes phylum) has been positively correlated to energy metabolism, promoting the homeostasis between microbiota and the host [60,61]. On the other hand, the proportion of Erwinia, Serratia, and Citrobacter decreased. Interestingly, an increased abundance of Serratia and Citrobacter has been associated with obesity [62,63]. The authors concluded that VLCKD could restore the microbiota after obesity-associated dysbiosis [56].
Additionally, in another study by Gutierrez-Repiso A. et al. (2021) [64], the authors compared the microbiota of subjects with different weight loss interventions (Mediterranean diet, VLCKD, and sleeve gastrectomy bariatric surgery) [64]. They found that VLCKD patients had an increased abundance of Alistipes and Parabacteroides. Correlations between the decreased proportion of Alistipes and Parabacteroides and obesity have been described [44,65]. On the contrary, the authors found a decrease in Lactobacillus [64]. The effect of the Lactobacillus genus on obesity appears to be strain dependent. For instance, studies have associated decreased visceral fat, BMI, and waist circumference with an increased abundance of L. gasseri [66]. Similarly, Wang M. et al. (2020) [67] described a correlation between the presence of L. fermentum, L. acidophilus, L. casei, L. paracasei, and L. rhamnosus and a decrease in body weight [67], whereas strains, such as L. reuteri, have been positively associated with obesity [68].
Basciani S. et al. (2020) [69] analyzed the effects of VLCKD on body composition parameters and gut microbiota of subjects with obesity. The authors enrolled forty-eight subjects and divided them into three groups: (1) VLCKD with whey protein, (2) VLCKD with vegetable protein, and (3) VLCKD with animal protein [69]. All groups showed a significant reduction in total fat mass, body weight, total and low-density lipoprotein cholesterol, BMI, triglycerides, and waist, thigh, and hip circumference. Moreover, at baseline, they found a higher abundance of Firmicutes, followed by Bacteroidetes, Proteobacteria, and Actinobacteria. However, at day 45 of the VLCKD, the authors found a decreased proportion of Firmicutes and Actinobacteria and an increased abundance of Bacteroidetes and Proteobacteria [69]. Alterations in the Firmicutes/Bacteroidetes ratio have been broadly associated with several diseases, including obesity [44,70]. For instance, Palmas V. et al. (2021) [65] described a positive association between obesity and an increased Firmicutes/Bacteroidetes ratio, reporting values more than twice in comparison with the microbiota of subjects with standard weight [65]. Similarly, an increased abundance of the Actinobacteria phylum has been correlated with obesity [71,72]. Moreover, the Proteobacteria phylum has been related to obesity as a potential inflammation driver [52]. Additionally, the authors found that the whey and vegetable protein groups showed the highest decrease in Firmicutes abundance, whereas the whey protein group had the highest increase in Bacteroidetes proportion [69].
Likewise, Deledda A. et al. (2022) [73] analyzed the dynamics of the gut microbiota by comparing the effect of a Mediterranean diet (MD) and a VLCKD [73]. The authors described that both diets influenced weight reduction, BMI, and waist circumference, and identified an increased abundance of the Verrucomicrobiota phylum, and characterized the Akkermansiaceae and Christensenellaceae families as microbial markers associated with VLCKD [73]. Interestingly, members of the Akkermansiaceae family as the A. municiphila have been correlated with anti-obesogenic effects in rodents and humans [74,75,76]. Even though the Christensenellales family belongs to the Firmicutes phylum, its abundance has been described as enriched in individuals with normal BMI in comparison with subjects with obesity [77]. The authors also described a depletion in the abundance of the Actinobacteria phylum, which has been associated with obesity [71,72]. In addition, the strongest gut microbiota positively associated pathways included non-homologous end-joining pathways and steroid and carotenoid biosynthesis. On the other hand, cephalosporin, penicillin biosynthesis, pinene, limonene, and ethylbenzene degradation were negatively associated with VLCKD. No significant taxa or pathway differences were found for the MD group [73].
The VLCKD Is an excellent alternative for weight loss on obesity management. Additionally, research has shown how VLCKD could improve microbiota homeostasis, promoting the abundance of bacteria associated with good health. Figure 1 and Supplementary Table S1 compare the reported effects that obesity and VLCKD have on gut microbiome abundance.
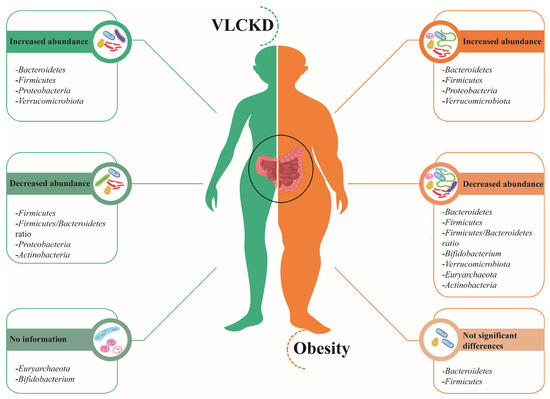
Figure 1.
Gut microbiota in VLCKD and obesity. On the left side (green), bacteria phyla with an increased or decreased abundance after a VLCKD are represented, whereas on the right side (orange), the phyla associated with obesity are depicted [42,43,44,45,46,55,63,68,72].
4. Discussion
Researchers consider the gut microbiota a “hidden metabolic organ” because of its role in host brain function, immunity, inflammation, nutrition, and metabolism [78,79]. Estimates suggest that there are around 500 to 1000 bacterial species at any given time in our gut, with each strain having a unique genome [80,81]. The bacteria in the microbiota produce an extensive range of metabolites, such as short-chain fatty acids (SCFAs), serotonin, and nitric oxide, which, based on their chemical structure similarity, can bind to host cell receptors and trigger hormonal signaling. For instance, it has been shown that strains of Lactobacilli can produce γ amino butyric acid (GABA), an inhibitory neurotransmitter whose modulation can influence depression and anxiety [82,83].
The human gut microbiota is mainly composed of Firmicutes and Bacteroidetes, which are the most abundant, followed by Proteobacteria and Actinobacteria phyla. Moreover, the principal enterotypes include Ruminococcus, Bacteroides, and Prevotella [84]. Interestingly, it has been shown that the host’s diet determines which genus is the most abundant [85]. Furthermore, microbiome dysbiosis has been correlated with several diseases, including epilepsy, Alzheimer, inflammatory bowel syndrome, and obesity [55,69]. Cuevas-Sierra A. et al. (2019) [86] reported a significant difference in gut microbiota between lean and subjects with obesity [86]. The gut microbiota is influenced by various factors, such as antibiotic use, genetics, age, and diet [33,36,73,80]. Additionally, this microbiota modulation could potentially help to treat these diseases [69].
Obesity has been associated with various metabolic diseases as hypertension and diabetes, among others. Several factors promote the pathogenicity of obesity as an energy imbalance, low-grade inflammation, and gut microbiota dysbiosis [87,88,89]. For instance, an increased Firmicutes/Bacteroidetes ratio is considered one of the hallmarks of obesity, although this statement has been disputed. Moreover, it has been shown that an altered microbiota increases gut permeability, which could lead to the passage of endotoxins, such as lipopolysaccharides (LPS). LPS may trigger a constant inflammatory and immune response [73,84,90]. In addition, Meijnikman AS. et al. (2020) [91] found that subjects with a higher BMI had a lower gut microbiota alpha diversity compared to non-obese individuals [91]. The authors also described a positive correlation between gut microbial amino acid metabolism and obesity [91].
The impact of diet on the microbial population has been well described; however, recent studies have highlighted that the microbiome may also influence nutritional status via their secreted metabolites, such as SCFAs, secondary bile acids (Bas), and indole metabolites, which could alter appetite regulation by modifying the activity of the enteric nervous system and the gut-brain axis, generating bi-directional crosstalk [84,92]. Enteroendocrine cells are located in the stomach, pancreas, and gut epithelium, and they can respond to nutrients, metabolites, and mechanical stimuli by secreting neurotransmitters, such as glucagon-like peptide 1, ghrelin, serotonin, and peptide YY. These neurotransmitters are associated with several essential functions in metabolism and can modulate gut motility, insulin and bile acids secretion, and food intake [84,92]. For instance, Modasia A. et al. (2020) [93] showed that the gut bacterium Bacteroides thetaiotaomicron could influence enteroendocrine cells in the gastrointestinal tract of a murine model [93].
Studies have compared the impact of different diets on the microbiome. For instance, as previously mentioned, Deledda A. et al. (2020) [73] compared the effects of a VLCKD and an MD. The authors found that, in addition to a reduction in body weight and BMI, the VLCKD had a statistically significant impact on gut microbiota, promoting the abundance of leanness-associated bacterium, such as A. municiphila [73]. Similarly, MD is based on the reduced consumption of red meat, and unprocessed and industrial foods may influence body weight and BMI. However, the effect on the microbiota before and after the nutritional intervention did not achieve statistically significant differences [73].
Likewise, Simões CD. et al. (2013) [94] analyzed the effects of a very low-energy diet (VLED) on gut microbiota. The authors found a two-fold decrease in Bifidobacterium after the nutritional intervention [94]. Interestingly, although Bifidobacterium belongs to the Actinobacteria phylum, which has been positively correlated to obesity, this genus is considered a probiotic associated with leanness and good health [94,95]. Moreover, the study also showed that the changes in gut microbiota were promoted by diet and not due to weight changes [94]. In comparison with VLCKD, the VLED changes in gut microbiota were only significant for one genus, whereas, for the VLCKD, the changes were significant in the increased or decreased abundance of different phyla. However, the approaches to evaluate the microbiome were different due to Simões CD. et al. 2014 used qPCR and FISH methods for VLED, and the VLCKD analyses mentioned in this paper were performed using next-generation sequencing. More research is needed to understand the differences in gut microbiota modulation between a VLED and a VLCKD.
Furthermore, Remely M. et al. (2015) [96] analyzed the effect of intermittent fasting (IF) on the microbiota. The authors did not report significant changes in total bacteria abundance; however, they found an increased proportion of the Faecalibacterium prausnitzii species and the Akkermansia and Bifidobacterium genera [96]. A decline in Faecalibacterium prausnitzii abundance has been associated with chronic inflammation and obesity [97]. Similarly, a reduction in the Akkermansia and Bifidobacterium genera has been correlated with obesity in different studies [95,98]. Similar to VLCKD, the influence of IF on gut microbiota looks promising; however, further studies should be conducted to identify the role of IF on the microbiome.
Miao Z. et al. (2022) [99] studied the effect of a plant-based diet on gut microbiota. The authors found an increased alpha and beta diversity after a healthy plant-based diet [99]. Similarly, Losasso C. et al. (2018) [100] compared the gut microbiota of three groups (vegetarian, vegan, and omnivore). The group found an increased microbiome diversity, increased Bacteroidetes abundance, and a decreased Firmicutes proportion. Although disputed, an increased microbiota diversity has been positively associated with greater functional diversity and host health [100]. In comparison with the VLCKD, a plant-based diet appears to have a stronger influence on the Firmicutes/Bacteroidetes ratio. More studies evaluating plant-based and VLCKDs must be conducted to associate the effect of diet on the microbiome.
5. Conclusions
There are several factors associated with the etiology of obesity, primarily associated with genetics, diet, and lifestyle. The prevalence of obesity is increasing and is estimated to affect half of the population in the future, which is immensely alarming. There is increasing evidence of an association between some bacterial strains and weight. VLCKD is a remarkable option for weight loss because, due to carbohydrate deprivation, triglycerides, and fatty acids are catabolized into ketone bodies, which are a more efficient energy source than glucose. Furthermore, besides the impact that VLCKD has on weight loss, it has been shown that VLCKD could significantly modulate gut microbiota and restore its homeostasis, which has been an essential and integral part of the treatment for several diseases, such as epilepsy, Alzheimer, inflammatory bowel syndrome, and obesity, as mentioned in the review. Additionally, by comparing the VLCKD with other types of diet, only the plant-based diets had a similar beneficial effect on gut microbiota, highlighting the impact that a VLCKD has on the microbiota.
6. Future Directions
Currently, there is increasing evidence of an association between gut microbiota dysbiosis and the pathophysiology of obesity. In addition, the molecular pathways, the role of metabolites, the interactions with viruses, and how microbiota modulation could be beneficial remain unclear, and more research is needed. Developments in the metagenomics field have allowed us to characterize each person’s microbiome, the metabolites produced, and their related pathways. This new information is vital to understand the association between microbiota and host health by identifying new disease biomarkers and how they can be modulated to promote homeostasis. However, there is a discrepancy regarding the “good” microbiota. For instance, some studies associate a decrease in the abundance of Firmicutes with a reduction in BMI, whereas others have related this effect to a drop in the Bacteroidetes proportion. Hence, it is highly important to elucidate which bacterial strains are beneficial for our health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15122728/s1, Table S1: Gut microbiota microorganisms’ abundance in individuals with obesity compared to individuals on a VLCKD.
Author Contributions
Conceptualization: A.K.Z., S.C.-U. and D.S.-R.; resources: A.K.Z. and D.S.-R.; writing—review and editing: A.K.Z., S.C.-U., P.G.-R., V.A.R.-P., E.P.-C., R.T.-T., E.F.-T., S.C., M.M., G.S., C.V.G. and D.S.-R.; supervision: A.K.Z. and D.S.-R.; project administration, A.K.Z.; funding acquisition: A.K.Z. and D.S.-R. All authors have read and agreed to the published version of the manuscript.
Funding
The publication fee of this article will be funded by Universidad UTE.
Data Availability Statement
The results are presented in the paper. For more information, please contact the corresponding author.
Acknowledgments
The authors are grateful to Universidad UTE for their support.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
Abbreviations
| Very low-calorie ketogenic diet | VLCKD |
| Ketogenic diet | KD |
| Low-calorie ketogenic diet | LCKD |
| Acetoacetate | AcAc |
| 3-hydroxybutyrate | BHB |
| World Health Organization | WHO |
| Body mass index | BMI |
| Cardiovascular disease | CVD |
| Short-chain fatty acids | SCFA |
| Mediterranean diet | MD |
| Gamma amino butyric acid | GABA |
| Lipopolysaccharides | LPS |
| Very low-energy diet | VLED |
| Intermittent fasting | IF |
References
- Dhamija, R.; Eckert, S.; Wirrell, E. Ketogenic Diet. Can. J. Neurol. Sci. 2022, 40, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Guarnotta, V.; Emanuele, F.; Amodei, R.; Giordano, C. Very Low-Calorie Ketogenic Diet: A Potential Application in the Treatment of Hypercortisolism Comorbidities. Nutrients 2022, 14, 2388. [Google Scholar] [CrossRef] [PubMed]
- Dilliraj, L.N.; Schiuma, G.; Lara, D.; Strazzabosco, G.; Clement, J.; Giovannini, P.; Trapella, C.; Narducci, M.; Rizzo, R. The Evolution of Ketosis: Potential Impact on Clinical Conditions. Nutrients 2022, 14, 3613. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.; Gupta, S. Biochemistry, Ketogenesis. In Biochemistry, Ketogenesis; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Alharbi, A.; Al-Sowayan, N.S. The Effect of Ketogenic-Diet on Health. Food Nutr. Sci. 2020, 11, 301–313. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177. [Google Scholar]
- Yao, A.; Li, Z.; Lyu, J.; Yu, L.; Wei, S.; Xue, L.; Wang, H.; Chen, G.-Q. On the nutritional and therapeutic effects of ketone body d-β-hydroxybutyrate. Appl. Microbiol. Biotechnol. 2021, 105, 6229–6243. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Ho, K.L.; Pherwani, S.; Ketema, E.B. Ketone metabolism in the failing heart. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158813. [Google Scholar] [CrossRef]
- Sarnyai, Z.; Palmer, C.M. Ketogenic Therapy in Serious Mental Illness: Emerging Evidence. Int. J. Neuropsychopharmacol. 2021, 23, 434–439. [Google Scholar] [CrossRef]
- Storoni, M.; Plant, G.T.; Patti, F. The Therapeutic Potential of the Ketogenic Diet in Treating Progressive Multiple Sclerosis. Mult. Scler. Int. 2015, 2015, 681289. [Google Scholar] [CrossRef]
- Henderson, S.T. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics 2008, 5, 470–480. [Google Scholar] [CrossRef]
- Grochowska, K.; Przeliorz, A. The Effect of the Ketogenic Diet on the Therapy of Neurodegenerative Diseases and Its Impact on Improving Cognitive Functions. Rev. Artic. Dement. Geriatr. Cogn. Disord. Extra 2022, 12, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Koutnik, A.P.; Goldberg, E.L.; Upadhyay, V.; Turnbaugh, P.J.; Verdin, E.; Newman, J.C. Investigating Ketone Bodies as Immunometabolic Countermeasures against Respiratory Viral Infections. Med 2020, 1, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, C.; Lattanzi, G.; Taylor, S.F.; Manfrini, S.; Khazrai, Y.M. Very low calorie ketogenic diets in overweight and obesity treatment: Effects on anthropometric parameters, body composition, satiety, lipid profile and microbiota. Obes. Res. Clin. Pract. 2020, 14, 491–503. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Vetrani, C.; Marino, F.; Aprano, S.; Savastano, S.; Colao, A.; Muscogiuri, G. VLCKD: A real time safety study in obesity. J. Transl. Med. 2022, 20, 23. [Google Scholar] [CrossRef]
- Barrea, L.; de Alteriis, G.; Muscogiuri, G.; Vetrani, C.; Verde, L.; Camajani, E.; Aprano, S.; Colao, A.; Savastano, S. Impact of a Very Low-Calorie Ketogenic Diet (VLCKD) on Changes in Handgrip Strength in Women with Obesity. Nutrients 2022, 14, 4213. [Google Scholar] [CrossRef]
- Goossens, C.; Weckx, R.; Derde, S.; Perre, S.V.; Derese, I.; Van Veldhoven, P.P.; Ghesquière, B.; Berghe, G.V.D.; Langouche, L. Additional file 1 of Altered cholesterol homeostasis in critical illness-induced muscle weakness: Effect of exogenous 3-hydroxybutyrate. Crit. Care 2021, 25, 252. [Google Scholar] [CrossRef]
- Koutnik, A.P.; Poff, A.M.; Ward, N.P.; DeBlasi, J.M.; Soliven, M.A.; Romero, M.A.; Roberson, P.A.; Fox, C.D.; Roberts, M.D.; D’Agostino, D.P. Ketone Bodies Attenuate Wasting in Models of Atrophy. J. Cachexia Sarcopenia Muscle 2020, 11, 973–996. [Google Scholar] [CrossRef]
- Guevara-Ramírez, P.; Cadena-Ullauri, S.; Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Paz-Cruz, E.; Simancas-Racines, D.; Zambrano, A.K. Genetics, genomics, and diet interactions in obesity in the Latin American environment. Front. Nutr. 2022, 9, 1063286. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. In Springer Reference; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Worldwide epidemic of obesity. In Obesity and Obstetrics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. [Google Scholar]
- Ignatieva, E.V.; Afonnikov, D.A.; Saik, O.V.; Rogaev, E.I.; Kolchanov, N.A. A compendium of human genes regulating feeding behavior and body weight, its functional characterization and identification of GWAS genes involved in brain-specific PPI network. BMC Genet. 2016, 17 (Suppl. S3), 89–116. [Google Scholar] [CrossRef]
- Cleveland Clinic. Triglycerides and Heart Health—How Triglycerides Impact Heart Health. 2022. Available online: https://my.clevelandclinic.org/health/articles/17583-triglycerides--heart-health (accessed on 16 April 2023).
- Division of Nutrition, Physical Activity, and Obesity. National Center for Chronic Disease Prevention and Health Promotion. Health Effects of Overweight and Obesity | Healthy Weight, Nutrition, and Physical Activity; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022.
- Cepeda-Lopez, A.C.; Baye, K. Obesity, iron deficiency and anaemia: A complex relationship. Public Health Nutr. 2020, 23, 1703–1704. [Google Scholar] [CrossRef] [PubMed]
- Campión, J.; Milagro, F.; Martínez, J.A. Epigenetics and Obesity. Prog. Mol. Biol. Transl. Sci. 2010, 94, 291–347. [Google Scholar] [PubMed]
- Seidell, J.C.; Halberstadt, J. The Global Burden of Obesity and the Challenges of Prevention. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 7–12. [Google Scholar] [CrossRef] [PubMed]
- Vetrani, C.; Di Nisio, A.; Paschou, S.A.; Barrea, L.; Muscogiuri, G.; Graziadio, C.; Savastano, S.; Colao, A.; on behalf of the Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group. From Gut Microbiota through Low-Grade Inflammation to Obesity: Key Players and Potential Targets. Nutrients 2022, 14, 2103. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Santangeli, P.; Lucà, S.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Very low-calorie ketogenic diet (VLCKD): An antihypertensive nutritional approach. J. Transl. Med. 2023, 21, 128. [Google Scholar] [CrossRef]
- Paoli, A. Ketogenic diet for obesity: Friend or foe? Int. J. Environ. Res. Public Health 2014, 11, 2092–2107. [Google Scholar] [CrossRef]
- Bezerra Bueno, N.; Vieira De Melo, I.S.; Lima De Oliveira, S.; Da, T.; Ataide, R. Systematic Review with Meta-analysis Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Attaye, I.; van Oppenraaij, S.; Warmbrunn, M.V.; Nieuwdorp, M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2022, 14, 191. [Google Scholar] [CrossRef]
- Tamayo-Trujillo, R.; Guevara-Ramírez, P.; Cadena-Ullauri, S.; Paz-Cruz, E.; Ruiz-Pozo, V.A.; Zambrano, A.K. Human virome: Implications in cancer. Heliyon 2023, 9, e14086. [Google Scholar] [CrossRef]
- Tagliabue, A.; Ferraris, C.; Uggeri, F.; Trentani, C.; Bertoli, S.; de Giorgis, V.; Veggiotti, P.; Elli, M. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 Deficiency Syndrome: A 3-month prospective observational study. Clin. Nutr. ESPEN 2017, 17, 33–37. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Cani, P.D. The gut microbiota in obesity and weight management: Microbes as friends or foe? Nat. Rev. Endocrinol. 2023, 19, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Backhed, F.; Fulton, L.; Gordon, J.I. Marked alterations in the distal gut microbiome linked to diet-induced obesity. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Heather, H.; Creasy, H.H.; Earl, A.M.; Fulton, R.S.; et al. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Waldram, A.; Holmes, E.; Wang, Y.; Rantalainen, M.; Wilson, I.D.; Tuohy, K.M.; McCartney, A.L.; Gibson, G.R.; Nicholson, J.K. Top-Down Systems Biology Modeling of Host Metabotype−Microbiome Associations in Obese Rodents. J. Proteome Res. 2009, 8, 2361–2375. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Sineok, L.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human Gut Microbiota in Obesity and after Gastric Bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog. 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Thuny, F.; Angelakis, E.; Casalta, J.-P.; Giorgi, R.; Habib, G.; Raoult, D. Lactobacillus reuteri and Escherichia coli in the human gut microbiota may predict weight gain associated with vancomycin treatment. Nutr. Diabetes 2013, 3, e87. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring Bacterial Community of Human Gut Microbiota Reveals an Increase in Lactobacillus in Obese Patients and Methanogens in Anorexic Patients. PLoS ONE 2009, 4, e7125. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.; Ng, S.C. Gut microbiota in patients with obesity and metabolic disorders—A systematic review. Genes Nutr. 2022, 17, 2. [Google Scholar] [CrossRef]
- Bai, J.; Hu, Y.; Bruner, D.W. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American Gut Project. Pediatr. Obes. 2019, 14, e12480. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Serino, M.; Blasco-Baque, V.; Azalbert, V.; Barton, R.H.; Cardellini, M.; Latorre, J.; Ortega, F.; Sabater-Masdeu, M.; Burcelin, R.; et al. Gut Microbiota Interacts with Markers of Adipose Tissue Browning, Insulin Action and Plasma Acetate in Morbid Obesity. Mol. Nutr. Food Res. 2018, 62, 1700721. [Google Scholar] [CrossRef]
- Lim, J.-M.; Letchumanan, V.; Tan, L.T.-H.; Hong, K.-W.; Wong, S.-H.; Ab Mutalib, N.-S.; Lee, L.-H.; Law, J.W.-F. Ketogenic Diet: A Dietary Intervention via Gut Microbiome Modulation for the Treatment of Neurological and Nutritional Disorders (a Narrative Review). Nutrients 2022, 14, 3566. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Hernández-García, C.; García-Almeida, J.M.; Bellido, D.; Martín-Núñez, G.M.; Sánchez-Alcoholado, L.; Alcaide-Torres, J.; Sajoux, I.; Tinahones, F.J.; Moreno-Indias, I. Effect of Synbiotic Supplementation in a Very-Low-Calorie Ketogenic Diet on Weight Loss Achievement and Gut Microbiota: A Randomized Controlled Pilot Study. Mol. Nutr. Food Res. 2019, 63, e1900167. [Google Scholar] [CrossRef] [PubMed]
- Konopiński, M.K. Shannon diversity index: A call to replace the original Shannon’s formula with unbiased estimator in the population genetics studies. PeerJ 2020, 8, e9391. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-R.; Zheng, H.-M.; Zhang, G.-X.; Chen, F.-L.; Chen, L.-D.; Yang, Z.-C. High Oscillospira abundance indicates constipation and low BMI in the Guangdong Gut Microbiome Project. Sci. Rep. 2020, 10, 9364. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Y.; Wen, Z.; Liu, W.; Meng, L.; Huang, H. Oscillospira—A candidate for the next-generation probiotics. Gut Microbes 2021, 13, 1987783. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Shen, Y.; Zhang, H.; Cao, M.; Guo, M.; He, J.; Zhang, B.; Xiao, C. Gut Microbiota Characteristics of People with Obesity by Meta-Analysis of Existing Datasets. Nutrients 2022, 14, 2993. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; An, J.; Kim, J.; Choi, D.; Song, Y.; Lee, C.-K.; Kong, H.; Kim, S.B.; Kim, K. A Novel Bacterium, Butyricimonas virosa, Preventing HFD-Induced Diabetes and Metabolic Disorders in Mice via GLP-1 Receptor. Front. Microbiol. 2022, 13, 858192. [Google Scholar] [CrossRef]
- Kashtanova, D.A.; Tkacheva, O.N.; Doudinskaya, E.N.; Strazhesko, I.D.; Kotovskaya, Y.V.; Popenko, A.S.; Tyakht, A.V.; Alexeev, D.G. Gut Microbiota in Patients with Different Metabolic Statuses: Moscow Study. Microorganisms 2018, 6, 98. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Li, M.; Sheng, Y.; Chen, L.; Cann, I.; Du, Z.-Y. Citrobacter Species Increase Energy Harvest by Modulating Intestinal Microbiota in Fish: Nondominant Species Play. mSystems 2020, 5, 3. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Molina-Vega, M.; Bernal-López, M.R.; Garrido-Sánchez, L.; García-Almeida, J.M.; Sajoux, I.; Moreno-Indias, I.; Tinahones, F.J. Different Weight Loss Intervention Approaches Reveal a Lack of a Common Pattern of Gut Microbiota Changes. J. Pers. Med. 2021, 11, 109. [Google Scholar] [CrossRef]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Vascellari, S.; Loviselli, A.; Manzin, A.; et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci. Rep. 2021, 11, 5532. [Google Scholar] [CrossRef]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. 2017, 41, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, B.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Intervention of five strains of Lactobacillus on obesity in mice induced by high-fat diet. J. Funct. Foods 2020, 72, 104078. [Google Scholar] [CrossRef]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2011, 36, 817–825. [Google Scholar] [CrossRef]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-Low-Calorie Ketogenic Diets With Whey, Vegetable, or Animal Protein in Patients With Obesity: A Randomized Pilot Study. J. Clin. Endocrinol. Metab. 2020, 105, 2939–2949. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Deledda, A.; Palmas, V.; Heidrich, V.; Fosci, M.; Lombardo, M.; Cambarau, G.; Lai, A.; Melis, M.; Loi, E.; Loviselli, A.; et al. Dynamics of Gut Microbiota and Clinical Variables after Ketogenic and Mediterranean Diets in Drug-Naïve Patients with Type 2 Diabetes Mellitus and Obesity. Metabolites 2022, 12, 1092. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guo, P.; Mao, R.; Ren, Z.; Wen, J.; Yang, Q.; Yan, T.; Yu, J.; Zhang, T.; Liu, Y. Gut Microbiota Signature of Obese Adults Across Different Classifications. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 3933–3947. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2016, 23, 107–113. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of Mammals and Their Gut Microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Göktas, Ö.; Reißhauer, A.; Neuhaus, J.; Weylandt, K.-H.; Guschin, A.; Bock, M. Reduced Mass and Diversity of the Colonic Microbiome in Patients with Multiple Sclerosis and Their Improvement with Ketogenic Diet. Front. Microbiol. 2017, 8, 1141. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; Stanton, C. Gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012, 113, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Rondanelli, M.; Gasparri, C.; Peroni, G.; Faliva, M.A.; Naso, M.; Perna, S.; Bazire, P.; Sajoux, I.; Maugeri, R.; Rigon, C. The Potential Roles of Very Low Calorie, Very Low Calorie Ketogenic Diets and Very Low Carbohydrate Diets on the Gut Microbiota Composition. Front. Endocrinol. 2021, 12, 662591. [Google Scholar]
- Arumugam, M.; Raes, J.; Pelletier, E.; Paslier, D.L.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Bertalan, M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Ramos-Lopez, O.; Riezu-Boj, J.I.; Milagro, F.I.; Martinez, J.A. Diet, Gut Microbiota, and Obesity: Links with Host Genetics and Epigenetics and Potential Applications. Adv. Nutr. 2019, 10, S17–S30. [Google Scholar] [CrossRef] [PubMed]
- Gohar, A.; Shakeel, M.; Atkinson, R.L.; Haleem, D.J. Potential mechanisms of improvement in body weight, metabolic profile, and liver metabolism by honey in rats on a high fat diet. PharmaNutrition 2020, 14, 100227. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Mamani-Ortiz, Y.; Sebastián, M.S.; Armaza, A.X.; Luizaga, J.M.; Illanes, D.E.; Ferrel, M.; Mosquera, P.A. Prevalence and determinants of cardiovascular disease risk factors using the WHO STEPS approach in Cochabamba, Bolivia. BMC Public Health 2019, 19, 786. [Google Scholar] [CrossRef] [PubMed]
- Van Son, J.; Koekkoek, L.L.; La Fleur, S.E.; Serlie, M.J.; Nieuwdorp, M. The Role of the Gut Microbiota in the Gut–Brain Axis in Obesity: Mechanisms and Future Implications. Int. J. Mol. Sci. 2021, 22, 2993. [Google Scholar] [CrossRef]
- Meijnikman, A.S.; Aydin, O.; Prodan, A.; Tremaroli, V.; Herrema, H.; Levin, E.; Acherman, Y.; Bruin, S.; Gerdes, V.; Backhed, F.; et al. Distinct differences in gut microbial composition and functional potential from lean to morbidly obese subjects. J. Intern. Med. 2020, 288, 699–710. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Modasia, A.; Parker, A.; Jones, E.; Stentz, R.; Brion, A.; Goldson, A.; Defernez, M.; Wileman, T.; Blackshaw, L.A.; Carding, S.R. Regulation of Enteroendocrine Cell Networks by the Major Human Gut Symbiont Bacteroides thetaiotaomicron. Front. Microbiol. 2020, 11, 575595. [Google Scholar] [CrossRef]
- Simões, C.D.; Maukonen, J.; Scott, K.P.; Virtanen, K.A.; Pietiläinen, K.H.; Saarela, M. Impact of a very low-energy diet on the fecal microbiota of obese individuals. Eur. J. Nutr. 2014, 53, 1421–1429. [Google Scholar] [CrossRef]
- Gong, H.; Gao, H.; Ren, Q.; He, J. The abundance of bifidobacterium in relation to visceral obesity and serum uric acid. Sci. Rep. 2022, 12, 13073. [Google Scholar] [CrossRef]
- Remely, M.; Hippe, B.; Geretschlaeger, I.; Stegmayer, S.; Hoefinger, I.; Haslberger, A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: A pilot study. Wien. Klin. Wochenschr. 2015, 127, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Maioli, T.U.; Borras-nogues, E.; Torres, L.; Barbosa, S.C. Possible Bene fits of Faecalibacterium prausnitzii for Obesity-Associated Gut Disorders. Front. Pharmacol. 2021, 12, 740636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Zhang, Y.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut bacteria Akkermansia is associated with reduced risk of obesity: Evidence from the American Gut Project. Nutr. Metab. 2020, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Du, W.; Xiao, C.; Su, C.; Gou, W.; Shen, L.; Zhang, J.; Fu, Y.; Jiang, Z.; Wang, Z.; et al. Gut microbiota signatures of long-term and short-term plant-based dietary pattern and cardiometabolic health: A prospective cohort study. BMC Med. 2022, 20, 204. [Google Scholar] [CrossRef]
- Losasso, C.; Eckert, E.M.; Mastrorilli, E.; Villiger, J.; Mancin, M.; Patuzzi, I.; Di Cesare, A.; Cibin, V.; Barrucci, F.; Pernthaler, J.; et al. Assessing the Influence of Vegan, Vegetarian and Omnivore Oriented Westernized Dietary Styles on Human Gut Microbiota: A Cross Sectional Study. Front. Microbiol. 2018, 9, 317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).