The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives
Abstract
1. Introduction
2. History of Bioceramics
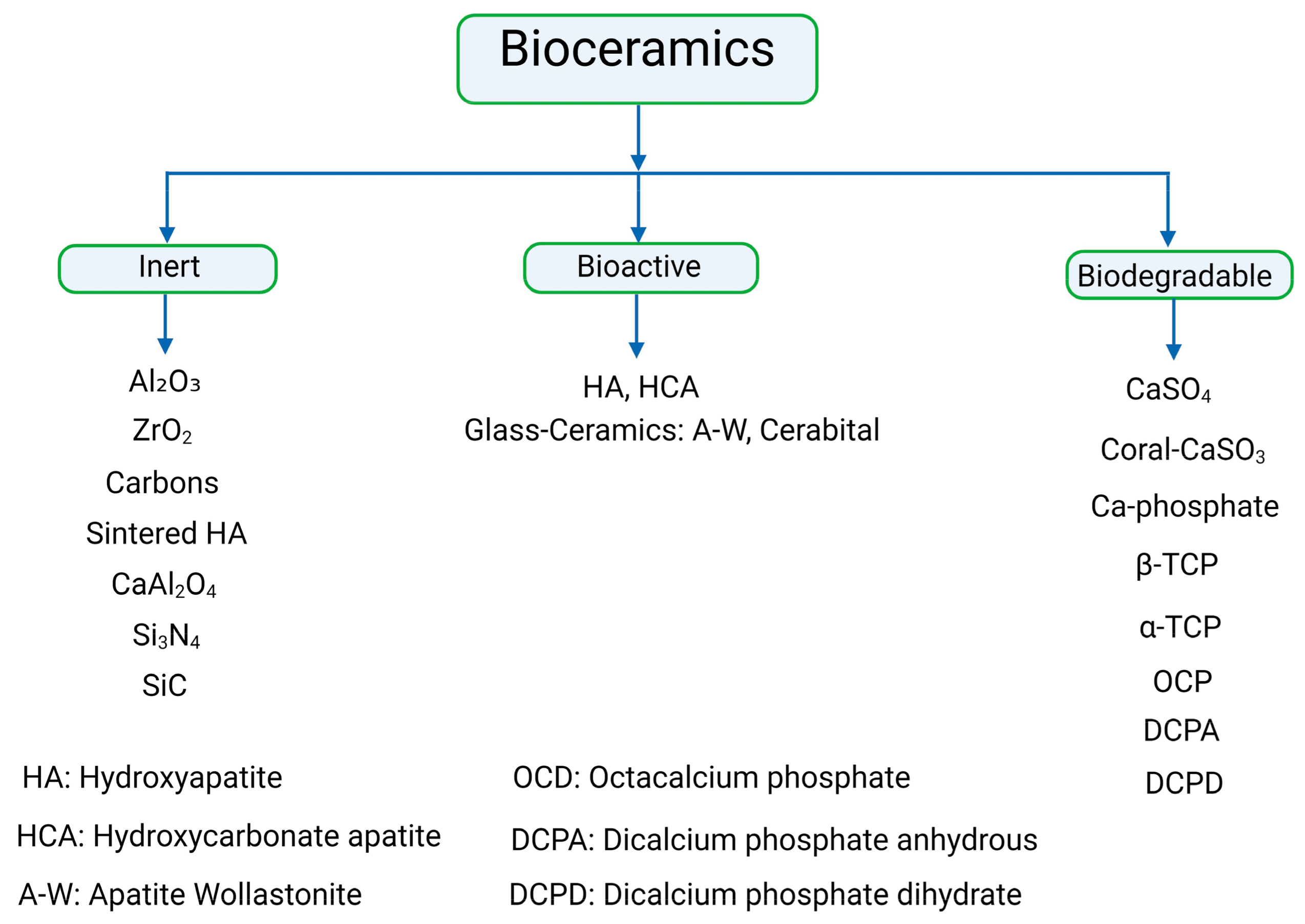
3. Classification of Bioceramics
4. Relationship of Different Types of Bioceramics
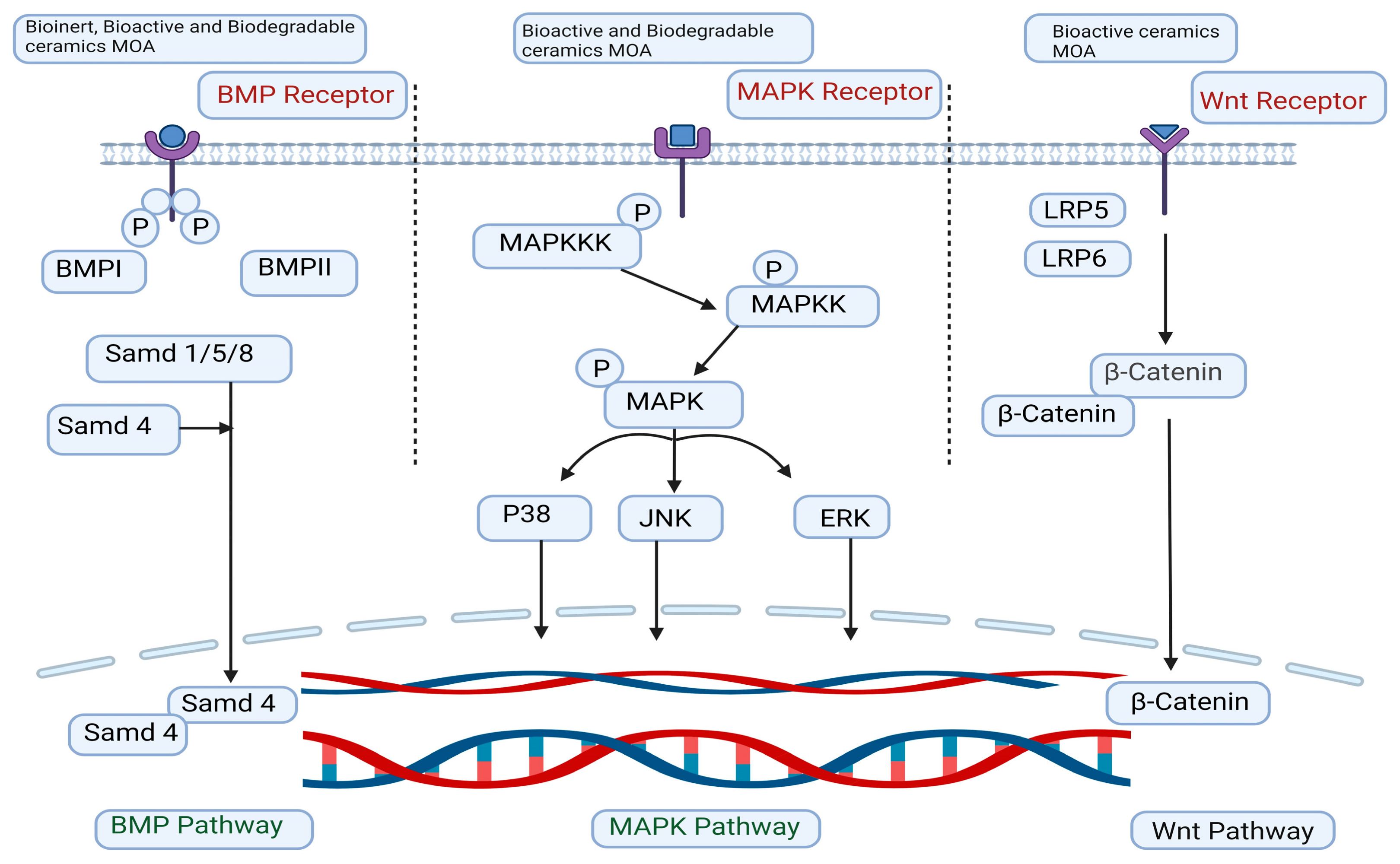
5. Mechanism of Action
5.1. The Signaling Pathway of MAPK
5.2. The Signaling Pathway of BMP
5.3. The Signaling Pathway of Wingless/Integrated (Wnt)/β-Catenin
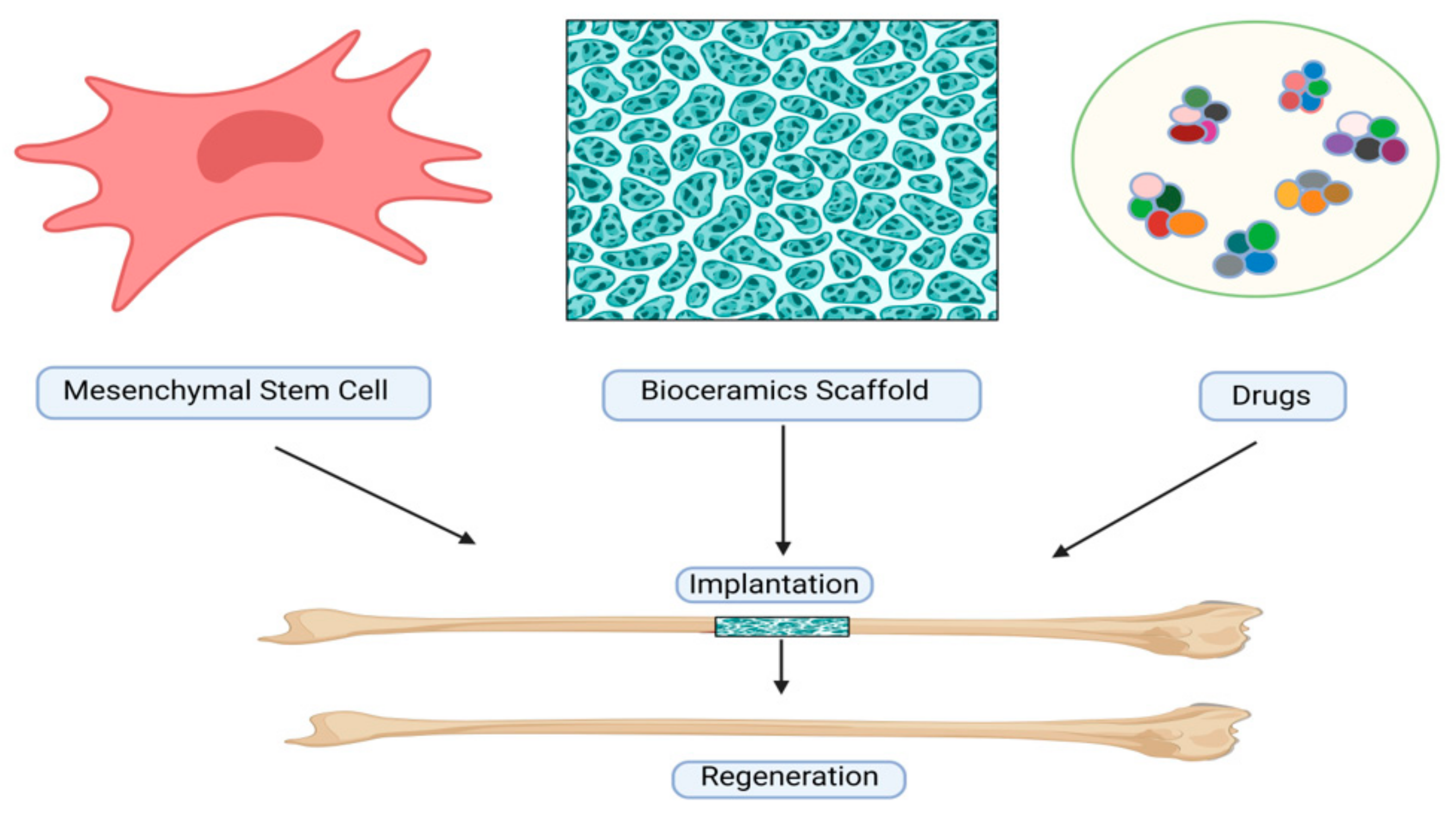
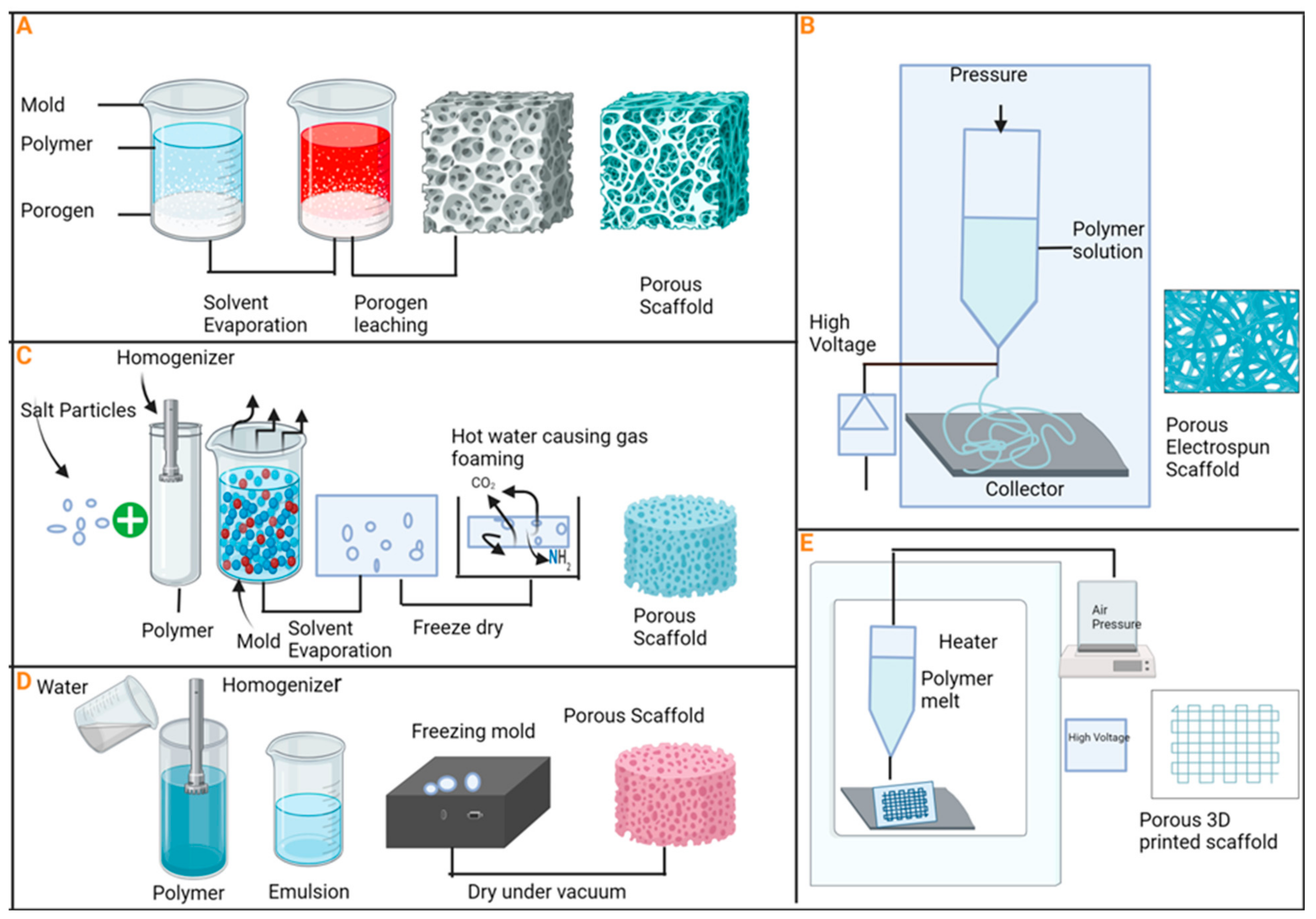
6. Uses of Porous Bioceramics
7. Application of Adipose-Derived MSCs
7.1. Application of Bone Marrow-Derived MSCs
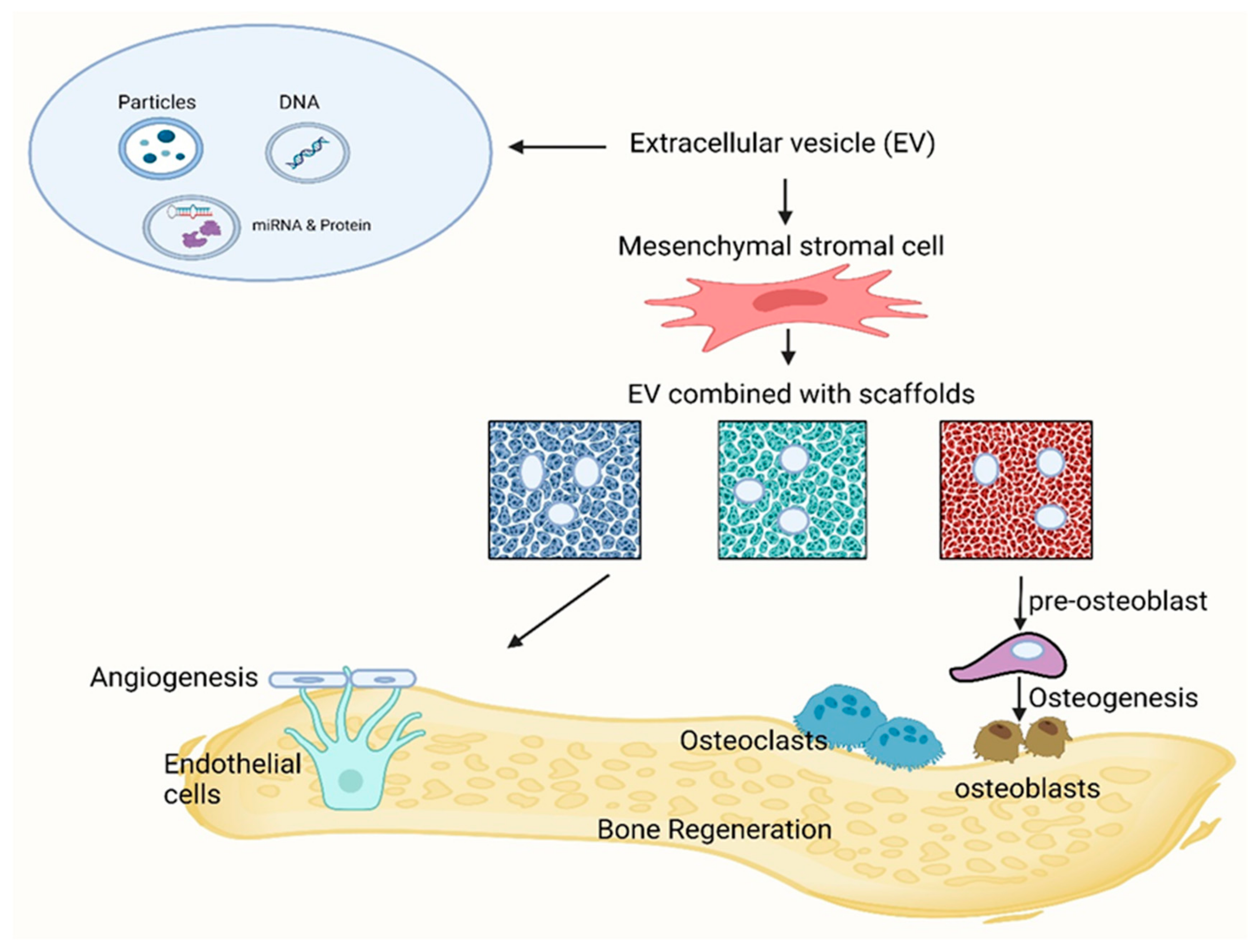
7.2. Application of Extracellular Vesicle-Derived MSCs
8. Impact of Environmental Factors on Bioceramics
9. Drug Delivery
10. Limitations and Future Potential Development of Bioceramics
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef] [PubMed]
- Downey, P.A.; Siegel, M.I. Bone biology and the clinical implications for osteoporosis. Phys. Ther. 2006, 86, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and Molecular Regulation of Bone Remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93 Pt B, 1354–1365. [Google Scholar] [CrossRef]
- Khaleque, M.A.; Kim, J.-H.; Lee, H.-H.; Kim, G.-H.; You, W.-Y.; Lee, W.-J.; Kim, Y.-Y. Comparative Analysis of Autophagy and Apoptosis in Disc Degeneration: Understanding the Dynamics of Temporary-Compression-Induced Early Autophagy and Sustained-Compression-Triggered Apoptosis. Int. J. Mol. Sci. 2024, 25, 2352. [Google Scholar] [CrossRef] [PubMed]
- Fairag, R.; Rosenzweig, D.H.; Ramirez-Garcialuna, J.L.; Weber, M.H.; Haglund, L. Three-Dimensional Printed Polylactic Acid Scaffolds Promote Bone-like Matrix Deposition in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 15306–15315. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Kong, Y.; Yu, L.; Li, Y.; Gao, C.; Peng, S.; Pan, H.; Zhao, Z.; Shuai, C. Molybdenum disulfide nanosheets embedded with nanodiamond particles: Co-dispersion nanostructures as reinforcements for polymer scaffolds. Appl. Mater. Today 2019, 17, 216–226. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Feroz, S.; Cathro, P.; Ivanovski, S.; Muhammad, N. Biomimetic bone grafts and substitutes: A review of recent advancements and applications. Biomed. Eng. Adv. 2023, 6, 100107. [Google Scholar] [CrossRef]
- The Committee on Biological Implants; Greenwald, A.S.; Boden, S.D.; Goldberg, V.M.; Khan, Y.; Laurencin, C.T.; Rosier, R.N. Bone-Graft Substitutes: Facts, Fictions, and Applications. JBJS J. Bone Jt. Surg. 2001, 83, S98–S103. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Garcia-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Peng, B.; Feng, P.; Yu, L.; Lai, R.; Min, A. In situ synthesis of hydroxyapatite nanorods on graphene oxide nanosheets and their reinforcement in biopolymer scaffold. J. Adv. Res. 2022, 35, 13–24. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, G.; Yang, Y.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. A strawberry-like Ag-decorated barium titanate enhances piezoelectric and antibacterial activities of polymer scaffold. Nano Energy 2020, 74, 104825. [Google Scholar] [CrossRef]
- Bayazit, V.; Bayazit, M.; Bayazit, E. Evaluation of bioceramic materials in biology and medicine. Dig. J. Nanomater. Biostruct. 2010, 7, 211–222. [Google Scholar]
- Bavya Devi, K.; Nandi, S.K.; Roy, M. Magnesium Silicate Bioceramics for Bone Regeneration: A Review. J. Indian Inst. Sci. 2019, 99, 261–288. [Google Scholar] [CrossRef]
- Ribas, R.G.; Schatkoski, V.M.; Montanheiro, T.L.d.A.; de Menezes, B.R.C.; Stegemann, C.; Leite, D.M.G.; Thim, G.P. Current advances in bone tissue engineering concerning ceramic and bioglass scaffolds: A review. Ceram. Int. 2019, 45, 21051–21061. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regí, M. Bioactive ceramics: From bone grafts to tissue engineering. RSC Adv. 2013, 3, 11116–11131. [Google Scholar] [CrossRef]
- Pina, S.; Rebelo, R.; Correlo, V.M.; Oliveira, J.M.; Reis, R.L. Bioceramics for Osteochondral Tissue Engineering and Regeneration. Adv. Exp. Med. Biol. 2018, 1058, 53–75. [Google Scholar] [CrossRef]
- Yeong, W.Y.; Chua, C.K.; Leong, K.F.; Chandrasekaran, M.; Lee, M.W. Comparison of drying methods in the fabrication of collagen scaffold via indirect rapid prototyping. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 260–266. [Google Scholar] [CrossRef]
- Marolt Presen, D.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal Stromal Cell-Based Bone Regeneration Therapies: From Cell Transplantation and Tissue Engineering to Therapeutic Secretomes and Extracellular Vesicles. Front. Bioeng. Biotechnol. 2019, 7, 352. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Edwards, E.R.; de Steiger, R.; Gabbe, B.J. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int. J. Environ. Res. Public Health 2018, 15, 2845. [Google Scholar] [CrossRef]
- Robey, P.G.; Kuznetsov, S.A.; Ren, J.; Klein, H.G.; Sabatino, M.; Stroncek, D.F. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone 2015, 70, 87–92. [Google Scholar] [CrossRef]
- Jakob, M.; Saxer, F.; Scotti, C.; Schreiner, S.; Studer, P.; Scherberich, A.; Heberer, M.; Martin, I. Perspective on the evolution of cell-based bone tissue engineering strategies. Eur. Surg. Res. 2012, 49, 1–7. [Google Scholar] [CrossRef]
- Tahara, Y.; Ishii, Y. Apatite cement containing cis-diamminedichloroplatinum implanted in rabbit femur for sustained release of the anticancer drug and bone formation. J. Orthop. Sci. 2001, 6, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Nishiguchi, S.; Neo, M.; Tamura, J.; Kawanabe, K.; Nakamura, T. Bone Bonding in Bioactive Glass Ceramics Combined with a New Synthesized Agent TAK-778. Key Eng. Mater. 2000, 192–195, 417–420. [Google Scholar] [CrossRef]
- de Groot, K. Ceramics of calcium phosphates: Preparation and properties. In Bioceramics Calcium Phosphate; CRC Press: Boca Raton, FL, USA, 2018; pp. 99–114. [Google Scholar]
- Vallet-Regi, M.; Ruiz-Hernandez, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef]
- Castner, D.; Ratner, B. Biomedical surface science: Foundations to frontiers. Surf. Sci. 2002, 500, 28–60. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Vallet-Regi, M. Revisiting ceramics for medical applications. Dalton Trans. 2006, 5211–5220. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Kamitakahara, M.; Kawashita, M.; Miyata, N.; Kokubo, T.; Nakamura, T. Bioactivity and Mechanical Properties of Polydimethylsiloxane (PDMS)-CaO-SiO2 Hybrids with Different PDMS Contents. J. Sol-Gel Sci. Technol. 2001, 21, 75–81. [Google Scholar] [CrossRef]
- Vallet-Regi, M. Nanostructured mesoporous silica matrices in nanomedicine. J. Intern. Med. 2010, 267, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Salinas, A.J. Sol–Gel Silica-Based Biomaterials and Bone Tissue Regeneration. In Handbook of Sol-Gel Science and Technology; Springer: Cham, Switzerland, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Tsuru, K.; Aburatani, Y.; Yabuta, T.; Hayakawa, S.; Ohtsuki, C.; Osaka, A. Synthesis and In Vitro Behavior of Organically Modified Silicate Containing Ca Ions. J. Sol-Gel Sci. Technol. 2001, 21, 89–96. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium Phosphate Ceramics as Hard Tissue Prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Damia, C.; Marchat, D.; Lemoine, C.; Douard, N.; Chaleix, V.; Sol, V.; Larochette, N.; Logeart-Avramoglou, D.; Brie, J.; Champion, E. Functionalization of phosphocalcic bioceramics for bone repair applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Heness, G.; Ben-Nissan, B. Innovative bioceramics. Mater. Forum 2004, 27, 104–114. [Google Scholar]
- Heimann, R.B. Structure, properties, and biomedical performance of osteoconductive bioceramic coatings. Surf. Coat. Technol. 2013, 233, 27–38. [Google Scholar] [CrossRef]
- Olofsson, J.; Pettersson, M.; Teuscher, N.; Heilmann, A.; Larsson, K.; Grandfield, K.; Persson, C.; Jacobson, S.; Engqvist, H. Fabrication and evaluation of SixNy coatings for total joint replacements. J. Mater. Sci. Mater. Med. 2012, 23, 1879–1889. [Google Scholar] [CrossRef]
- Mazzocchi, M.; Bellosi, A. On the possibility of silicon nitride as a ceramic for structural orthopaedic implants. Part I: Processing, microstructure, mechanical properties, cytotoxicity. J. Mater. Sci. Mater. Med. 2008, 19, 2881–2887. [Google Scholar] [CrossRef]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Lobel, K.; Hench, L. In vitro adsorption and activity of enzymes on reaction layers of bioactive glass substrates. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. 1998, 39, 575–579. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Evolution of bioceramics within the field of biomaterials. Comptes Rendus Chim. 2010, 13, 174–185. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J. Ceramics as bone repair materials. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–178. [Google Scholar]
- Dorozhkin, S. Current state of bioceramics. J. Ceram. Sci. Technol. 2018, 9, 353–370. [Google Scholar]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef]
- Voisin, L.; Saba-El-Leil, M.K.; Julien, C.; Fremin, C.; Meloche, S. Genetic demonstration of a redundant role of extracellular signal-regulated kinase 1 (ERK1) and ERK2 mitogen-activated protein kinases in promoting fibroblast proliferation. Mol. Cell. Biol. 2010, 30, 2918–2932. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Li, M.; He, P.; Wu, Y.; Zhang, Y.; Xia, H.; Zheng, Y.; Han, Y. Stimulatory effects of the degradation products from Mg-Ca-Sr alloy on the osteogenesis through regulating ERK signaling pathway. Sci. Rep. 2016, 6, 32323. [Google Scholar] [CrossRef]
- Lin, S.; Yang, G.; Jiang, F.; Zhou, M.; Yin, S.; Tang, Y.; Tang, T.; Zhang, Z.; Zhang, W.; Jiang, X. A Magnesium-Enriched 3D Culture System that Mimics the Bone Development Microenvironment for Vascularized Bone Regeneration. Adv. Sci. 2019, 6, 1900209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Liu, C.; Tan, W.; Tang, M.; Zhou, X.; Sun, T.; Deng, Y. Mg(2+) in beta-TCP/Mg-Zn composite enhances the differentiation of human bone marrow stromal cells into osteoblasts through MAPK-regulated Runx2/Osx. J. Cell. Physiol. 2020, 235, 5182–5191. [Google Scholar] [CrossRef]
- Wu, B.C.; Kao, C.T.; Huang, T.H.; Hung, C.J.; Shie, M.Y.; Chung, H.Y. Effect of verapamil, a calcium channel blocker, on the odontogenic activity of human dental pulp cells cultured with silicate-based materials. J. Endod. 2014, 40, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Hung, C.J.; Huang, T.H.; Lin, C.C.; Kao, C.T.; Shie, M.Y. Odontogenic differentiation of human dental pulp cells by calcium silicate materials stimulating via FGFR/ERK signaling pathway. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, E.; Rajasekharan, S.; Chitturi, R.T.; Declercq, H.; Martens, L.; De Coster, P. Gene Expression Profiling and Molecular Signaling of Various Cells in Response to Tricalcium Silicate Cements: A Systematic Review. J. Endod. 2016, 42, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Srinath, P.; Abdul Azeem, P.; Venugopal Reddy, K. Review on calcium silicate-based bioceramics in bone tissue engineering. Int. J. Appl. Ceram. Technol. 2020, 17, 2450–2464. [Google Scholar] [CrossRef]
- Lai, S.; Chen, L.; Cao, W.; Cui, S.; Li, X.; Zhong, W.; Ma, M.; Zhang, Q. Dicalcium Silicate Induced Proinflammatory Responses through TLR2-Mediated NF-kappaB and JNK Pathways in the Murine RAW 264.7 Macrophage Cell Line. Mediat. Inflamm. 2018, 2018, 8167932. [Google Scholar] [CrossRef]
- Luther, G.; Wagner, E.; Zhu, G.; Kang, Q.; Luo, Q.; Lamplot, J.; Bi, Y.; Luo, X.; Luo, J.; Teven, C.; et al. BMP-9 Induced Osteogenic Differentiation of Mesenchymal Stem Cells: Molecular Mechanism and Therapeutic Potential. Curr. Gene Ther. 2011, 11, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.; Tahmasebi Birgani, Z.; Habibovic, P. Biomaterial-induced pathway modulation for bone regeneration. Biomaterials 2022, 283, 121431. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cell Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Wu, J.; Feng, C.; Wang, M.; Wu, H.; Zhu, X.; Li, X.; Chen, X.; Zhang, X. Whisker of biphasic calcium phosphate ceramics: Osteo-immunomodulatory behaviors. Nano Res. 2022, 15, 9169–9182. [Google Scholar] [CrossRef]
- Han, F.; Li, T.; Li, M.; Zhang, B.; Wang, Y.; Zhu, Y.; Wu, C. Nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing. Bioact. Mater. 2023, 20, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Nethala, S.; Tripathi, A.; Saravanan, S.; Moorthi, A.; Selvamurugan, N. Chitosan scaffolds containing silicon dioxide and zirconia nano particles for bone tissue engineering. Int. J. Biol. Macromol. 2011, 49, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Roohani-Esfahani, S.-I.; Lu, Z.; Zreiqat, H.; Dunstan, C.R. Zirconium ions up-regulate the BMP/SMAD signaling pathway and promote the proliferation and differentiation of human osteoblasts. PLoS ONE 2015, 10, e0113426. [Google Scholar] [CrossRef] [PubMed]
- Janda, C.Y.; Waghray, D.; Levin, A.M.; Thomas, C.; Garcia, K.C. Structural basis of Wnt recognition by Frizzled. Science 2012, 337, 59–64. [Google Scholar] [CrossRef]
- Mao, L.; Liu, J.; Zhao, J.; Chang, J.; Xia, L.; Jiang, L.; Wang, X.; Lin, K.; Fang, B. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int. J. Nanomed. 2015, 10, 7031–7044. [Google Scholar] [CrossRef]
- Ruolan, W.; Liangjiao, C.; Longquan, S. The mTOR/ULK1 signaling pathway mediates the autophagy-promoting and osteogenic effects of dicalcium silicate nanoparticles. J. Nanobiotechnol. 2020, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, Y.; Wang, J.; Huang, C.; Wang, Y.; Yang, H.; Liu, W.; Wang, T.; Wang, D.; Wang, G.; et al. Strontium modulates the osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating the Wnt/beta-catenin signaling pathway. Bioact. Mater. 2020, 5, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, L.; Zhang, Q.; Wang, Q.; Wang, X.; Yan, G. Mechanism and application of 3D-printed degradable bioceramic scaffolds for bone repair. Biomater. Sci. 2023, 11, 7034–7050. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, A.; Barradas, A.; de Boer, J.; Moroni, L.; van Blitterswijk, C.; Habibovic, P. Combining technologies to create bioactive hybrid scaffolds for bone tissue engineering. Biomatter 2013, 3, e23705. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, M.; Sharrock, P.; Fiallo, M.; ElFeki, H. Hydroxyapatite bioceramic with large porosity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 985–990. [Google Scholar] [CrossRef]
- De Boer, J.; van Blitterswijk, C.; Thomsen, P.; Hubbell, J.; Cancedda, R.; De Bruijn, J.; Lindahl, A.; Sohier, J.; Williams, D.F. Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Hegde, C.; Shetty, V.; Wasnik, S.; Ahammed, I.; Shetty, V. Use of bone graft substitute in the treatment for distal radius fractures in elderly. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.R.; Bhagat, S.B.; Shukla, D.D. Bovine cancellous xenograft in the treatment of tibial plateau fractures in elderly patients. Int. Orthop. 2009, 33, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R.; Hench, L.L. Factors affecting the structure and properties of bioactive foam scaffolds for tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 68, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Asadollahi, M.A.; Nasri-Nasrabadi, B.; Ghaedi, K.; Salehi, H.; Dolatshahi-Pirouz, A.; Arpanaei, A. Incorporation of mesoporous silica nanoparticles into random electrospun PLGA and PLGA/gelatin nanofibrous scaffolds enhances mechanical and cell proliferation properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 25–32. [Google Scholar] [CrossRef]
- Bodenberger, N.; Kubiczek, D.; Abrosimova, I.; Scharm, A.; Kipper, F.; Walther, P.; Rosenau, F. Evaluation of methods for pore generation and their influence on physio-chemical properties of a protein-based hydrogel. Biotechnol. Rep. 2016, 12, 6–12. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kamioka, H.; Honjo, T.; Tezuka, K.-i.; Takano-Yamamoto, T. Three-dimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone 2005, 36, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Vehof, J.W.; Dean, D.; van der Waerden, J.P.; Holland, T.A.; Mikos, A.G.; Jansen, J.A. Soft and hard tissue response to photo crosslinked poly(propylene fumarate) scaffolds in a rabbit model. J. Biomed. Mater. Res. 2002, 59, 547–556. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Xu, H.; Hu, K.; Sun, J.; Liu, M.; Zhang, F.; Gu, N. Pre-vascularization in fibrin Gel/PLGA microsphere scaffolds designed for bone regeneration. NPG Asia Mater. 2018, 10, 827–839. [Google Scholar] [CrossRef]
- Cheng, M.Q.; Wahafu, T.; Jiang, G.F.; Liu, W.; Qiao, Y.Q.; Peng, X.C.; Cheng, T.; Zhang, X.L.; He, G.; Liu, X.Y. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef]
- Shrestha, S.; Yeon Lee, S.; Shrestha, D.; Kandel, R.; Yoo, Y.-J.; Tae, H.-J.; Kumar Shrestha, B.; Hee Park, C.; Sang Kim, C. Micro/nanometer-sized porous structure of zinc phosphate incorporated Ti(HPO4)2 hydrate bioceramic induces osteogenic gene expression and enhances osteoporotic bone regeneration. Chem. Eng. J. 2022, 450, 138360. [Google Scholar] [CrossRef]
- Cao, H.; Kuboyama, N. A biodegradable porous composite scaffold of PGA/beta-TCP for bone tissue engineering. Bone 2010, 46, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Ren, J.; Chen, C.; Ren, T.B.; Zhou, X.Y. Preparation and properties of poly(lactide-co-glycolide) (PLGA)/nano-hydroxyapatite (NHA) scaffolds by thermally induced phase separation and rabbit MSCs culture on scaffolds. J. Biomater. Appl. 2008, 22, 409–432. [Google Scholar] [CrossRef]
- Lee, U.L.; Lim, J.Y.; Park, S.N.; Choi, B.H.; Kang, H.; Choi, W.C. A Clinical Trial to Evaluate the Efficacy and Safety of 3D Printed Bioceramic Implants for the Reconstruction of Zygomatic Bone Defects. Materials 2020, 13, 4515. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.L.; Gaspar, V.M.; Serra, I.R.; Diogo, G.S.; Fradique, R.; Silva, A.P.; Correia, I.J. Bioactive polymeric-ceramic hybrid 3D scaffold for application in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4460–4469. [Google Scholar] [CrossRef]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; et al. Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. Acta Biomater. 2011, 7, 2327–2336. [Google Scholar] [CrossRef]
- Barbanti Brodano, G.; Mazzoni, E.; Tognon, M.; Griffoni, C.; Manfrini, M. Human mesenchymal stem cells and biomaterials interaction: A promising synergy to improve spine fusion. Eur. Spine J. 2012, 21 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, D. Adipose-Derived Mesenchymal Stem Cells: A New Tool for the Treatment of Renal Fibrosis. Stem Cells Dev. 2018, 27, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.; Talovic, M.; Patel, K.; Patel, A.; Marcinczyk, M.; Garg, K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J. Orthop. Res. 2019, 37, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, J.; Chen, H.; Shi, X.; Wang, X.; Zhu, Y.; Tan, Z. The topography of fibrous scaffolds modulates the paracrine function of Ad-MSCs in the regeneration of skin tissues. Biomater. Sci. 2019, 7, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.M.; Mozdzen, L.C.; Harley, B.A.; Bailey, R.C. The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells. Biomaterials 2014, 35, 8951–8959. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: Comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Izquierdo, M.; Cabeza, L.; Lainez-Ramos-Bossini, A.; Quesada, R.; Perazzoli, G.; Alvarez, P.; Prados, J.; Melguizo, C. An updated review of adipose derived-mesenchymal stem cells and their applications in musculoskeletal disorders. Expert Opin. Biol. Ther. 2019, 19, 233–248. [Google Scholar] [CrossRef]
- McCullen, S.D.; Zhu, Y.; Bernacki, S.H.; Narayan, R.J.; Pourdeyhimi, B.; Gorga, R.E.; Loboa, E.G. Electrospun composite poly(L-lactic acid)/tricalcium phosphate scaffolds induce proliferation and osteogenic differentiation of human adipose-derived stem cells. Biomed. Mater. 2009, 4, 035002. [Google Scholar] [CrossRef]
- Sanchooli, T.; Norouzian, M.; Ardeshirylajimi, A.; Ghoreishi, S.K.; Abdollahifar, M.A.; Nazarian, H.; Piryaei, A. Adipose Derived Stem Cells Conditioned Media in Combination with Bioceramic-Collagen Scaffolds Improved Calvarial Bone Healing in Hypothyroid Rats. Iran. Red Crescent Med. J. 2017, 19, e45516. [Google Scholar] [CrossRef]
- Xia, L.; Lin, K.; Jiang, X.; Fang, B.; Xu, Y.; Liu, J.; Zeng, D.; Zhang, M.; Zhang, X.; Chang, J.; et al. Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose-derived stem cells. Biomaterials 2014, 35, 8514–8527. [Google Scholar] [CrossRef] [PubMed]
- Daei-Farshbaf, N.; Ardeshirylajimi, A.; Seyedjafari, E.; Piryaei, A.; Fadaei Fathabady, F.; Hedayati, M.; Salehi, M.; Soleimani, M.; Nazarian, H.; Moradi, S.L.; et al. Bioceramic-collagen scaffolds loaded with human adipose-tissue derived stem cells for bone tissue engineering. Mol. Biol. Rep. 2014, 41, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Sandor, G.K.; Numminen, J.; Wolff, J.; Thesleff, T.; Miettinen, A.; Tuovinen, V.J.; Mannerstrom, B.; Patrikoski, M.; Seppanen, R.; Miettinen, S.; et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl. Med. 2014, 3, 530–540. [Google Scholar] [CrossRef]
- Prins, H.J.; Schulten, E.A.; Ten Bruggenkate, C.M.; Klein-Nulend, J.; Helder, M.N. Bone Regeneration Using the Freshly Isolated Autologous Stromal Vascular Fraction of Adipose Tissue in Combination With Calcium Phosphate Ceramics. Stem Cells Transl. Med. 2016, 5, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef] [PubMed]
- Requicha, J.F.; Viegas, C.A.; Albuquerque, C.M.; Azevedo, J.M.; Reis, R.L.; Gomes, M.E. Effect of anatomical origin and cell passage number on the stemness and osteogenic differentiation potential of canine adipose-derived stem cells. Stem Cell Rev. Rep. 2012, 8, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Meza-Zepeda, L.A.; Noer, A.; Dahl, J.A.; Micci, F.; Myklebost, O.; Collas, P. High-resolution analysis of genetic stability of human adipose tissue stem cells cultured to senescence. J. Cell. Mol. Med. 2008, 12, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.T.; Chen, C.T. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J. Stem Cells 2014, 6, 288–295. [Google Scholar] [CrossRef]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J. Stem Cells 2019, 11, 347–374. [Google Scholar] [CrossRef]
- Amati, E.; Perbellini, O.; Rotta, G.; Bernardi, M.; Chieregato, K.; Sella, S.; Rodeghiero, F.; Ruggeri, M.; Astori, G. High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: Identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources. Stem Cell Res. Ther. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Neen, D.; Noyes, D.; Shaw, M.; Gwilym, S.; Fairlie, N.; Birch, N. Healos and Bone Marrow Aspirate Used for Lumbar Spine Fusion: A Case Controlled Study Comparing Healos With Autograft. Spine 2006, 31, E636–E640. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Emadedin, M.; Labibzadeh, N.; Fazeli, R.; Mohseni, F.; Hosseini, S.E.; Moghadasali, R.; Mardpour, S.; Azimian, V.; Goodarzi, A.; Ghorbani Liastani, M.; et al. Percutaneous Autologous Bone Marrow-Derived Mesenchymal Stromal Cell Implantation Is Safe for Reconstruction of Human Lower Limb Long Bone Atrophic Nonunion. Cell J. 2017, 19, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barrena, E.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebe, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; Padilla-Eguiluz, N.; et al. Feasibility and safety of treating non-unions in the tibia, femur, and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials 2019, 196, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Su, P.; Wang, Y.; Chen, X.; Meng, Y.; Liu, C.; Yu, X.; Yang, X.; Yu, W.; Zhang, X.; et al. A novel biomimetic composite scaffold hybridized with mesenchymal stem cells in repair of rat bone defects models. J. Biomed. Mater. Res. Part A 2010, 95A, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C.; Lin, K.; Fan, W.; Chen, L.; Xiao, Y.; Chang, J. Biological responses of human bone marrow mesenchymal stem cells to Sr-M-Si (M = Zn, Mg) silicate bioceramics. J. Biomed. Mater. Res. A 2012, 100, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wu, C.; Dai, K.; Chang, J.; Tang, T. Proliferation and osteoblastic differentiation of human bone marrow-derived stromal cells on akermanite-bioactive ceramics. Biomaterials 2006, 27, 5651–5657. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Yin, Z.; Mao, L.; Wang, X.; Liu, J.; Jiang, X.; Zhang, Z.; Lin, K.; Chang, J.; Fang, B. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci. Rep. 2016, 6, 22005. [Google Scholar] [CrossRef]
- Maiti, S.K.; Shivakumar, M.U.; Mohan, D.; Kumar, N.; Singh, K.P. Mesenchymal Stem Cells of Different Origin-Seeded Bioceramic Construct in Regeneration of Bone Defect in Rabbit. Tissue Eng. Regen. Med. 2018, 15, 477–492. [Google Scholar] [CrossRef]
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 2013, 34, 10028–10042. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.K.; Ninu, A.R.; Sangeetha, P.; Mathew, D.D.; Tamilmahan, P.; Kritaniya, D.; Kumar, N.; Hescheler, J. Mesenchymal stem cells-seeded bio-ceramic construct for bone regeneration in large critical-size bone defect in rabbit. J. Stem Cells Regen. Med. 2016, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Barrena, E.; Padilla-Eguiluz, N.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebe, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; et al. Early efficacy evaluation of mesenchymal stromal cells (MSC) combined to biomaterials to treat long bone non-unions. Injury 2020, 51 (Suppl. S1), S63–S73. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Balbi, C. Extracellular Vesicles: From Biomarkers to Therapeutic Tools. Biology 2020, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Teixeira, J.H.; Almeida, M.I.; Goncalves, R.M.; Barbosa, M.A.; Santos, S.G. Extracellular Vesicles: Immunomodulatory messengers in the context of tissue repair/regeneration. Eur. J. Pharm. Sci. 2017, 98, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Li, H.; Chen, C.; Hu, B.; Niu, X.; Li, Q.; Zhao, B.; Xie, Z.; Wang, Y. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res. Ther. 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.B.; Brennan, M.Á.; Lötvall, J.; Breakefield, X.O.; EL Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, eaav8521. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.F.; Yang, G.H.; Pan, X.H.; Zhang, S.J.; Zhao, C.; Qiu, B.S.; Gu, H.F.; Hong, J.F.; Cao, L.; Chen, Y.; et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS ONE 2014, 9, e114627. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, H.; Wang, S.; Li, T.; Fan, J.; Liang, X.; Li, J.; Han, Q.; Zhu, L.; Fan, L.; et al. let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 2014, 23, 1452–1463. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Y.; Lin, Z.; Wan, J.; Xu, C.; Zeng, Y.; Zhu, Y. miR-199b-5p modulates BMSC osteogenesis via suppressing GSK-3beta/beta-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2016, 477, 749–754. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Maeda, Y.; Taipaleenmaki, H.; Zhang, W.; Jafferji, M.; Gordon, J.A.; Li, Z.; Croce, C.M.; van Wijnen, A.J.; Stein, J.L.; et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J. Biol. Chem. 2012, 287, 42084–42092. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; McBain, S.; Dobson, J.; El Haj, A.J. Selective activation of mechanosensitive ion channels using magnetic particles. J. R. Soc. Interface 2008, 5, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jung, H.J.; Wong, D.S.H.; Kim, S.K.; Lin, S.; Chan, K.F.; Zhang, L.; Li, G.; Dravid, V.P.; Bian, L. Remote Control of Heterodimeric Magnetic Nanoswitch Regulates the Adhesion and Differentiation of Stem Cells. J. Am. Chem. Soc. 2018, 140, 5909–5913. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Chen, C. Remote Control and Modulation of Cellular Events by Plasmonic Gold Nanoparticles: Implications and Opportunities for Biomedical Applications. ACS Nano 2017, 11, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, L.; Li, Z.; Ma, G.; Zhou, Y.; Han, G. Illuminating cell signaling with near-infrared light-responsive nanomaterials. ACS Nano 2016, 10, 3881–3885. [Google Scholar] [CrossRef] [PubMed]
- Taghian, T.; Narmoneva, D.A.; Kogan, A.B. Modulation of cell function by electric field: A high-resolution analysis. J. R. Soc. Interface 2015, 12, 20150153. [Google Scholar] [CrossRef]
- Wong, D.S.; Li, J.; Yan, X.; Wang, B.; Li, R.; Zhang, L.; Bian, L. Magnetically Tuning Tether Mobility of Integrin Ligand Regulates Adhesion, Spreading, and Differentiation of Stem Cells. Nano Lett. 2017, 17, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Lee, E.J.; Son, M.; Lee, J.H.; Yoo, D.; Kim, J.W.; Park, S.W.; Shin, J.S.; Cheon, J. A magnetic switch for the control of cell death signaling in vitro and in vivo systems. Nat. Mater. 2012, 11, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Deepagan, V.G.; You, D.G.; Um, W.; Ko, H.; Kwon, S.; Choi, K.Y.; Yi, G.R.; Lee, J.Y.; Lee, D.S.; Kim, K.; et al. Long-Circulating Au-TiO(2) Nanocomposite as a Sonosensitizer for ROS-Mediated Eradication of Cancer. Nano Lett. 2016, 16, 6257–6264. [Google Scholar] [CrossRef]
- Lin, L.; Liu, L.; Zhao, B.; Xie, R.; Lin, W.; Li, H.; Li, Y.; Shi, M.; Chen, Y.G.; Springer, T.A.; et al. Carbon nanotube-assisted optical activation of TGF-beta signalling by near-infrared light. Nat. Nanotechnol. 2015, 10, 465–471. [Google Scholar] [CrossRef]
- Fu, J.; Liu, X.; Tan, L.; Cui, Z.; Zheng, Y.; Liang, Y.; Li, Z.; Zhu, S.; Yeung, K.W.K.; Feng, X.; et al. Photoelectric-Responsive Extracellular Matrix for Bone Engineering. ACS Nano 2019, 13, 13581–13594. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Huang, T.H.; Kao, C.T.; Huang, C.H.; Ding, S.J. The effect of a physiologic solution pH on properties of white mineral trioxide aggregate. J. Endod. 2009, 35, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Stark, J. Early hydration of ordinary Portland cement with an alkaline shotcrete accelerator. Adv. Cem. Res. 2005, 17, 1–8. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Lotfi, M.; Saghiri, A.M.; Vosoughhosseini, S.; Fatemi, A.; Shiezadeh, V.; Ranjkesh, B. Effect of pH on sealing ability of white mineral trioxide aggregate as a root-end filling material. J. Endod. 2008, 34, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Namazikhah, M.S.; Nekoofar, M.H.; Sheykhrezae, M.S.; Salariyeh, S.; Hayes, S.J.; Bryant, S.T.; Mohammadi, M.M.; Dummer, P.M. The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int. Endod. J. 2008, 41, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, e12195. [Google Scholar] [CrossRef] [PubMed]
- Kazemipoor, M.; Sanati, E. Surface Microstructure of Two Bioceramics: Calcium-Enriched Mixture and Cold Ceramic in Setting Environments with Different pH Values. Int. J. Dent. 2023, 2023, 7130619. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Kluin, O.S.; van der Mei, H.C.; Busscher, H.J.; Neut, D. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert. Opin. Drug Deliv. 2013, 10, 341–351. [Google Scholar] [CrossRef]
- Giavaresi, G.; Bertazzoni Minelli, E.; Sartori, M.; Benini, A.; Della Bora, T.; Sambri, V.; Gaibani, P.; Borsari, V.; Salamanna, F.; Martini, L.; et al. Microbiological and pharmacological tests on new antibiotic-loaded PMMA-based composites for the treatment of osteomyelitis. J. Orthop. Res. 2012, 30, 348–355. [Google Scholar] [CrossRef]
- Ferraz, M.P.; Mateus, A.Y.; Sousa, J.C.; Monteiro, F.J. Nanohydroxyapatite microspheres as delivery system for antibiotics: Release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res. A 2007, 81, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Neut, D.; van de Belt, H.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials 2003, 24, 1829–1831. [Google Scholar] [CrossRef] [PubMed]
- Trécant, M.; Guicheux, J.m.; Grimandi, G.; Leroy, M.; Daculsi, G. Dynamic compaction: A new process to compact therapeutic agent-loaded calcium phosphates. Biomaterials 1997, 18, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kawanabe, K.; Okada, Y.; Matsusue, Y.; Iida, H.; Nakamura, T. Treatment of osteomyelitis with antibiotic-soaked porous glass ceramic. J. Bone Jt. Surg. Br. Vol. 1998; 80B, 527–530. [Google Scholar] [CrossRef]
- Solberg, B.D.; Gutow, A.P.; Baumgaertner, M.R. Efficacy of Gentamycin-Impregnated Resorbable Hydroxyapatite Cement in Treating Osteomyelitis in a Rat Model. J. Orthop. Trauma 1999, 13, 102–106. [Google Scholar] [CrossRef]
- McLaren, A.C. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin. Orthop. Relat. Res. 2004, 427, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jiang, D.; Yan, L.; Wu, J. In vitro and in vivo osteogenic activity of the novel vancomycin-loaded bone-like hydroxyapatite/poly(amino acid) scaffold. J. Biomater. Appl. 2016, 30, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Jiang, D.; Yan, L.; Wu, J. In vitro and in vivo drug release and antibacterial properties of the novel vancomycin-loaded bone-like hydroxyapatite/poly amino acid scaffold. Int. J. Nanomed. 2017, 12, 1841–1851. [Google Scholar] [CrossRef]
- Joosten, U.; Joist, A.; Frebel, T.; Brandt, B.; Diederichs, S.; von Eiff, C. Evaluation of an in situ setting injectable calcium phosphate as a new carrier material for gentamicin in the treatment of chronic osteomyelitis: Studies in vitro and in vivo. Biomaterials 2004, 25, 4287–4295. [Google Scholar] [CrossRef]
- Dvorzhinskiy, A.; Perino, G.; Chojnowski, R.; van der Meulen, M.C.H.; Bostrom, M.P.G.; Yang, X. Ceramic composite with gentamicin decreases persistent infection and increases bone formation in a rat model of debrided osteomyelitis. J. Bone Jt. Infect. 2021, 6, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Wang, C.X.; Zhou, X.; Wang, M. Influence of sintering temperatures on hardness and Young’s modulus of tricalcium phosphate bioceramic by nanoindentation technique. Mater. Charact. 2004, 52, 301–307. [Google Scholar] [CrossRef]
- Bouslama, N.; Ben Ayed, F.; Bouaziz, J. Sintering and mechanical properties of tricalcium phosphate–fluorapatite composites. Ceram. Int. 2009, 35, 1909–1917. [Google Scholar] [CrossRef]
| Name of the Materials | Applications | Biological Behavior | References |
|---|---|---|---|
| Alumina | Femoral balls, inserts of acetabular cups, artificial heart valves, dental roots, bone screws, and endoscope | Bioinert | [39] |
| Al2O3 | Coatings for tissue growth: orthopedic | Bioinert | [39] |
| Zirconia (Y-TZP) | Femoral balls, dental veneers, and tooth inlays | Bioinert | [39] |
| Titanium nitride | Antiwear coating of femoral balls and knee prostheses | Bioinert | [40] |
| Zirconium nitride | Antiwear coating of femoral balls and knee prostheses, and coating for coronary stents | Bioinert | [40] |
| Silicon nitride | Antiwear coatings of femoral balls | Bioinert | [41,42] |
| Hydroxyapatite | Bone cavity fillings, ear implants, vertebrae replacement, hip implant coatings, bone scaffolds, and orthopedic | Bioactive | [43,44] |
| Bioglass | Bone replacement | Bioactive | [45] |
| Tricalcium phosphate | Bone replacement | Bioactive/biodegradable | [46] |
| Hydroxyapatite/PCL | Tissue engineering scaffolds | Biodegradable | [46] |
| β-Tricalcium phosphate (β-TCP) | Bone regeneration, bioactivity | Biodegradable | [47] |
| Dicalcium phosphate dehydrate (DCPD) | Bone regeneration, osteoconductivity | Biodegradable | [47] |
| Calcium phosphate | Promotes tissue ingrowth and vascularization | Biodegradable | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanvir, M.A.H.; Khaleque, M.A.; Kim, G.-H.; Yoo, W.-Y.; Kim, Y.-Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. https://doi.org/10.3390/biomimetics9040230
Tanvir MAH, Khaleque MA, Kim G-H, Yoo W-Y, Kim Y-Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics. 2024; 9(4):230. https://doi.org/10.3390/biomimetics9040230
Chicago/Turabian StyleTanvir, Md Amit Hasan, Md Abdul Khaleque, Ga-Hyun Kim, Whang-Yong Yoo, and Young-Yul Kim. 2024. "The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives" Biomimetics 9, no. 4: 230. https://doi.org/10.3390/biomimetics9040230
APA StyleTanvir, M. A. H., Khaleque, M. A., Kim, G.-H., Yoo, W.-Y., & Kim, Y.-Y. (2024). The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics, 9(4), 230. https://doi.org/10.3390/biomimetics9040230









