Vitamin K and Hallmarks of Ageing: Focus on Diet and Gut Microbiome
Abstract
1. Introduction
2. Vitamin K, Diet, and Healthy Ageing
2.1. Healthy Diet and Ageing
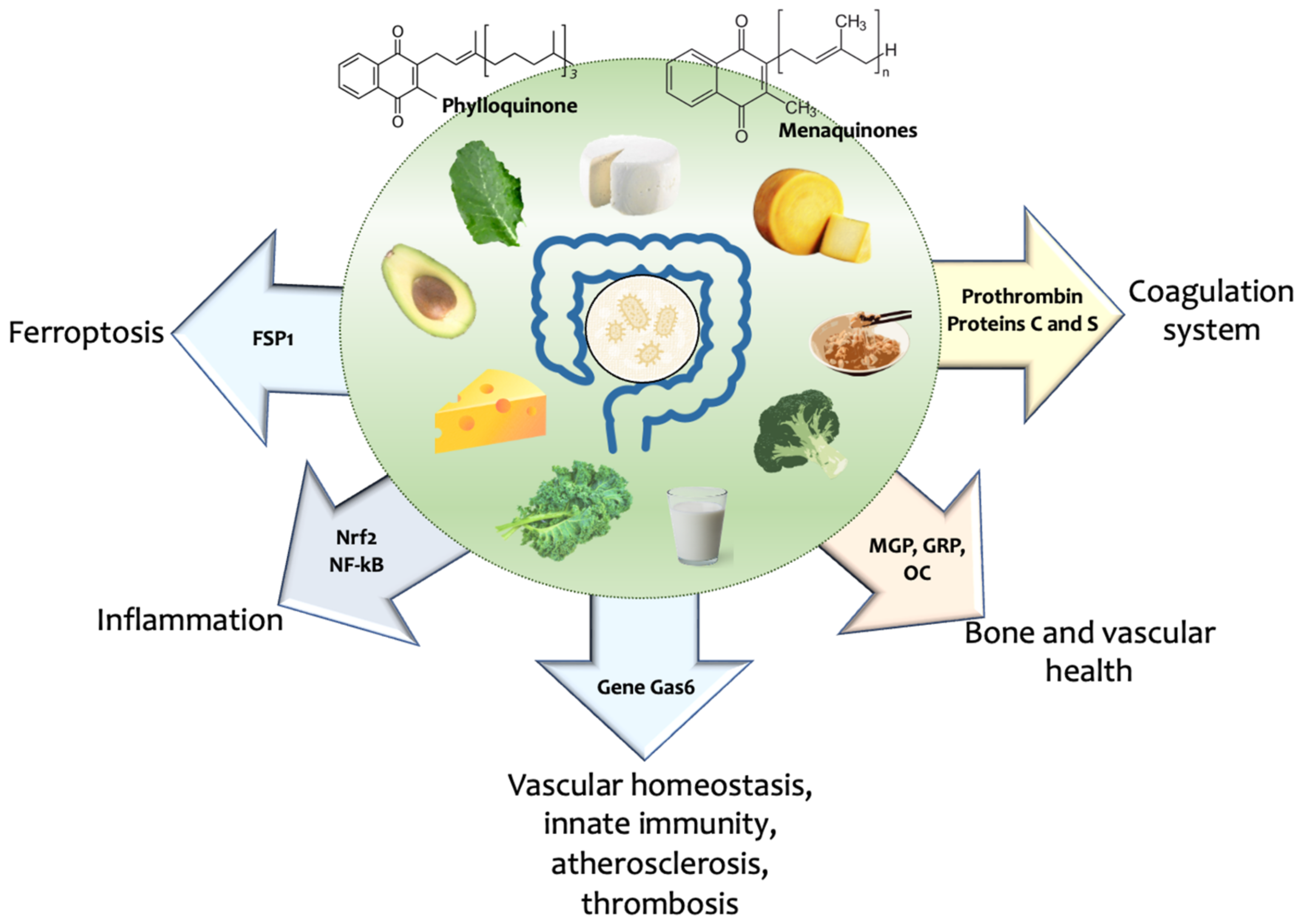
2.2. Vitamin K Source, Metabolism, Recycling, and Its Role in Ageing
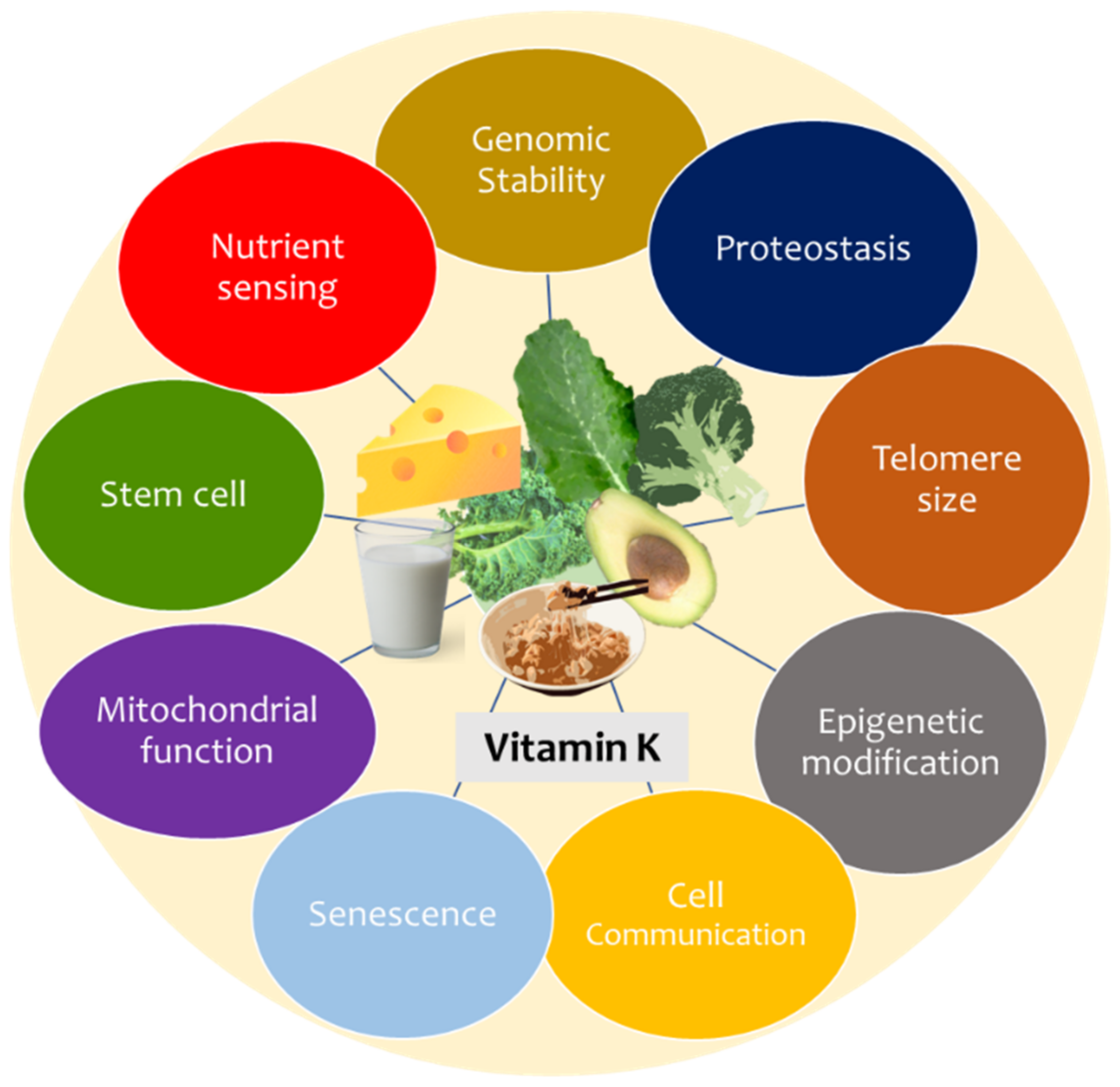
3. Vitamin K and Hallmarks of Ageing
3.1. Dietary Source of Vitamin K and Health—Food Pattern Matters
3.2. Vitamin K and Health Outcome—Nutrient vs. Food Intake
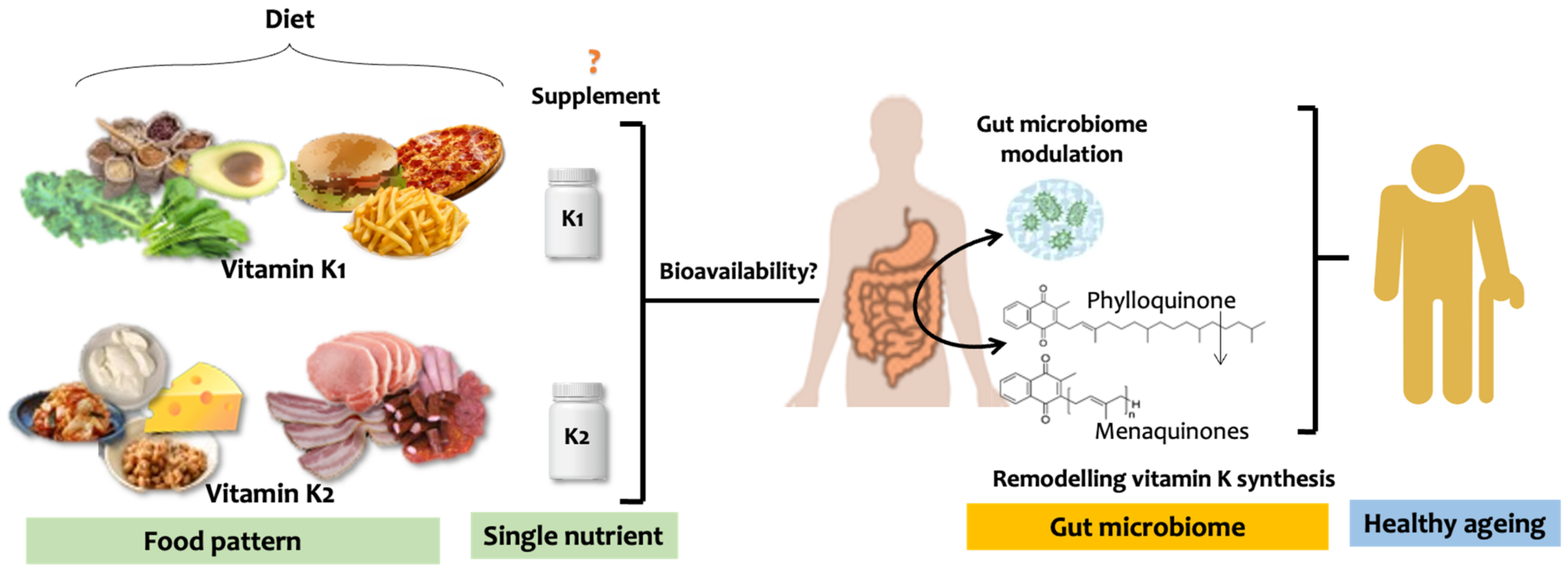
4. Vitamin K, Gut Microbiome, and Ageing
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Decade of Healthy Ageing 2020–2030; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization (WHO). World Report on Ageing and Health; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization (WHO). Decade of Healthy Ageing: Baseline Report; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking Aging to Chronic Disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Preventing Chronic Diseases: A Vital Investment—WHO Global Report; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.L.; Ezzati, M. The Preventable Causes of Death in the United States: Comparative Risk Assessment of Dietary, Lifestyle, and Metabolic Risk Factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Machado, D.B.; Cislaghi, B.; Salman, O.M.; Karanikolos, M.; McKee, M.; Abbas, K.M.; Brady, O.J.; Larson, H.J.; Trias-Llimós, S.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Wilmanski, T.; Diener, C.; Rappaport, N.; Patwardhan, S.; Wiedrick, J.; Lapidus, J.; Earls, J.C.; Zimmer, A.; Glusman, G.; Robinson, M.; et al. Gut Microbiome Pattern Reflects Healthy Ageing and Predicts Survival in Humans. Nat. Metab. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Popa, D.S.; Bigman, G.; Rusu, M.E. The Role of Vitamin k in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912. [Google Scholar] [CrossRef]
- Dai, L.; Schurgers, L.; Shiels, P.G.; Stenvinkel, P. A Biomimetic Natural Sciences Approach to Understanding the Mechanisms of Ageing in Burden of Lifestyle Diseases. Clin. Sci. 2021, 135, 1251–1272. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef]
- Snelson, M.; Tan, S.M.; Clarke, R.E.; de Pasquale, C.; Thallas-Bonke, V.; Nguyen, T.-V.; Penfold, S.A.; Harcourt, B.E.; Sourris, K.C.; Lindblom, R.S.; et al. Processed Foods Drive Intestinal Barrier Permeability and Microvascular Diseases. Sci. Adv. 2021, 7, eabe4841. [Google Scholar] [CrossRef]
- Zhang, H.; Greenwood, D.C.; Risch, H.A.; Bunce, D.; Hardie, L.J.; Cade, J.E. Meat Consumption and Risk of Incident Dementia: Cohort Study of 493,888 UK Biobank Participants. Am. J. Clin. Nutr. 2021, 114, 175–184. [Google Scholar] [CrossRef]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food Based Dietary Patterns and Chronic Disease Prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv. Nutr. Int. Rev. J. 2017, 8, 17–26. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and Vegetable Intake and Mortality. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef]
- Harshman, S.G.; Finnan, E.G.; Barger, K.J.; Bailey, R.L.; Haytowitz, D.B.; Gilhooly, C.H.; Booth, S.L. Vegetables and Mixed Dishes Are Top Contributors to Phylloquinone Intake in US Adults: Data from the 2011-2012 NHANES. J. Nutr. 2017, 147, 1308–1313. [Google Scholar] [CrossRef]
- Juanola-Falgarona, M.; Salas-Salvadó, J.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary Intake of Vitamin K Is Inversely Associated with Mortality Risk. J. Nutr. 2014, 144, 743–750. [Google Scholar] [CrossRef]
- Bishop, N.J.; Ullevig, S.L.; Wang, K.; Zuniga, K.E. Dietary Quality Modifies the Association between Multimorbidity and Change in Mobility Limitations among Older Americans. Prev. Med. 2021, 153, 106721. [Google Scholar] [CrossRef]
- McCann, A.; Jeffery, I.B.; Ouliass, B.; Ferland, G.; Fu, X.; Booth, S.L.; Tran, T.T.T.; O’Toole, P.W.; O’Connor, E.M. Exploratory Analysis of Covariation of Microbiota-Derived Vitamin K and Cognition in Older Adults. Am. J. Clin. Nutr. 2019, 110, 1404–1415. [Google Scholar] [CrossRef]
- Huang, S.-H.; Fang, S.-T.; Chen, Y.-C. Molecular Mechanism of Vitamin K2 Protection against Amyloid-β-Induced Cytotoxicity. Biomolecules 2021, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Ugochukwu, S.A.; Borges, N.A.; Cardozo, L.F.M.F.; Stenvinkel, P.; Shiels, P.G. Food for Healthier Aging: Power on Your Plate. Crit. Rev. Food Sci. Nutr. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as Medicine: Targeting the Uraemic Phenotype in Chronic Kidney Disease. Nat. Rev. Nephrol. 2021, 17, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Determination of Phylloquinone and Menaquinones in Food. Pathophysiol. Haemost Thromb. 2000, 30, 298–307. [Google Scholar] [CrossRef]
- Piironen, V.; Koivu, T.; Tammisalo, O.; Mattila, P. Determination of Phylloquinone in Oils, Margarines and Butter by High-Performance Liquid Chromatography with Electrochemical Detection. Food Chem. 1997, 59, 473–480. [Google Scholar] [CrossRef]
- Bøe, C.A.; Holo, H. Engineering Lactococcus Lactis for Increased Vitamin K2 Production. Front. Bioeng. Biotechnol. 2020, 8, 191. [Google Scholar] [CrossRef]
- Elder, S.J.; Haytowitz, D.B.; Howe, J.; Peterson, J.W.; Booth, S.L. Vitamin K Contents of Meat, Dairy, and Fast Food in the U.S. Diet. J. Agric. Food Chem. 2006, 54, 463–467. [Google Scholar] [CrossRef]
- Walther, B.; Karl, J.P.; Booth, S.L.; Boyaval, P. Menaquinones, Bacteria, and the Food Supply: The Relevance of Dairy and Fermented Food Products to Vitamin K Requirements. Adv. Nutr. 2013, 4, 463–473. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Allison, P.M.; Mummah-Schendel, L.L.; Kindberg, C.G.; Harms, C.S.; Bang, N.U.; Suttie, J.W. Effects of a Vitamin K-Deficient Diet and Antibiotics in Normal Human Volunteers. J. Lab. Clin. Med. 1987, 110, 180–188. [Google Scholar]
- Komai, M.; Shirakawa, H.; Kimura, S. Newly Developed Model for Vitamin K Deficiency in Germfree Mice. Int. J. Vitam. Nutr. Res. 1988, 58, 55–59. [Google Scholar]
- Uchida, K.; Komeno, T. Relationships between Dietary and Intestinal Vitamin K, Clotting Factor Levels, Plasma Vitamin K and Urinary Gla. In Current Advances in Vitamin K Research; Elsevier: Amsterdam, The Netherlands, 1988; pp. 477–492. [Google Scholar]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Dietary Reference Values for Vitamin K. EFSA J. 2017, 15, e04780. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Vermeer, C. Differential Lipoprotein Transport Pathways of K-Vitamins in Healthy Subjects. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2002, 1570, 27–32. [Google Scholar] [CrossRef]
- Hemker, H.C.; Muller, A.D.; Loeliger, E.A. Two Types of Prothrombin in Vitamin K Deficiency. Thromb. Diath Haemorrh. 1970, 23, 633–637. [Google Scholar] [CrossRef]
- Silva, A.P.; Viegas, C.S.B.; Mendes, F.; Macedo, A.; Guilherme, P.; Tavares, N.; Dias, C.; Rato, F.; Santos, N.; Faísca, M.; et al. Gla-Rich Protein (GRP) as an Early and Novel Marker of Vascular Calcification and Kidney Dysfunction in Diabetic Patients with CKD: A Pilot Cross-Sectional Study. J. Clin. Med. 2020, 9, 635. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous Calcification of Arteries and Cartilage in Mice Lacking Matrix GLA Protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased Bone Formation in Osteocalcin-Deficient Mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Hemostasis, V.; Laurance, S.; Lemarié, C.A.; Blostein, M.D. Growth Arrest-Specific Gene 6 (gas6) and Vascular Hemostasis. Adv. Nutr. 2012, 3, 196–203. [Google Scholar] [CrossRef]
- Dihingia, A.; Ozah, D.; Baruah, P.K.; Kalita, J.; Manna, P. Prophylactic Role of Vitamin K Supplementation on Vascular Inflammation in Type 2 Diabetes by Regulating the NF-ΚB/Nrf2 Pathway via Activating Gla Proteins. Food Funct. 2018, 9, 450–462. [Google Scholar] [CrossRef]
- Petsophonsakul, P.; Furmanik, M.; Forsythe, R.; Dweck, M.; Schurink, G.W.; Natour, E.; Reutelingsperger, C.; Jacobs, M.; Mees, B.; Schurgers, L. Role of Vascular Smooth Muscle Cell Phenotypic Switching and Calcification in Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1351–1368. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A Non-Canonical Vitamin K Cycle Is a Potent Ferroptosis Suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Yogiashi, Y.; Mihara, M.; Takada, I.; Kitagawa, H.; Kato, S. Vitamin K Induces Osteoblast Differentiation through Pregnane X Receptor-Mediated Transcriptional Control of the Msx2 Gene. Mol. Cell. Biol. 2007, 27, 7947–7954. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Horie-Inoue, K.; Ikeda, K.; Blumberg, B.; Inoue, S. Vitamin K2 Induces Phosphorylation of Protein Kinase A and Expression of Novel Target Genes in Osteoblastic Cells. J. Mol. Endocrinol. 2007, 39, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Powerful Micronutrient in Aging and Age-Related Diseases: Pros and Cons from Clinical Studies. Int. J. Mol. Sci. 2019, 20, 4150. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.C.; Wang, H.; Peterson, J.W.; Furie, B.C.; Furie, B.; Booth, S.L.; Volpe, J.J.; Rosenberg, P.A. Novel Role of Vitamin K in Preventing Oxidative Injury to Developing Oligodendrocytes and Neurons. J. Neurosci. 2003, 23, 5816–5826. [Google Scholar] [CrossRef]
- Shiels, P.G.; Painer, J.; Natterson-Horowitz, B.; Johnson, R.J.; Miranda, J.J.; Stenvinkel, P. Manipulating the Exposome to Enable Better Ageing. Biochem. J. 2021, 478, 2889–2898. [Google Scholar] [CrossRef]
- Dai, L.; Schurgers, L.J.; Shiels, P.G.; Stenvinkel, P. Early Vascular Ageing in Chronic Kidney Disease: Impact of Inflammation, Vitamin K, Senescence and Genomic Damage. Nephrol. Dial. Transplant. 2020, 35, ii31–ii37. [Google Scholar] [CrossRef]
- Shiels, P.G.; McGuinness, D.; Eriksson, M.; Kooman, J.P.; Stenvinkel, P. The Role of Epigenetics in Renal Ageing. Nat. Rev. Nephrol. 2017, 13, 471–482. [Google Scholar] [CrossRef]
- Dawood, M.; Hegazy, M.-E.F.; Elbadawi, M.; Fleischer, E.; Klinger, A.; Bringmann, G.; Kuntner, C.; Shan, L.; Efferth, T. Vitamin K3 Chloro Derivative (VKT-2) Inhibits HDAC6, Activates Autophagy and Apoptosis, and Inhibits Aggresome Formation in Hepatocellular Carcinoma Cells. Biochem. Pharmacol. 2020, 180, 114176. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Caluwé, R.; Pyfferoen, L.; De Bacquer, D.; De Boeck, K.; Delanote, J.; De Surgeloose, D.; Van Hoenacker, P.; Van Vlem, B.; Verbeke, F. Multicenter Randomized Controlled Trial of Vitamin K Antagonist Replacement by Rivaroxaban with or without Vitamin K2 in Hemodialysis Patients with Atrial Fibrillation: The Valkyrie Study. J. Am. Soc. Nephrol. 2020, 31, 186–196. [Google Scholar] [CrossRef]
- Oikonomaki, T.; Papasotiriou, M.; Ntrinias, T.; Kalogeropoulou, C.; Zabakis, P.; Kalavrizioti, D.; Papadakis, I.; Goumenos, D.S.; Papachristou, E. The Effect of Vitamin K2 Supplementation on Vascular Calcification in Haemodialysis Patients: A 1-Year Follow-up Randomized Trial. Int. Urol. Nephrol. 2019, 51, 2037–2044. [Google Scholar] [CrossRef]
- Vlasschaert, C.; Goss, C.J.; Pilkey, N.G.; McKeown, S.; Holden, R.M. Vitamin k Supplementation for the Prevention of Cardiovascular Disease: Where Is the Evidence? A Systematic Review of Controlled Trials. Nutrients 2020, 12, 2909. [Google Scholar] [CrossRef]
- Shea, M.K.; Barger, K.; Booth, S.L.; Matuszek, G.; Cushman, M.; Benjamin, E.J.; Kritchevsky, S.B.; Weiner, D.E. Vitamin K Status, Cardiovascular Disease, and All-Cause Mortality: A Participant-Level Meta-Analysis of 3 US Cohorts. Am. J. Clin. Nutr. 2020, 111, 1170–1177. [Google Scholar] [CrossRef]
- Westerman, K.; Kelly, J.M.; Ordovás, J.M.; Booth, S.L.; DeMeo, D.L. Epigenome-Wide Association Study Reveals a Molecular Signature of Response to Phylloquinone (Vitamin K1) Supplementation. Epigenetics 2020, 15, 859–870. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture; Agricultural Research Service. What We Eat in America Food Categories 2011–2012; USDA: Washington, DC, USA, 2015.
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.J.; van der Meer, I.M.; Hofman, A.; Witteman, J.C.M. Dietary Intake of Menaquinone Is Associated with a Reduced Risk of Coronary Heart Disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef]
- Booth, S.L.; Lichtenstein, A.H.; Dallal, G.E. Phylloquinone Absorption from Phylloquinone-Fortified Oil Is Greater than from a Vegetable in Younger and Older Men and Women. J. Nutr. 2002, 132, 2609–2612. [Google Scholar] [CrossRef]
- Jones, K.S.; Bluck, L.J.C.; Wang, L.Y.; Stephen, A.M.; Prynne, C.J.; Coward, W.A. The Effect of Different Meals on the Absorption of Stable Isotope-Labelled Phylloquinone. Br. J. Nutr. 2009, 102, 1195–1202. [Google Scholar] [CrossRef]
- Fu, X.; Shen, X.; Finnan, E.G.; Haytowitz, D.B.; Booth, S.L. Measurement of Multiple Vitamin K Forms in Processed and Fresh-Cut Pork Products in the U.S. Food Supply. J. Agric. Food Chem. 2016, 64, 4531–4535. [Google Scholar] [CrossRef]
- USDA Food Data Central. Available online: https://fdc.nal.usda.gov (accessed on 7 June 2023).
- Papier, K.; Fensom, G.K.; Knuppel, A.; Appleby, P.N.; Tong, T.Y.N.; Schmidt, J.A.; Travis, R.C.; Key, T.J.; Perez-Cornago, A. Meat Consumption and Risk of 25 Common Conditions: Outcome-Wide Analyses in 475,000 Men and Women in the UK Biobank Study. BMC Med. 2021, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.L.; Fu, X.; Al Rajabi, A.; Grusak, M.A.; Shearer, M.J.; Naumova, E.N.; Saltzman, E.; Barger, K.; Booth, S.L. Plasma Response to Deuterium-Labeled Vitamin K Intake Varies by TG Response, but Not Age or Vitamin K Status, in Older and Younger Adults. J. Nutr. 2019, 149, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, A.C.; Pavlic, A.; Petsophonsakul, P.; Halder, M.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K2 Needs an RDI Separate from Vitamin K1. Nutrients 2020, 12, 1852. [Google Scholar] [CrossRef] [PubMed]
- Gast, G.C.M.; de Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.J.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; Peeters, P.H.M.; van der Schouw, Y.T. A High Menaquinone Intake Reduces the Incidence of Coronary Heart Disease. Nutr. Metab. Cardiovasc. Diseases 2009, 19, 504–510. [Google Scholar] [CrossRef]
- Chen, F.; Du, M.; Blumberg, J.B.; Ho Chui, K.K.; Ruan, M.; Rogers, G.; Shan, Z.; Zeng, L.; Zhang, F.F. Association among Dietary Supplement Use, Nutrient Intake, and Mortality among U.S. Adults. Ann. Intern. Med. 2019, 170, 604. [Google Scholar] [CrossRef]
- Adebamowo, S.N.; Feskanich, D.; Stampfer, M.; Rexrode, K.; Willett, W.C. Multivitamin Use and Risk of Stroke Incidence and Mortality amongst Women. Eur. J. Neurol. 2017, 24, 1266–1273. [Google Scholar] [CrossRef]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and Mineral Supplements in the Primary Prevention of Cardiovascular Disease and Cancer: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Boeing, H.; Stelmach-Mardas, M.; Gottschald, M.; Dietrich, S.; Hoffmann, G.; Chaimani, A. Dietary Supplements and Risk of Cause-Specific Death, Cardiovascular Disease, and Cancer: A Systematic Review and Meta-Analysis of Primary Prevention Trials. Adv. Nutr. Int. Rev. J. 2017, 8, 27–39. [Google Scholar] [CrossRef]
- Craven, H.; McGuinness, D.; Buchanan, S.; Galbraith, N.; McGuinness, D.H.; Jones, B.; Combet, E.; Mafra, D.; Bergman, P.; Ellaway, A.; et al. Socioeconomic Position Links Circulatory Microbiota Differences with Biological Age. Sci. Rep. 2021, 11, 12629. [Google Scholar] [CrossRef]
- Wibowo, M.C.; Yang, Z.; Borry, M.; Hübner, A.; Huang, K.D.; Tierney, B.T.; Zimmerman, S.; Barajas-Olmos, F.; Contreras-Cubas, C.; García-Ortiz, H.; et al. Reconstruction of Ancient Microbial Genomes from the Human Gut. Nature 2021, 594, 234–239. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. The Ancestral and Industrialized Gut Microbiota and Implications for Human Health. Nat. Rev. Microbiol. 2019, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Tett, A.; Huang, K.D.; Asnicar, F.; Fehlner-Peach, H.; Pasolli, E.; Karcher, N.; Armanini, F.; Manghi, P.; Bonham, K.; Zolfo, M.; et al. The Prevotella Copri Complex Comprises Four Distinct Clades Underrepresented in Westernized Populations. Cell Host Microbe 2019, 26, 666–679.e7. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef]
- Sharma, V.; Rodionov, D.A.; Leyn, S.A.; Tran, D.; Iablokov, S.N.; Ding, H.; Peterson, D.A.; Osterman, A.L.; Peterson, S.N. B-Vitamin Sharing Promotes Stability of Gut Microbial Communities. Front. Microbiol. 2019, 10, 1485. [Google Scholar] [CrossRef]
- Fenn, K.; Strandwitz, P.; Stewart, E.J.; Dimise, E.; Rubin, S.; Gurubacharya, S.; Clardy, J.; Lewis, K. Quinones Are Growth Factors for the Human Gut Microbiota. Microbiome 2017, 5, 161. [Google Scholar] [CrossRef]
- Ellis, J.L.; Karl, J.P.; Oliverio, A.M.; Fu, X.; Soares, J.W.; Wolfe, B.E.; Hernandez, C.J.; Mason, J.B.; Booth, S.L. Dietary Vitamin K Is Remodeled by Gut Microbiota and Influences Community Composition. Gut Microbes 2021, 13, 1887721. [Google Scholar] [CrossRef]
- Karl, J.P.; Meydani, M.; Barnett, J.B.; Vanegas, S.M.; Barger, K.; Fu, X.; Goldin, B.; Kane, A.; Rasmussen, H.; Vangay, P.; et al. Fecal Concentrations of Bacterially Derived Vitamin K Forms Are Associated with Gut Microbiota Composition but Not Plasma or Fecal Cytokine Concentrations in Healthy Adults. Am. J. Clin. Nutr. 2017, 106, 1052–1061. [Google Scholar] [CrossRef]
- Usui, Y.; Tanimura, H.; Nishimura, N.; Kobayashi, N.; Okanoue, T.; Ozawa, K. Vitamin K Concentrations in the Plasma and Liver of Surgical Patients. Am. J. Clin. Nutr. 1990, 51, 846–852. [Google Scholar] [CrossRef]
- Klinder, A.; Shen, Q.; Heppel, S.; Lovegrove, J.A.; Rowland, I.; Tuohy, K.M. Impact of Increasing Fruit and Vegetables and Flavonoid Intake on the Human Gut Microbiota. Food Funct. 2016, 7, 1788–1796. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Mafra, D.; Shiels, P.G.; Hackeng, T.M.; Stenvinkel, P.; Schurgers, L.J. Vitamin K and Hallmarks of Ageing: Focus on Diet and Gut Microbiome. Nutrients 2023, 15, 2727. https://doi.org/10.3390/nu15122727
Dai L, Mafra D, Shiels PG, Hackeng TM, Stenvinkel P, Schurgers LJ. Vitamin K and Hallmarks of Ageing: Focus on Diet and Gut Microbiome. Nutrients. 2023; 15(12):2727. https://doi.org/10.3390/nu15122727
Chicago/Turabian StyleDai, Lu, Denise Mafra, Paul G. Shiels, Tilman M. Hackeng, Peter Stenvinkel, and Leon J. Schurgers. 2023. "Vitamin K and Hallmarks of Ageing: Focus on Diet and Gut Microbiome" Nutrients 15, no. 12: 2727. https://doi.org/10.3390/nu15122727
APA StyleDai, L., Mafra, D., Shiels, P. G., Hackeng, T. M., Stenvinkel, P., & Schurgers, L. J. (2023). Vitamin K and Hallmarks of Ageing: Focus on Diet and Gut Microbiome. Nutrients, 15(12), 2727. https://doi.org/10.3390/nu15122727







