Abstract
β-tricalcium phosphate (β-TCP) is a promising material in regenerative traumatology for the creation of bone implants. Previously, it was established that doping the structure with certain cations can reduce the growth of bacterial activity. Recently, much attention has been paid to co-doped β-TCP, that is explained by their ability, on the one hand, to reduce cytotoxicity for cells of the human organism, on the other hand, to achieve a successful antibacterial effect. Sr, Cu-co-doped solid solutions of the composition Ca9.5–xSrxCu(PO4)7 was obtained by the method of solid-phase reactions. The Rietveld method of structural refinement revealed the presence of Sr2+ ions in four crystal sites: M1, M2, M3, and M4. The M5 site is completely occupied by Cu2+. Isomorphic substitution of Ca2+ → (Sr2+and Cu2+) expands the concentration limits of the existence of the solid solution with the β-TCP structure. No additional phases were formed up to x = 4.5 in Ca9.5–xSrxCu(PO4)7. Biocompatibility tests were performed on cell lines of human bone marrow mesenchymal stromal cells (hMSC), human fibroblasts (MRC-5) and osteoblasts (U-2OS). It was demonstrated that cytotoxicity exhibited a concentration dependence, along with an increase in osteogenesis and cell proliferation. Ca9.5–xSrxCu(PO4)7 powders showed significant inhibitory activity against pathogenic strains Escherichia coli and Staphylococcus aureus. Piezoelectric properties of Ca9.5–xSrxCu(PO4)7 were investigated. Possible ways to achieve high piezoelectric response are discussed. The combination of bioactive properties of Ca9.5–xSrxCu(PO4)7 renders them multifunctional materials suitable for bone substitutes.
1. Introduction
The regenerative approach to restoring damaged bone tissues is emerging as a compelling and promising method in osteosurgery. This method involves utilizing implants with compositions similar to human bones. Calcium phosphate (CaP)-based substances have demonstrated efficacy as bone substitutes owing to their excellent biocompatibility. However, postoperative complications, often caused by harmful bacteria, remain a challenging issue to address.
To mitigate the proliferation of undesirable pathogens, compounds based on the β-tricalcium phosphate (β-Ca3(PO4)2, β-TCP) (space group R3c, unit cell parameters: a = b = 10.439 Å and c = 37.375 Å) [1] with various types of dopants applied to biomedical implants can prove useful. The low-temperature modification β-TCP has high biocompatibility [2], resorbable properties [3,4] and osteoinductive characteristics [5]. The crystallochemical aspect of β-TCP is also of considerable interest. The β-TCP structure consists of five cationic sites (M1–M5) [6,7]. The variety of crystallographic positions and rigid structure of phosphates allows for isomorphic substitutions of host Ca2+ ions by iso- or heterovalent ions, and co-doping, as well [8,9,10].
Copper ions (Cu2+) may become suitable candidates for partial replacement of calcium. Their action contributes not only to osteogenesis but also to a decrease in bacterial growth. Strong antibacterial properties of copper ions could prevent peri-implantitis—inflammation around a newly installed implant [11]. Previous studies have demonstrated a clear antibacterial effect, with a death rate of about 22% at a concentration of Cu2+ ions at approximately 12 mol.% in the β-TCP structure, and acceptable cytocompatibility [12]. However, such a bacteria death rate might not be sufficient, raising the question of how to enhance the antibacterial effectiveness.
One of the ways for increasing antibacterial activity while maintaining biocompatibility is through co-substitution in CPs [13,14,15]. Co-substituted CPs demonstrate a higher antibacterial effect than mono-substituted ones, without a negative effect on cell cultures [16], Ag and Zn or Ti, for instance. As a co-dopant, Strontium (Sr) is of interest because it can also inhibit several strains of microorganisms, such as Escherichia coli and Staphylococcus aureus [17,18]. Thereby, the antibacterial effect can be enhanced. Additionally, the presence of Sr2+ ions in the CPs-based implants can positively impact bone tissue due to their physiological similarity to calcium ions [19]. The presence of Sr2+ ions in the implant can stimulate osteogenesis [20], and increase adhesion, proliferation and differentiation of osteoblasts [21,22]. The controlled release of a specific amount of Sr2+ ions can facilitate the healing of osteoporotic bone defects [23]. Furthermore, it was shown that Sr-doped β-TCP scaffolds promote early angiogenesis [24]. Sr2+ ions prevented the formation of biofilms [25] without exhibiting cytotoxicity for mesenchymal stem cells [26].
Incorporation of Sr2+ ions into the β-TCP structure above 2.25 mol% also enhances the phase purity of the reaction product. This is achieved as the synthesis temperature range expands to 1125 °C, thereby preventing the α→β transition in the β-TCP structure [27]. The doping of the β-TCP structure by 10% mol. of Sr2+ ions does not affect the α-TCP phase stabilization [28].
In this work, to validate the hypothesis regarding the positive synergetic effect of Cu2+ and Sr2+ co-doping in the β-TCP, we synthesized a series of solid solutions Ca9.5–xSrxCu(PO4)7. Based on our previous findings [12], the concentration of Cu2+ ions was held constant at 9.5 mol.% to achieve a pronounced antibacterial effect. Meanwhile, the concentration of Sr2+ ions was varied in a wide range from 0 to 42 mol.% corresponding to the formula Ca9.5–xSrxCu(PO4)7. The area of isomorphic substitution was investigated and the crystal structures were refined using the Rietveld method. The bioactive properties, including cytotoxicity study and cell proliferation, of the Ca9.5–xSrxCu(PO4)7 powders were evaluated across various cell lines, including mesenchymal stromal cells (hMSC), human fibroblasts (MRC-5) and osteoblasts (U-2 OS). Additionally, the antibacterial effect was determined against Escherichia coli and Staphylococcus aureus. Biomimetic properties were also examined through the piezoelectric effect on the Ca9.5–xSrxCu(PO4)7 powders. Finally, the influence of the crystal structure and concentration of doping ions are discussed.
2. Materials and Methods
2.1. Synthetic Route
Strontium-copper-substituted phosphates with a general formula Ca9.5–xSrxCu(PO4)7 were obtained by solid-phase synthesis at high temperatures (Table 1). Before the synthesis, we calculated and weighed the initial quantities of substances according to the stoichiometry of the reaction. The initial reagents for solid-state synthesis CaHPO4⋅2H2O (99.9%), SrCO3 (99.9%), CaCO3 (99.9%), (NH4)2H2PO4 (99.9%) and CuO (99.9%) were purchased from the Sigma-Aldrich (Gillingram, UK). Raw reagents were mixed and homogenized. The mixtures were preheated at 773 K for 12 h with a low heating rate for slow removal of gas products. Then the samples were annealed at 1173 K and kept for 18 h. After that, the final stage of synthesis was carried out at 1173 K and kept for 18 h. Between each stage, grinding and homogenization was carried out at room temperature in the presence of acetone in an agate mortar [12]. Before grinding, the mixtures were slowly cooled. Synthesis of Sr2+, Cu2+-doped TCP was performed according to the following reactions:
14CaHPO4·2H2O + 2xSrCO3 + (5 − 2x)CaCO3 + 2CuO → 2Ca9.5–xSrxCu(PO4)7 + 5CO2↑ + 35H2O (0 ≤ x ≤ 4.5).

Table 1.
Chemical formula, doping mol.%, unit cell (a, c) parameters and volume (V) in Ca9.5–xSrxCu(PO4)7 0 ≤ x ≤ 4.5 samples.
The parameters of the unit cells were calculated by describing the profile using the least squares method. The error is calculated using the charge flipping method (Table 1).
2.2. Characterization
2.2.1. Powder X-Ray Diffraction Study
Powder X-ray diffraction (PXRD) patterns were collected on a Rigaku SmartLab SE: 3 kW sealed X-ray tube, D/teX Ultra 250 silicon strip detector (Rigaku, Tokyo, Japan), vertical type θ-θ geometry, CuKα radiation, HyPix-400 (2D HPAD) detector (Rigaku, Tokyo, Japan). PXRD data were collected at room temperature in the 2θ range between 3° and 80° with a step interval of 0.02°. The X-ray data were reproduced three times. The LeBail decomposition and structure refinement by the Rietveld methods were applied using the JANA2006 software (Version 2020) [29]. The crystal structures of Ca9.5–xSrxCu(PO4)7 samples were refined using the Rietveld method [30]. The crystal structure of Ca9.5Cu(PO4)7 (CSD Deposition Number 2220569) [12] served as a starting model for the refinement. The space group R3c was chosen [12,31]. The background was described by a Chebyshev polynomial function (31st order). Pseudo-Voigt functions were employed for fitting the reflection profiles. The refined structural parameters included the atomic coordinates and the isotropic temperature factor. The isotropic temperature factors were constrained to be the same for all oxygen atoms. The main crystallographic data from the Rietveld refinement and experimental details for Ca9.5–xSrxCu(PO4)7 samples are listed in Tables S3–S12 of the Supplementary Information.
2.2.2. Fourier-Transform Infrared (FT-IR) Study
The FT-IR spectra of the samples were recorded on an FT-803 Fourier spectrometer (Simeks Research and Production Company 2022 Novosibirsk, Russia) in the wavenumber region of 4000–400 cm−1, with 1 cm−1 spectral resolution. The standard KBr disc method was applied to obtained the spectra.
2.2.3. The Ion Release Behavior
For the study of Ca2+, Sr2+ and Cu2+ release into solutions behavior, the powder samples were pressed into pallets (d = 0.4 cm, m ~ 0.2 g). A hydraulic oil press was used to produce the tablets. The release of Ca2+, Sr2+ and Cu2+ from Ca9.5–xSrxCu(PO4)7 was investigated by soaking of Ca9.5Cu(PO4)7 and Ca5Sr4.5Cu(PO4)7 pallets in the 0.05 M Tris-HCl (pH = 7.4) buffer solution (75 mL). The soaking systems were placed in flasks for 4, 7, 14 and 18 days. The accumulative release amount of Ca2+, Sr2+ and Cu2+ ions was measured using inductively coupled plasma optical emission spectroscopy (ICP-OES, 720-ES axial spectrometer (Agilent Technologies, New York, NY, USA). The obtained data were reproduced three times and reported as mean ± standard deviation.
2.2.4. In Vitro Biological Response to the Ceramics
Cytocompatibility of the studied samples was evaluated on human mesenchymal bone marrow stromal cells (MSCs), human lung fibroblasts (MRC-5) and U-2 OS (human osteosarcoma cell line, resemble pre-osteoblasts). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Gibco) under standard culture conditions (humidified atmosphere, 5% CO2 and 95% air, at 37 °C). Before the biocompatibility study, U-2 OS cell lines were differentiated into osteoblasts for a 3-week culture period. Differentiation was performed by adding ascorbic acid (50 μg/mL) on day 4 and β-glycerolphosphate (5 mM) on day 11 to the culture medium.
Cell Cultivation on a Powder Layer
Sterile cover glasses with a diameter of 1 cm were placed in a 12-well plate, onto which powder samples were evenly applied in a thin continuous layer. The samples were then incubated with 50 µL of culture medium to obtain a dense layer. MSCs and MRC-5 cells were seeded at a density of 10 × 103 per 100 mL/glass in medium (DMEM + 10%FBS + antibiotic/antimycotic). After 24 h, 1 mL of cellular medium was added, and the cells were incubated for 3 days. Analysis of cell number and viability was determined using fluorescent dyes Hoechst 33342 (a fluorescent DNA intercalating dye that stains the nuclei of all cells) and PI (stains only the nuclei of dead cells). Cells were analyzed by fluorescence microscopy (Zeiss, Axio observer Z1, Oberkochen, Germany). The acquired images were processed using ZEN blue software (ZEN3.1. blue edition) (Zeiss, Oberkochen, Germany) (Figure 1).
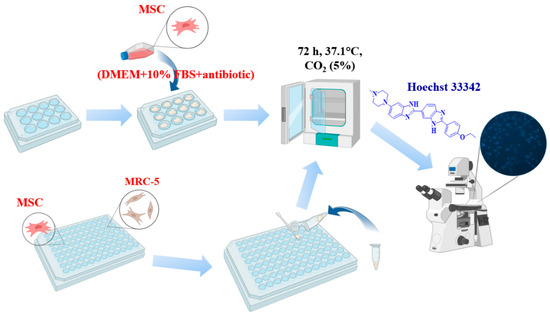
Figure 1.
The sequence of research on biological cell cultures. Created with BioRender.com.
Adding Powders of Ceramics to Cell Medium
MSCs and MRC-5 cells were seeded in 96-well plates at a density of 1 × 104 cells per 100 µL/well. After 24 h, powders were added on top of the cells into the wells, and the cells were then incubated with the powders for 3 days. Cell viability was assessed by fluorescent microscopy, with cell nuclei stained by Hoechst 33342.
The addition of elements such as Ca, Zn, Sr and Cu to bone implants is intended to improve their osteogenic properties. Copper is an essential metal in the human body including playing an important functional role in bone growth and development. It was shown that Cu at certain concentrations increases osteogenic differentiation of mesenchymal stromal cells (MSC) [32,33]. In turn, strontium is also an extremely important element in osteogenesis. Strontium ions (Sr2+) promote osteogenesis by enhancing the expression of osteogenesis-related genes in mesenchymal stem cells through activation of signaling pathways such as Wnt/β-catenin and RAS/MAPK [34]. And of course, calcium ions play an essential role in the formation of bone tissue. Ca2+ activates various intracellular signaling pathways such as yes-associated protein (YAP), wingless integration site (WNT) and mitogen-activated protein kinase (MAPK)—extracellular signal-regulated kinase (ERK)1/2. Through these pathways, Ca2+ stimulates the expression of osteogenic genes, osteoblast proliferation and MSCs differentiation [35,36].
So, the effect of ions released into the culture medium on cell viability was also determined. MSCs and U-2 OS cells were seeded in 96-well plates (1 × 104 cells/100 µL/well). The powders (100 mg) were placed in 1.5 mL Eppendorf tubes, mixed with 1 mL of cell medium, and then incubated at 37 °C for 24 h. After incubation, the tubes containing the powder suspension were centrifuged at 10,000 g, and the supernatant containing diluted Cu2+, Sr2+ and Ca2+ cations was added to cells, replacing the old medium. Different concentrations were used, with a two-fold dilution step. The cells were incubated then for 2 days, after which the MTT test was performed. The MTT test based on the reduction of MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) in the cell cytoplasm to a strongly light-absorbing formazan is among the most commonly used methods for the determination of cell viability and activity of NAD-dependent oxidoreductases. MTT test was performed according to the standard protocol. Optical density was measured using a Multiscan Sky device (Thermo Fisher Scientific Inc. Waltham, MA, USA) at a wavelength of 540 nm.
2.2.5. Antimicrobial Activity Study
The antimicrobial activity of Ca9.5–xSrxCu(PO4)7 was investigated using E. coli and S. aureus. The study employed a dense nutrient-rich medium, GRM-agar, produced by the FSB SSC PMB (Obolensk, Russia). A physiological solution (PS), consisting of sodium chloride (NaCl = 9 g/l), was used as a buffer. Night cultures of the reference strains were fully looped and suspended in 0.5 mL of PS. Subsequently, 0.1 mL of all dilutions were seeded onto GRM-agar in Petri dishes to count the number of colony-forming units (CFU) at the beginning of the experiment (0 h). Each sample, weighing 0.1 g, was placed in a 1.5 mL Eppendorf test tube (Eppendorf, Germany) and 0.99 mL of PS was added. Additionally, PS (0.9 mL) was added to another test tube as a control, without the powder under investigation. The test tubes were sealed with Parafilm M sealing film (Bemis Company, Inc., USA) without closing the lid and shaken on a vortex for 2 min. The tubes were then incubated at 37 °C for 24 h. At the end of the incubation period, 0.1 mL of suspension was extracted from each tube, and 0.1 mL of all dilutions was plated onto GRM-agar in Petri dishes to count the number of CFUs (24 h).
2.2.6. Piezoelectric Properties
The local piezoelectric properties were examined using the Piezoresponse Force Microscopy (PFM) technique with a commercial Ntegra II scanning probe microscope from NT-MDT Spectrum Instruments, Russia. Commercial cantilevers NSG01/Pt were used for these measurements. The HD-PFM scanning regime was employed to mitigate the undesirable effect of particle shifting by the tip. The modulation amplitude and frequency were set at 10 V and 550 kHz, respectively.
3. Results
3.1. PXRD Study
The PXRD patterns of synthesized Ca9.5–xSrxCu(PO4)7 0 ≤ x ≤ 4.5 solid solutions are presented in Figure 2a. The data analysis revealed that all the powder samples belong to the β-TPC structural type. No impurities from either the apatite type or pyrophosphate phases were detected, which confirms a complete reaction. In [37], the maximum doping concentrations of both Cu2+ and Sr2+ ions were ~ 2.52 mol.%. The absence of impurity phases confirmed the complete incorporation of these ions into the β-TCP structure, represented by the formula Ca9.97Sr0.265Cu0.265(PO4)7, as determinate by elemental analysis [37]. In the single Sr2+-doped solid solution Ca10.5–xSrx(PO4)7 [31], the limit of Sr2+ substitution before a structural change was determinate to be x = 6, corresponding to Ca4.5Sr6(PO4)7. According to our results, the reduction of the unit cell caused by Cu2+ incorporation does not hinder the formation of single-phase solid solutions throughout the studied range in Ca9.5–xSrxCu(PO4)7 (0 ≤ x ≤ 4.5).
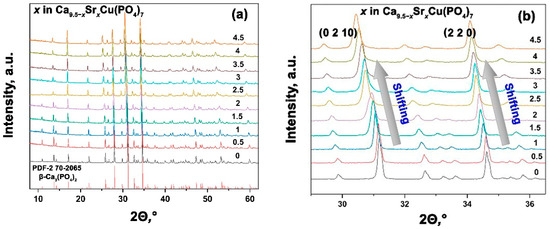
Figure 2.
PXRD patterns of Ca9.5–xSrxCu(PO4)7 with 0 ≤ x ≤ 4.5 (a). Shifting of the main diffraction reflections in Ca9.5–xSrxCu(PO4)7, 0 ≤ x ≤ 4.5 (b).
In the smaller-angle scale of the PXRD pattern, one can notice a shift in reflexes towards decreasing angles θ, that indicates an increase in the parameters of the unit cell. This effect correlates well with the classical Bragg law and confirms the successful isomorphic substitution in the structure (Figure 2b).
The calculation of the unit cells parameters a, c and the volumes V is consistent with the Vegard’s law. This suggests the creation of a continuous series of solid solutions Ca9.5–xSrxCu(PO4)7, confirming the intended isomorphic substitution (Figure 3). The precise values of the elementary cell parameters are detailed in Table 1.
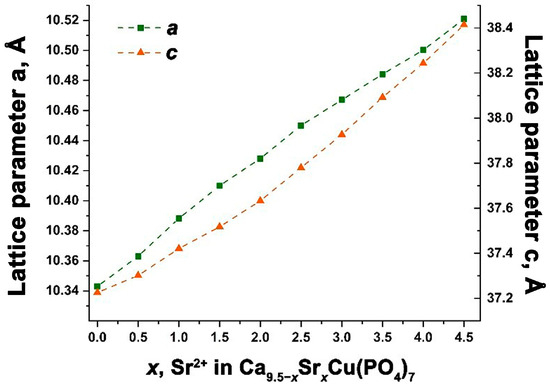
Figure 3.
The dependence of the unit cell parameters a and c for Ca9.5–xSrxCu(PO4)7 solid solutions.
3.2. Fourier-Transform Infrared Study
Figure 4a,b shows the FT-IR spectra of Ca9.5–xSrxCu(PO4)7. All the presented spectra are very similar. Absorption bands of PO43– groups are noticeable in all spectra. The vibrational modes in the phosphate stretching and bending regions appear in the range of 939–999 cm−1 for ν1s, 1128–1021 cm−1 for ν3as and 611–544 cm−1 for ν4 [38]. However, transmission peaks at 1203 and 729 cm−1 were identified in Ca9.5Cu(PO4)7. These low-intensity bands suggest the presence of the ν [P–O–P] from P2O74–. However, the PXRD patterns did not show reflections attributed to the pyrophosphate phase, its amount being below the sensitivity level of the instrument. Thus, P2O74– ions likely do not significantly contribute to the properties.
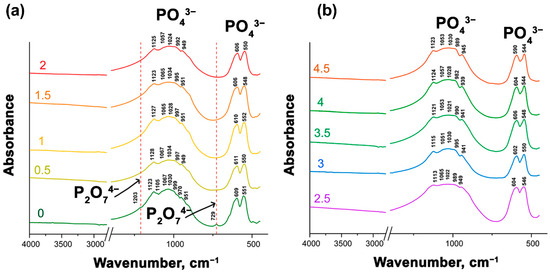
Figure 4.
FT-IR spectra of Ca9.5–xSrxCu(PO4)7 with 0 ≤ x ≤ 2 (a) and 2 ≤ x ≤ 4.5 (b).
3.3. The Rietveld Refinement
Firstly, the occupancies of the crystal sites were refined. Cu2+ ions were determined to occupy the smallest octahedral M5 site due to the value of the ionic radii (rVI = 0.73 Å). Next, Ca and Sr atoms were randomly placed over the M1–M3 sites. Their occupancies (ai) were refined with total occupancy restrained to unity. For the M4 site, the occupancy was restricted to a value of 0.5 [7]. According to the refinement, Sr2+ with ionic radii (rVIII = 1.26 Å) [39] primarily locate in the M1–M3 sites, while in the M4 site, ai(Sr) does not exceed 0.1 (Figure 5a). Our results are in a good agreement with the literature data from [37], which refined the chemical formula to Ca9.632Sr0.497Cu0.371(PO4)7 (or β-Ca2.752Sr0.142Cu0.106(PO4)2), and noted the occupancy of the M4 site by Sr2+ ions as 0.110. Further increases in the Sr2+ ion concentration results in its distribution through M1–M3, with a preference for the M3 site. This same trend of Sr2+ location was observed in the single-doped solid solution Ca9.5–xSrxCu(PO4)7 [31].
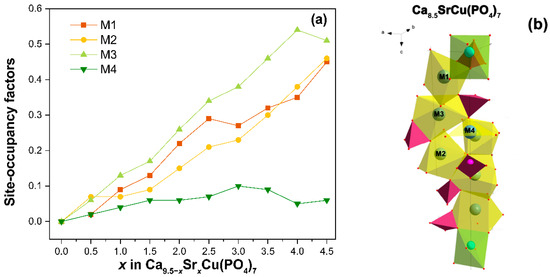
Figure 5.
The dependence of the occupation site on the composition Ca9.5–xSrxCu(PO4)7 (a). The fragment of structure Ca8.5SrCu(PO4)7 (b).
3.4. The Release Behavior of Sr2+, Cu2+, Ca2+ Ions
The accumulative release amount of Ca2+, Cu2+, and Sr2+ from Ca9.5–xSrxCu(PO4)7 samples after soaking in Tris-HCl buffer solution is shown in Figure 6. After 18 days of soaking, the release of Cu2+ from the samples exhibits roughly a linear dependency (Figure 6). The amount of Cu2+ ion released reaches 1.7 mg/L. There is no significant difference between the Ca9.5Cu(PO4)7 (Figure 6a) and Ca5Sr4.5Cu(PO4)7 (Figure 6b) samples in the value of the released Cu2+. The notably lower concentrations of Cu2+ in the soaking solution, compared to Ca2+ and Sr2+ ions, are related to its site-selective arrangement in the smallest octahedral M5 site, as proposed by us in [12], and according to crystal structure refinement. The placement of Sr2+ in the voluminous M1O8–M3O8 and M4O6 polyhedra (Figure 5b) contributes to its high dissolution into the liquid. The measurements were repeated three times in each step.
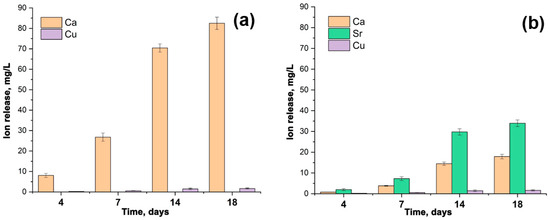
Figure 6.
The accumulative release amount of Ca2+, Cu2+ and Sr2+ ions from Ca9.5Cu(PO4)7 (a) and Ca5Sr4.5Cu(PO4)7 (b) samples after soaking in Tris-HCl buffer solution for 4, 7, 14 and 18 days.
3.5. Biocompatibility Tests
Staining with Hoechst 33342 dye makes it possible to determine the total number of cells, as well as to identify apoptotic cell nuclei. The tests were carried out on samples Ca9.5Cu(PO4)7 and Ca5Sr4.5Cu(PO4)7. When using the method of cell cultivation of hMSCs on a powder layer, it was observed that culturing cells on such a layer exhibited a highly cytotoxic effect, evidenced by the presence of individual dead cells after 3 days (Figure 7a). In the second case, it was observed that upon the addition of the studied powders, the proliferative activity significantly decreased, with the morphology of the nuclei indicating complete cell death for the Ca9.5Cu(PO4)7 sample, and partial death for Ca5Sr4.5Cu(PO4)7 (Figure 7b). Weakly stained cells with Hoechst 33342 dye remained viable, mainly located on the glass in the gaps between the blocks of powders (Figure 8).
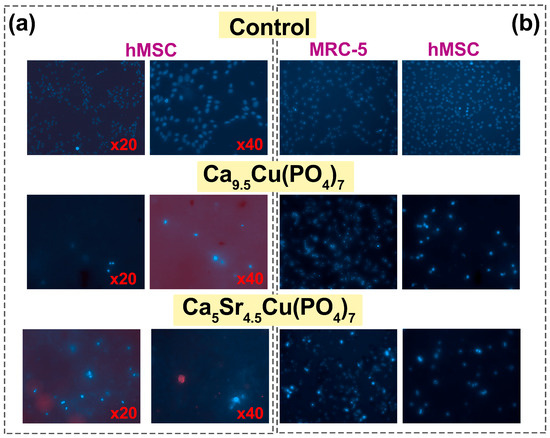
Figure 7.
Viability of hMSCs cells after cultivation on a powder layer of Ca9.5Cu(PO4)7 and Ca5Sr4.5Cu(PO4)7 samples (a). Viability of MRC-5 and hMSCs while adding ceramic powders to cell medium for 3 days (magnification ×20) (b).
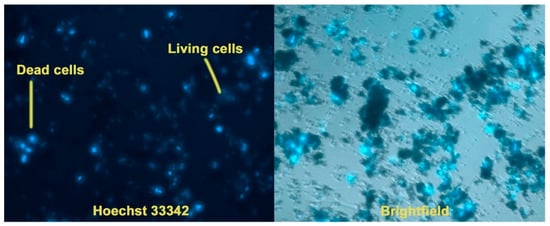
Figure 8.
Viability of hMSCs cells after cultivation on a powder layer of Ca5Sr4.5Cu(PO4)7 (magnification ×20).
The MTT test demonstrated high toxicity of aqueous extracts of Ca5Sr4.5Cu(PO4)7 powder in maximum concentrations of 25, 50 and 100 mg/mL. Solutions of Ca9.5Cu(PO4)7 powder are less toxic (Figure 9a) even at 100 mg/mL concentration. It is interesting to note that solutions of Ca9.5Cu(PO4)7 exhibit a less toxic effect on osteoblasts, with concentrations exceeding 50 mg/mL showing a stimulating effect on osteoblast proliferation, significantly surpassing control values (Figure 9b). However, the presence of a large quantity of Sr2+ ions from the β-TCP structure in Ca5Sr4.5Cu(PO4)7 has a negative impact on the cells.
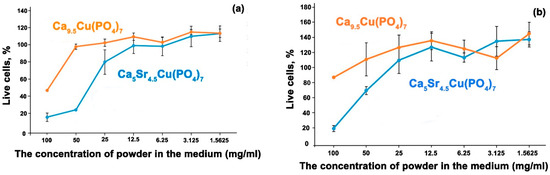
Figure 9.
The cytotoxicity of the solution derived from Ca9.5Cu(PO4)7 and Ca5Sr4.5Cu(PO4)7 powders on hMSCs cells (a), and U-2 OS cells (b) assessed using the MTT test.
3.6. Antibacterial Activity
Ca9.5–xSrxCu(PO4)7 samples showed antibacterial effects against test strains of E. coli and S. aureus following 24 h of incubation (Figure 10). The study was carried out on the samples where x = 0, 1, 3 and 4.5. All samples effectively suppressed bacterial growth. Comparison with our previous study on single doped Ca10.5–xCux(PO4)7 solid solutions [12] revealed similar results for bacteria inhibition, particularly for a sample with x = 0 (Figure 10). This similarity was also observed in samples Ca8.5SrCu(PO4)7 (or Ca2.42Sr2.86Cu2.86(PO4)2, Figure 10) and Ca2.5Cu0.25Sr0.25(PO4)2 [16]. The inhibition of the bacteria growth of approximately 90% on S. aureus and 60% on E. coli was observed in Ca2.8Cu0.1Sr0.1(PO4)2 [16].
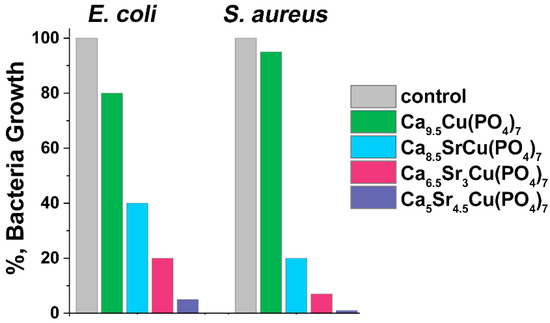
Figure 10.
The inhibition of bacteria (E. coli and S. aureus) grown for 24 h in the presence of Ca9.5–xSrxCu(PO4)7. The positive control (ctr) is represented by the growth of each bacteria strain in the absence of phosphates.
A significant reduction in bacterial growth was observed when x ≥ 1. In our previous study [16], it was suggested that active dopant ions distributed in various crystal sites of the β-TCP structure might augment antimicrobial efficacy. This could be due to the increase in possible concentration of dopant ions and the expansion of the unit cell, leading to a facilitated release of ions. The single-doped Ca9.5Cu(PO4)7 sample inhibits the growth of E. coli by 20%, while S. aureus shows lower susceptibility to this phosphate with only a 5% inhibition observed (Figure 10). The addition of Sr2+ ions to the β-TCP significantly increases its antibacterial activity. For instance, Ca8.5SrCu(PO4)7 inhibits E. coli growth by 60%, and S. aureus inhibition reaches 80%. A further increase in Sr2+ concentration in Ca9.5–xSrxCu(PO4)7 results in almost complete suppression of bacterial growth: 93% of inhibition for E. coli, and nearly a total reduction in S. aureus growth (Figure 10). Notably, according to the results, S. aureus is more sensitive to the presence of Sr2+ ions in the solution than E. coli, and almost indifferent to Cu2+ ions. In this study, Ca9.5–xSrxCu(PO4)7 exhibited a significant antibacterial activity related to a synergetic effect of Sr2+ and Cu2+ co-doping.
3.7. Piezoelectric Properties
Scanning probe microscopy (SPM) is a commonly used method for investigation of the local piezoelectric properties at the micro(nano)scale, applied for films and (micro)nano particles, and connected to the piezoforce microscopy (PFM) technique. One key challenge in conducting PFM studies on micro(nano) particles is their attachment to a conductive substrate. To address this issue, the particle agglomerates were mixed in isopropyl alcohol and sprayed onto a conductive tape used as the substrate. Through a series of test scans, particles with better adhesion were identified and subsequently utilized for further experiments.
Figure 11 shows AFM scans obtained for a sample prepared according to the procedure described above. As can be seen, the particles of Ca9Sr0.5Cu(PO4)7 are inhomogeneously distributed on the substrate and are interconnected into agglomerates, as evident from the 3D scan map (Figure 11b). The linear dimensions of some of the largest particle agglomerates are about 2×3 μm with a height of up to 1 μm.
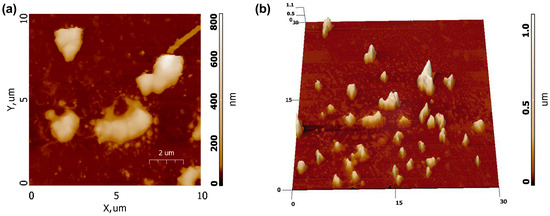
Figure 11.
AFM images of Ca9Sr0.5Cu(PO4)7 sample: (a) 2D AFM map, (b) 3D AFM map.
To confirm the piezoelectric nature of the Ca9Sr0.5Cu(PO4)7, PFM scans of the amplitude and phase in vertical (Figure 12a,b) and horizontal (Figure 12c,d) modes were conducted. A weak piezoresponse was observed on some particles and their agglomerates. The low output signal is also associated with a low amplitude of modulated voltage. The piezoelectric coefficients are proportional to PFM amplitude, and the main challenge in their correct calculations lies in the quantification of PFM signals, which requires calibration. Moreover, the sample demonstrates an «exotic» type of piezoelectricity with the d14 piezoelectric coefficient, while more conventional piezoelectric coefficients, such as d33 and d31, correspond to transverse and longitudinal modes of piezoelectricity. Macroscopic piezoelectricity in calcium phosphates was investigated by S. A. M. Tofail [40], who estimated a shear piezoelectric coefficient of d14 = 14pC/N. It should be noted that experiments aimed at directly measuring the macroscopic piezoelectric coefficient d33 in Ca9Sr0.5Cu(PO4)7 ceramics did not demonstrate piezoelectricity, which may be related to macroscopic anisotropy. However, at micro and nano scales using the PFM technique, piezoelectricity in the sample was observed [41]. For example, the presence of the piezoelectric polarization in tooth enamel was observed by Kalinin in several isolated 50–200 nm regions [42,43]. Finally, the analysis of the obtained results allows for some preliminary conclusions regarding the presence of piezoelectricity in co-doped β-TCP, which necessitates further studies for the accurate quantification of PFM signals to determine piezoelectric coefficients.
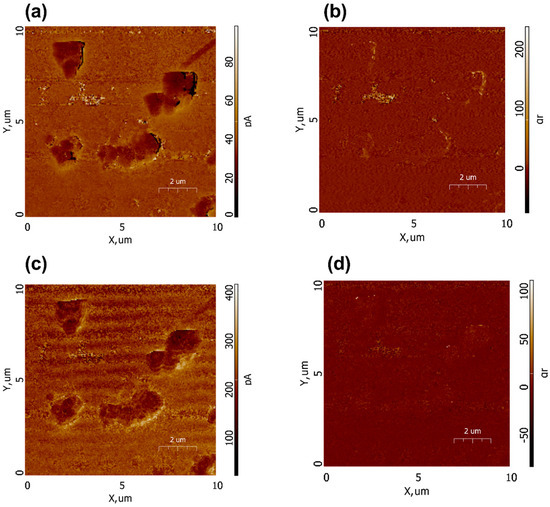
Figure 12.
PFM images of Ca9Sr0.5Cu(PO4)7 sample: amplitude (a,c) and phase (b,d) in vertical (a,b) and horizontal (c,d) modes.
4. Discussion
In the present study, we demonstrate that in the Ca9.5–xSrxCu(PO4)7 solid solution, a continuous series of solid solutions can be formed up to x = 4.5 (42.9 mol.% of Sr/(Ca+Sr+Cu)).
In the Ca10.5–xSrx(PO4)7 series, the limit of isomorphism for β-TCP, upon substitution with strontium ions, was found at x = 6. This corresponds to the transition of the structure to the palmierite-type Sr3(PO4)2 [44]. The full incorporation of the Cu2+ ions into the M5 site and, consequently, the decrease in the unit cell volume does not affect the isomorphic capacity of the Sr2+ ions in Ca9.5–xSrxCu(PO4)7. Therefore, within the studied concentration range, a continuous series of solid solutions was confirmed by PXRD and FT-IR studies. The Rietveld refinement revealed that Sr2+ and Ca2+ ions jointly occupy M1–M4 crystal sites. Strontium prefers the M3 site. The difference in the Sr2+ orientation is attributed to the reduced interatomic distances in the M4 polyhedra. In the Ca9.45Sr1.05(PO4)7 structure (PDF-4+ № 04-021-3537), the mean distance M4–O (dM4–O) is 2.622 Å. Conversely, according to the refinement, the dM4–O value in Ca8.5SrCu(PO4)7 is 2.386 Å. Given the sum of the ionic radii (rVIII(Sr2+) = 1.26 Å and rII(O2–) = 1.35 Å), the Sr2+ location in the M4 site is not suitable for the β-TCP structure due to the nearest oxygen environment is a flat triangle (Figure 5b), which is not loyal to the substitution for large ions. However, the possibility of splitting this position [45] with a change in coordination allows ions with a radius greater than Ca2+ to be located there.
Surface morphology significantly influences the adhesion, proliferation and functional activity of cells, including osteoblasts, which play a direct role in osteogenesis. Since the materials in question are powders, their high toxicity is most likely related to physical parameters. When cells are introduced to powders, cell adhesion is disrupted, leading to low survival rates. Conversely, adding powders to cells that are already attached has a significantly less cytotoxic effect. The use of these powders in the form of materials, such as scaffolds, will significantly reduce toxicity [46].
Ca9.5–xSrxCu(PO4)7 solid solutions show high antibacterial activity, which can be attributed to the significant release of ions into the solution. The increase in the antibacterial activity of the co-doped CPs [16] was confirmed in the present study. In [16], the inhibition of bacterial growth was reduced due to the presence of the apatite-type phase, known for its lower solubility. The minimal concentration of Sr2+ in β-TCP that exhibited effective antibacterial activity was found at x = 0.35 in Ca10.15Sr0.35(PO4)7 [26]. Importantly, this concentration did not induce cytotoxic effects [47].
Some criteria for enhancing antibacterial activity can be postulated as follows:
- (1)
- Co-doping results in the expansion of the boundaries of single-phase solid solution, thereby enabling the incorporation of a larger number of active ions.
- (2)
- The expansion of the unit cell leads to a more pronounced release of ions into the solution.
- (3)
- Both ions are required to contribute to the antibacterial properties.
- (4)
- The ionic radii should differ significantly to facilitate the preferential localization of co-doped ions at different crystal sites of the host structure.
This study demonstrated that the developed ceramic samples, when incubated in a nutrient medium at 37% under CO2 incubator conditions (5% CO2, 37 Cº, 95% humidity), in the liquid containing Ca2+, Sr2+ and Cu2+ ions, promoted the active proliferation of osteoblasts. This finding is interesting and will be further investigated, particularly when evaluating solid surfaces obtained from these powders.
Piezoelectric materials have significant potential for various biomedical applications [48]. In the medical field, these materials can function as smart biomaterials that affect cell interactions and biological processes, providing therapy through mechanical stimuli. Charge carriers produced by piezostimulation can catalyze redox reactions and affect biological activity in decontamination, sterilization and therapy processes [49]. Furthermore, studies have shown that piezoelectric signals may affect collagen chemistry or play a direct role in cellular activity [50,51]. β-TCP-based materials are promising candidates for biological applications due to their excellent biocompatibility, bone formation characteristics and cell adhesion ability, as well as their piezocatalytic properties [52].
To enhance piezoelectric properties, polyvinylidene fluoride (PVDF) coatings [53], composites [54] and scaffolds [55] are often employed. These materials have demonstrated positive effects on osteogenesis and cell proliferation [56] as well as bone treatment [57]. In the Ca9Sr0.5Cu(PO4)7 sample, a weak piezoelectric response was observed. Previously, Mg2+-doped β-TCP phosphates [55,58] were studied. Piezoelectric materials are characterized by crystal structures that lack central symmetry. In the case of hydroxyapatite, polar and noncentrosymmetric structures arise due to ferroelectric ordering of hydroxyl ions (OH) along the crystallographic c axis (direction [001]) [52] The enhancement of piezoelectricity in phosphates can be attributed to several factors, including the crystal structure and symmetry, the presence of certain ions or defects, electric polarization and mechanical stress. Additionally, the alignment and rotation of the phosphate groups (PO4) within the crystal structure can significantly influence the piezoelectric behavior. Another important aspect is the charge distribution within the phosphate groups. The uneven distribution of charge can lead to an electric dipole moment, which is a key factor in piezoelectricity. The piezoelectric properties can also be influenced by the presence of impurities or doped ions in the crystal lattice. Numerous studies have explored the impact of doping on the piezoelectric properties of materials [59]. These impurities can perturb the regular arrangement of atoms and create local regions of polarization, enhancing the overall piezoelectric response.
The incorporation of Sr and Cu ions leads to a certain shift in the electron density in the crystal sites due to the difference in the electronegativity (χ) of Ca2+ (χ = 1.00) host ions and Cu (χ = 1.9). The increase in the difference in the electronegativity value can contribute to the piezoelectric properties. It is important to note that the piezoelectric properties of the material are affected not only by the electronegativity of the substituted atoms but also by their ionic radius. For instance, isovalent substitution of Ca2+ ions (rVI = 1.00 Å) with larger Sr2+ ions (rVI = 1.18 Å) [39] can affect the properties. The absence of second-order phase transitions, combined with chemical pressure, leads to lattice distortion and the emergence of an additional dipole moment.
5. Conclusions
Multifunctional biomimetic co-doped Ca9.5–xSrxCu(PO4)7 solid solutions were synthesized in the powder form by solid-state synthesis. Up to x =4.5 in Ca9.5–xSrxCu(PO4)7, structural saturation by Sr2+ ions was not observed, as confirmed by PXRD studies. A very small amount of pyrophosphate impurity detected by the FT-IR method was found only in the β-Ca3(PO4)2 sample. The Rietveld refinement revealed that Sr2+ ions are distributed among M1–M4 sites, while M5 is fully occupied by Cu2+. The biological study showed a dose-dependent effect on the hMSCs and U-2 OS cell lines. At concentration less than 25 mg/mL, a positive effect on the cells’ proliferation was observed. A significant antibacterial effect was detected against E. coli and S. aureus, which is associated with the synergetic effect of co-doping with Sr2+ and Cu2+ releases into the solution. Solid solutions Ca9.5–xSrxCu(PO4)7 with x ≤ 3 can be used as bone substitutes. The presence of the piezoelectric response contributes to confer biomimetic properties to Ca9.5–xSrxCu(PO4)7 material.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomimetics9040252/s1, Figure S1: The dependence of the unit cell parameters a and c for synthesized solid solutions Ca9.5–xSrxCu(PO4)7; Intensity profiles for the powder X-ray Rietveld refinement of Figure S2: Ca9.5Cu(PO4)7; Figure S3: Ca9Sr0.5Cu(PO4)7; Figure S4: Ca8.5SrCu(PO4)7; Figure S5: Ca8Sr1.5Cu(PO4)7; Figure S6: Ca7.5Sr2Cu(PO4)7; Figure S7: Ca7Sr2.5Cu(PO4)7; Figure S8: Ca6.5Sr3Cu(PO4)7; Figure S9: Ca6Sr3.5Cu(PO4)7; Figure S10: Ca5.5Sr4Cu(PO4)7; Figure S11: Intensity profiles for the powder X-ray Rietveld refinement of Ca5Sr4.5Cu(PO4)7; Table S1: Chemical formula, sample code, unit cell (a, c) parameters and volume (V) in Ca9.5–xSrxCu(PO4)7 0 ≤ x ≤ 4.5 samples; Table S2: Main crystallographic and experimental data on Ca9.5–xSrxCu(PO4)7 (2.5 ≤ x ≤ 4.5); Atomic coordinates, displacement parameters (Å2) and site-occupancy factors (SOFs) in the structure of Table S3: Ca9.5Cu(PO4)7; Table S4: Ca9Sr0.5Cu(PO4)7; Table S5: Ca8.5SrCu(PO4)7; Table S6: Ca8Sr1.5Cu(PO4)7; Table S7: Ca7.5Sr2Cu(PO4)7; Table S8: Ca7Sr2.5Cu(PO4)7; Table S9: Ca6.5Sr3.5Cu(PO4)7; Table S10: Ca6Sr3.5Cu(PO4)7; Table S11: Ca5.5Sr4Cu(PO4)7; Table S12: Ca5Sr4.5Cu(PO4)7.
Author Contributions
Conceptualization, D.V.D. and J.V.R.; methodology, I.V.F., D.V.D., V.N.L., M.I.K., A.O.S. and J.V.R.; validation, I.V.F., D.V.D., V.N.L., M.I.K., A.O.S., A.A.A., M.A.K., F.F.O., O.V.B., V.G.Y. and J.V.R.; formal analysis, I.V.F., D.V.D., V.N.L., M.I.K., A.O.S., A.A.A., M.A.K., F.F.O., O.V.B., V.G.Y. and B.I.L.; investigation, I.V.F., D.V.D., V.N.L., M.I.K., A.O.S., A.A.A., M.A.K., F.F.O., O.V.B., V.G.Y. and B.I.L.; resources, D.V.D. and A.O.S.; data curation, D.V.D., V.N.L., O.V.B., and J.V.R.; writing—original draft preparation, D.V.D. and J.V.R.; writing—review and editing, D.V.D., B.I.L., V.N.L., A.O.S. and J.V.R.; supervision, D.V.D.; funding acquisition, D.V.D., B.I.L. and J.V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the RSF (Project 23-73-10007), https://www.rscf.ru/project/23-73-10007/.
Institutional Review Board Statement
Human fibroblast cells (MRC-5 line) and U-2 OS cell lines were purchased from the State Research Center of Virology and Biotechnology VECTOR (Novosibirsk, Russian). Human MSCs (4–6 passages) were obtained from the culture bank of RICEL—branch of ICG SB RAS, which were extracted from the bone marrow by standard protocol (the study was approved by the Ethics Committee of the RICEL-branch of ICG SBRAS (No 115 from 24 December 2015).
Data Availability Statement
The research data are available upon an official reasonable request.
Acknowledgments
The cytotoxicity assays were supported by the state funding of the RICEL–Branch of IC&G SB RAS.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dickens, B.; Schroeder, L.W.; Brown, W.E. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. The crystal structure of pure β-Ca3(PO4)2. J. Solid State Chem. 1974, 10, 232–248. [Google Scholar] [CrossRef]
- Cheung, H.S.; Haak, M.H. Growth of osteoblasts on porous calcium phosphate ceramic: An in vitro model for biocompatibility study. Biomaterials 1989, 10, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Koerten, H.K.; van der Meulen, J. Degradation of calcium phosphate ceramics. J. Biomed. Mater. Res. 1999, 44, 78–86. [Google Scholar] [CrossRef]
- Davison, N.L.; Harkel, B.T.; Schoenmaker, T.; Luo, X.; Yuan, H.; Everts, V.; Groot, F.B.-D.; de Bruijn, J.D. Osteoclast resorption of beta-tricalcium phosphate controlled by surface architecture. Biomaterials 2014, 35, 7441–7451. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Barbieri, D.; Luo, X.; Weng, J.; Bao, C.; de Bruijn, J.D.; Yuan, H. Variation of the bone forming ability with the physicochemical properties of calcium phosphate bone substitutes. Biomater. Sci. 2018, 6, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Sblendorio, G.A.; Le Gars Santoni, B.; Alexander, D.T.L.; Bowen, P.; Bohner, M.; Döbelin, N. Towards an improved understanding of the β-TCP crystal structure by means of ‘checkerboard’ atomistic simulations. J. Eur. Ceram. Soc. 2023, 43, 3746–3754. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Singh, R.K.; Kannan, S. Synthesis, Structural analysis, Mechanical, antibacterial and Hemolytic activity of Mg2+ and Cu2+ co-substitutions in β-Ca3(PO4)2. Mater. Sci. Eng. C 2014, 45, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Srivastava, M.; Prasad, N.K.; Shetty, P.H.; Kannan, S. Hyperthermia effect and antibacterial efficacy of Fe3+/Co2+ co-substitutions in β-Ca3(PO4)2 for bone cancer and defect therapy. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2018, 106, 1317–1328. [Google Scholar] [CrossRef]
- Matsumoto, N.; Sato, K.; Yoshida, K.; Hashimoto, K.; Toda, Y. Preparation and characterization of β-tricalcium phosphate co-doped with monovalent and divalent antibacterial metal ions. Acta Biomater. 2009, 5, 3157–3164. [Google Scholar] [CrossRef]
- Prosolov, K.; Lastovka, V.; Khimich, M.; Glukhov, I.; Kashin, A.; Luginin, N.; Sharkeev, Y. Influence of Cu Substitution on the Properties of Hydroxyapatite Targets and Deposited Coatings. Coatings 2023, 13, 1410. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Zheng, Y.; Barbaro, K.; Lebedev, V.N.; Aksenov, S.M.; Borovikova, E.Y.; Gafurov, M.R.; Fadeeva, I.V.; Lazoryak, B.I.; Di Giacomo, G.; et al. Dependence of antimicrobial properties on site-selective arrangement and concentration of bioactive Cu2+ ions in tricalcium phosphate. Ceram. Int. 2023, 49, 21308–21323. [Google Scholar] [CrossRef]
- Chou, Y.-J.; Ningsih, H.S.; Shih, S.-J. Preparation, characterization and investigation of antibacterial silver-zinc co-doped β-tricalcium phosphate by spray pyrolysis. Ceram. Int. 2020, 46, 16708–16715. [Google Scholar] [CrossRef]
- Stipniece, L.; Skadins, I.; Mosina, M. Silver- and/or titanium-doped β-tricalcium phosphate bioceramic with antibacterial activity against Staphylococcus aureus. Ceram. Int. 2022, 48, 10195–10201. [Google Scholar] [CrossRef]
- Shokri, M.; Kharaziha, M.; Tafti, H.A.; Eslaminejad, M.B.; Aghdam, R.M. Synergic role of zinc and gallium doping in hydroxyapatite nanoparticles to improve osteogenesis and antibacterial activity. Mater. Sci. Eng. C 2022, 134, 112684. [Google Scholar] [CrossRef] [PubMed]
- Deyneko, D.V.; Fadeeva, I.V.; Borovikova, E.Y.; Dzhevakov, P.B.; Slukin, P.V.; Zheng, Y.; Xia, D.; Lazoryak, B.I.; Rau, J.V. Antimicrobial properties of co-doped tricalcium phosphates Ca3-2x(M′M″)x(PO4)2 (M = Zn2+, Cu2+, Mn2+ and Sr2+). Ceram. Int. 2022, 48, 29770–29781. [Google Scholar] [CrossRef]
- Ranga, N.; Poonia, E.; Jakhar, S.; Sharma, A.K.; Kumar, A.; Devi, S.; Duhan, S. Enhanced antimicrobial properties of bioactive glass using strontium and silver oxide nanocomposites. J. Asian Ceram. Soc. 2019, 7, 75–81. [Google Scholar] [CrossRef]
- Li, Y.; Stone, W.; Schemitsch, E.H.; Zalzal, P.; Papini, M.; Waldman, S.D.; Towler, M.R. Antibacterial and osteo-stimulatory effects of a borate-based glass series doped with strontium ions. J. Biomater. Appl. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Lu, T.; Fang, X.; Qiu, C.; Tian, Y.; Li, Y.; Zuo, F.; Ye, J. Study on MgxSr3-x(PO4)2 bioceramics as potential bone grafts. Colloids Surf. B Biointerfaces 2019, 175, 158–165. [Google Scholar] [CrossRef]
- Ran, L.; Liu, L.; Gao, J.; Pan, Y.; Ramalingam, M.; Du, X.; Liu, Y.; Cheng, L.; Shi, Z. Strontium-doped hydroxyapatite and its role in osteogenesis and angiogenesis. Int. J. Dev. Biol. 2023, 67, 137–146. [Google Scholar] [CrossRef]
- Silva, A.V.; dos Gomes, D.S.; de Victor, R.S.; de Santana, L.N.L.; Neves, G.A.; Menezes, R.R. Influence of Strontium on the Biological Behavior of Bioactive Glasses for Bone Regeneration. Materials 2023, 16, 7654. [Google Scholar] [CrossRef] [PubMed]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Henß, A.; Rohnke, M.; Gelinsky, M. A novel and easy-to-prepare strontium(II) modified calcium phosphate bone cement with enhanced mechanical properties. Acta Biomater. 2013, 9, 7536–7544. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Yang, X.; Diao, J.; Ding, H.; Wu, Y.; Ren, X.; Gao, J.; Ma, M.; Yang, S. 3D printed strontium-doped calcium phosphate ceramic scaffold enhances early angiogenesis and promotes bone repair through the regulation of macrophage polarization. Mater. Today Bio 2023, 23, 100871. [Google Scholar] [CrossRef] [PubMed]
- Dapporto, M.; Tavoni, M.; Restivo, E.; Carella, F.; Bruni, G.; Mercatali, L.; Visai, L.; Tampieri, A.; Iafisco, M.; Sprio, S. Strontium-doped apatitic bone cements with tunable antibacterial and antibiofilm ability. Front. Bioeng. Biotechnol. 2022, 10, 969641. [Google Scholar] [CrossRef]
- Ghezzi, D.; Graziani, G.; Cappelletti, M.; Fadeeva, I.V.; Montesissa, M.; Sassoni, E.; Borciani, G.; Barbaro, K.; Boi, M.; Baldini, N.; et al. New strontium-based coatings show activity against pathogenic bacteria in spine infection. Front. Bioeng. Biotechnol. 2024, 12, 1347811. [Google Scholar] [CrossRef]
- Somers, N.; Jean, F.; Lasgorceix, M.; Curto, H.; Urruth, G.; Thuault, A.; Petit, F.; Leriche, A. Influence of dopants on thermal stability and densification of β-tricalcium phosphate powders. Open Ceram. 2021, 7, 100168. [Google Scholar] [CrossRef]
- Sinusaite, L.; Kareiva, A.; Zarkov, A. Thermally Induced Crystallization and Phase Evolution of Amorphous Calcium Phosphate Substituted with Divalent Cations Having Different Sizes. Cryst. Growth Des. 2021, 21, 1242–1248. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L.; Petrícek, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Fur Krist. 2014, 229, 345–352. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Belik, A.A.; Izumi, F.; Stefanovich, S.Y.; Malakho, A.P.; Lazoryak, B.I.; Leonidov, I.A.; Leonidova, O.N.; Davydov, S.A. Polar and Centrosymmetric Phases in Solid Solutions Ca3-xSrx(PO4)2 (0 ≤ x ≤ 16/7). Chem. Mater. 2002, 14, 3197–3205. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Ríos, S.; González, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jin, S.; Qi, X.; Chen, X.; Zhang, W.; Yang, K.; Zhong, H. Osteogenesis stimulation by copper-containing 316L stainless steel via activation of akt cell signaling pathway and Runx2 upregulation. J. Mater. Sci. Technol. 2019, 35, 2727–2733. [Google Scholar] [CrossRef]
- Yang, F.; Yang, D.; Tu, J.; Zheng, Q.; Cai, L.; Wang, L. Strontium Enhances Osteogenic Differentiation of Mesenchymal Stem Cells and In Vivo Bone Formation by Activating Wnt/Catenin Signaling. Stem Cells 2011, 29, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Khalilimeybodi, A.; Fraley, S.I.; Rangamani, P. Mechanisms underlying divergent relationships between Ca2+ and YAP/TAZ signalling. J. Physiol. 2023, 601, 483–515. [Google Scholar] [CrossRef] [PubMed]
- Agell, N.; Bachs, O.; Rocamora, N.; Villalonga, P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and Calmodulin. Cell. Signal. 2002, 14, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.N.; Boovarasan, M.; Singh, R.K.; Kannan, S. Synthesis, structural analysis and fabrication of coatings of the Cu2+ and Sr2+ co-substitutions in β-Ca3(PO4)2. RSC Adv. 2013, 3, 22469–22479. [Google Scholar] [CrossRef]
- Reddy, B.J.; Frost, R.L.; Palmer, S.J. A near-infrared spectroscopic study of the phosphate mineral pyromorphite Pb5(PO4)3Cl. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 430–435. [Google Scholar] [CrossRef][Green Version]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Tofail, S.A.M.; Haverty, D.; Cox, F.; Erhart, J.; Hána, P.; Ryzhenko, V. Direct and ultrasonic measurements of macroscopic piezoelectricity in sintered hydroxyapatite. J. Appl. Phys. 2009, 105, 064103. [Google Scholar] [CrossRef]
- Gandhi, A.A.; Wojtas, M.; Lang, S.B.; Kholkin, A.L.; Tofail, S.A.M. Piezoelectricity in Poled Hydroxyapatite Ceramics. J. Am. Ceram. Soc. 2014, 97, 2867–2872. [Google Scholar] [CrossRef]
- Kalinin, S.V.; Rodriguez, B.; Shin, J.; Jesse, S.; Grichko, V.; Thundat, T.; Baddorf, A.; Gruverman, A. Bioelectromechanical imaging by scanning probe microscopy: Galvani’s experiment at the nanoscale. Ultramicroscopy 2006, 106, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, S.V.; Rodriguez, B.J.; Jesse, S.; Thundat, T.; Gruverman, A. Electromechanical imaging of biological systems with sub-10nm resolution. Appl. Phys. Lett. 2005, 87, 053901. [Google Scholar] [CrossRef]
- Tissot, R.G.; Rodriguez, M.A.; Sipola, D.L.; Voigt, J.A. X-ray powder diffraction study of synthetic Palmierite, K2Pb(SO4)2. Powder Diffr. 2001, 16, 92–97. [Google Scholar] [CrossRef]
- Stefanovich, S.Y.; Petrova, D.A.; Morozov, V.A.; Fortalnova, E.A.; Belov, D.A.; Deyneko, D.V.; Barishnikova, O.V.; Belik, A.A.; Lazoryak, B.I. Enhanced nonlinear optical activity and Ca2+-conductivity in Ca10.5-xPbx(VO4)7 ferroelectrics. J. Alloys Compd. 2018, 735, 1826–1837. [Google Scholar] [CrossRef]
- Li, J.; Zhao, C.; Liu, C.; Wang, Z.; Ling, Z.; Lin, B.; Tan, B.; Zhou, L.; Chen, Y.; Liu, D.; et al. Cobalt-doped bioceramic scaffolds fabricated by 3D printing show enhanced osteogenic and angiogenic properties for bone repair. Biomed. Eng. Online 2021, 20, 1–24. [Google Scholar] [CrossRef]
- Bača, Ľ.; Sivčáková, T.; Nováková, Z.V.; Matejdes, M.; Orlovská, M.H.; Thurzo, A.; Danišovič, Ľ.; Janek, M. Synthesis, sintering, radiopacity and cytotoxicity of Ca, Sr and Ba—Phosphate bioceramics. J. Eur. Ceram. Soc. 2024, 44, 5298–5307. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, P.; Mao, L.; Wu, W.; Lin, H.; Xu, D.; Lu, X.; Shi, J. Piezocatalytic Medicine: An Emerging Frontier using Piezoelectric Materials for Biomedical Applications. Adv. Mater. 2023, 35, e2208256. [Google Scholar] [CrossRef]
- Lam, P.-L.; Wong, R.S.-M.; Lam, K.-H.; Hung, L.-K.; Wong, M.-M.; Yung, L.-H.; Ho, Y.-W.; Wong, W.-Y.; Hau, D.K.-P.; Gambari, R.; et al. The role of reactive oxygen species in the biological activity of antimicrobial agents: An updated mini review. Chem. Biol. Interact. 2020, 320, 109023. [Google Scholar] [CrossRef]
- Bassett, C.A.L. Biologic significance of piezoelectricity. Calcif. Tissue Res. 1967, 1, 252–272. [Google Scholar] [CrossRef]
- Marino, A.A.; Becker, R.O. Piezoelectric Effect and Growth Control in Bone. Nature 1970, 228, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.B.; Tofail, S.A.M.; Kholkin, A.L.; Wojtaś, M.; Gregor, M.; Gandhi, A.A.; Wang, Y.; Bauer, S.; Krause, M.; Plecenik, A. Ferroelectric Polarization in Nanocrystalline Hydroxyapatite Thin Films on Silicon. Sci. Rep. 2013, 3, 2215. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Suryani, L.; Zhou, X.; Muthukumaran, P.; Rakshit, M.; Yang, F.; Wen, F.; Hassanbhai, A.M.; Parida, K.; Simon, D.T.; et al. Synergistic Effect of PVDF-Coated PCL-TCP Scaffolds and Pulsed Electromagnetic Field on Osteogenesis. Int. J. Mol. Sci. 2021, 22, 6438. [Google Scholar] [CrossRef] [PubMed]
- Khazani, Y.; Rafiee, E.; Samadi, A.; Mahmoodi, M. Alginate-PVDF piezoelectric hydrogel containing calcium copper titanate- hydroxyapatite as a self-powered scaffold for bone tissue engineering and energy harvesting. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 687, 133537. [Google Scholar] [CrossRef]
- Wang, L.; Pang, Y.; Tang, Y.; Wang, X.; Zhang, D.; Zhang, X.; Yu, Y.; Yang, X.; Cai, Q. A biomimetic piezoelectric scaffold with sustained Mg2+ release promotes neurogenic and angiogenic differentiation for enhanced bone regeneration. Bioact. Mater. 2022, 25, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.H.; van der Jagt, O.P.; Punt, B.J.; Verhaar, J.A.; van Leeuwen, J.P.; Weinans, H.; Jahr, H. Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: An in vitro study. BMC Musculoskelet. Disord. 2010, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wan, X.; Sui, B.; Hu, Q.; Liu, Z.; Ding, T.; Zhao, J.; Chen, Y.; Wang, Z.L.; Li, L. Piezoelectric hydrogel for treatment of periodontitis through bioenergetic activation. Bioact. Mater. 2024, 35, 346–361. [Google Scholar] [CrossRef]
- Kaliannagounder, V.K.; Raj, N.P.M.J.; Unnithan, A.R.; Park, J.; Park, S.S.; Kim, S.-J.; Park, C.H.; Kim, C.S.; Sasikala, A.R.K. Remotely controlled self-powering electrical stimulators for osteogenic differentiation using bone inspired bioactive piezoelectric whitlockite nanoparticles. Nano Energy 2021, 85, 105901. [Google Scholar] [CrossRef]
- Bhoi, B.; Purohit, P. A Study on Effect of Doping on Piezoelectric Materials. In Proceedings of the 2022 International Interdisciplinary Conference on Mathematics, Engineering and Science (MESIICON), Durgapur, India, 11–12 November 2022; pp. 1–6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).