Effects of Sodium Alginate and Calcium Chloride on Fungal Growth and Viability in Biomass-Fungi Composite Materials Used for 3D Printing
Abstract
1. Introduction
2. Effects on Fungal Growth in Different Medium Solutions
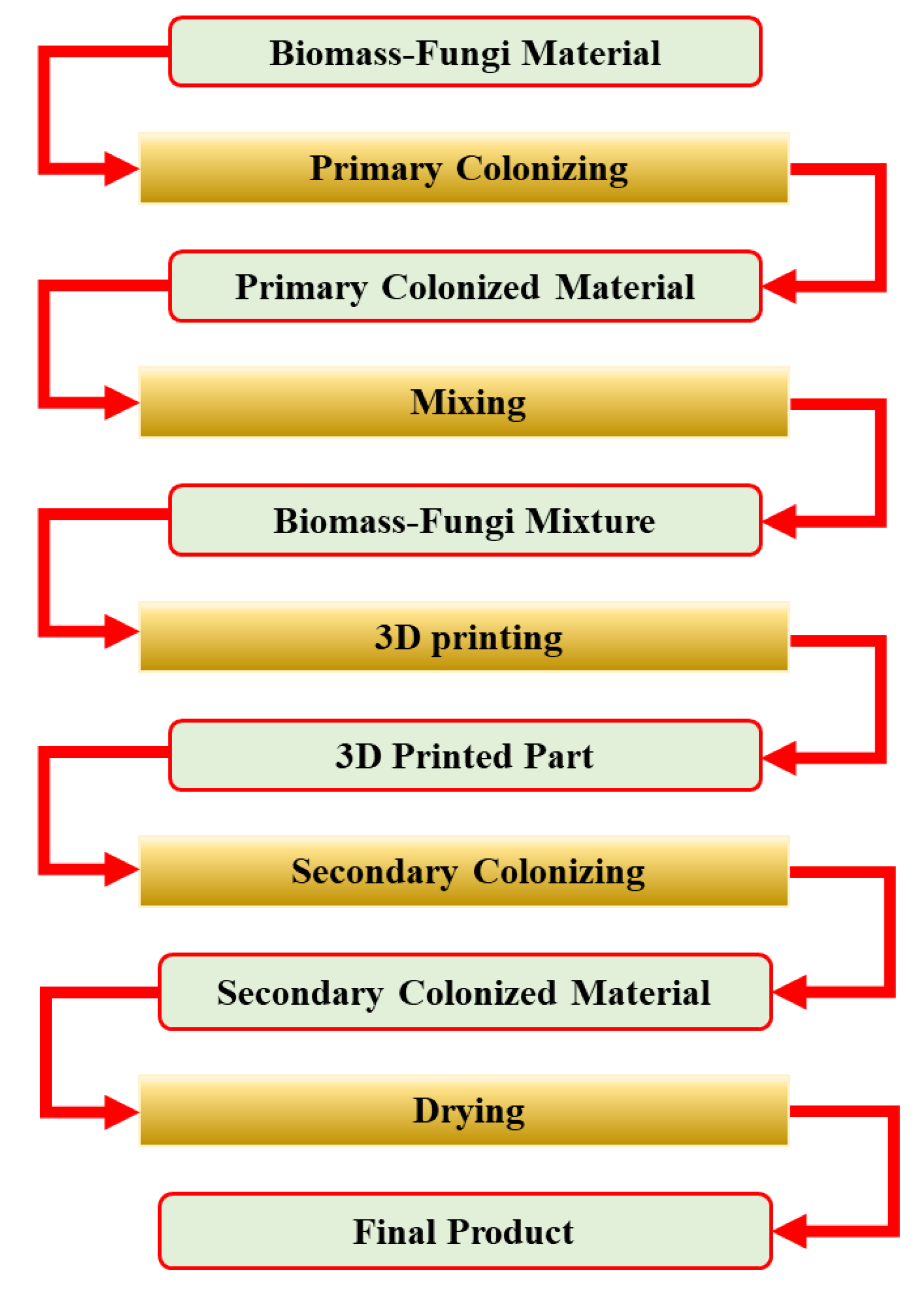
2.1. Experimental Procedure
2.1.1. Procurement of Materials
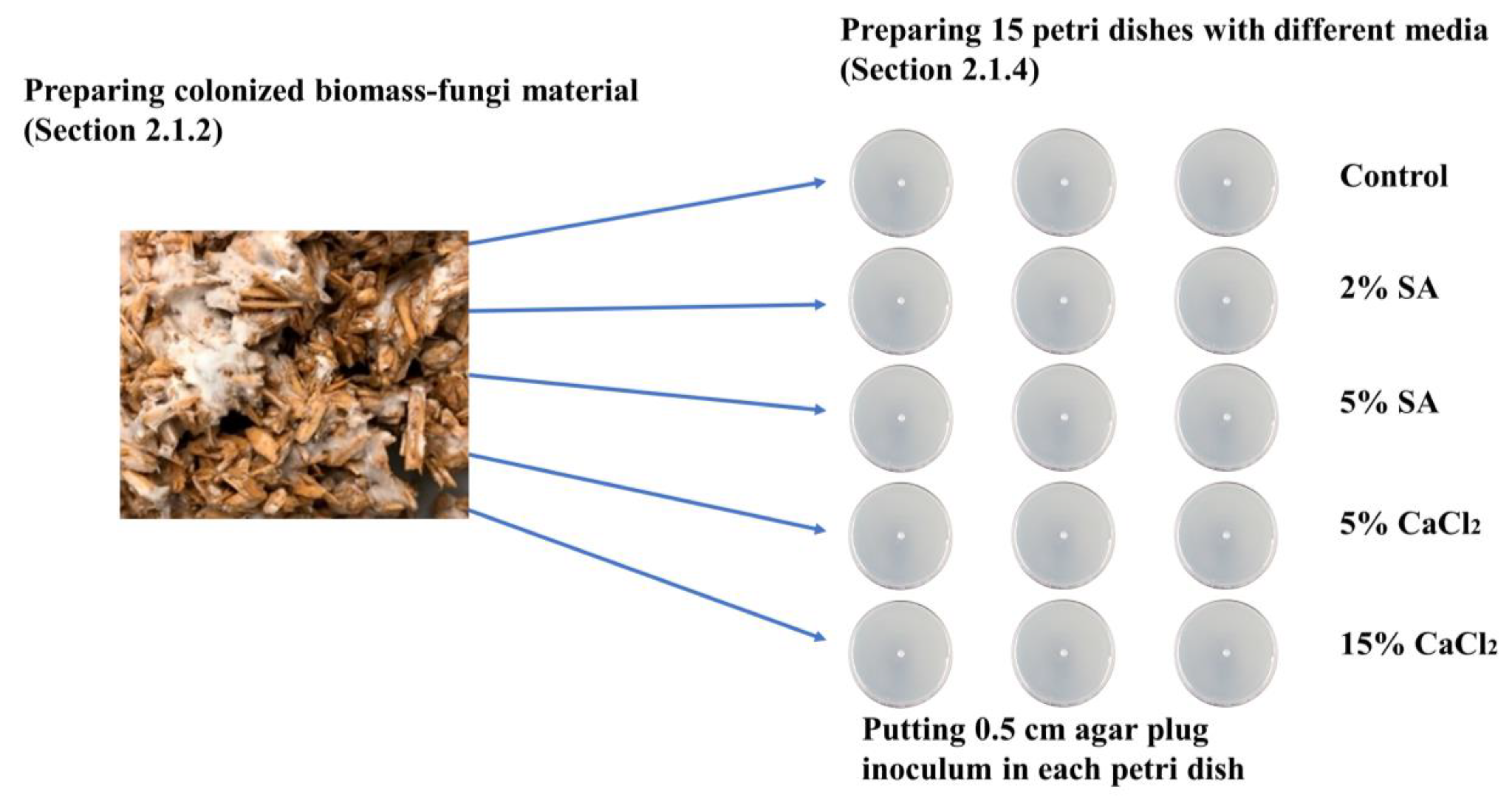
2.1.2. Preparation of Primary Colonized Biomass-Fungi Material
2.1.3. Preparation of HDPA Plates Containing Pure Fungi Culture
2.1.4. Preparation of Petri Dishes with Different Concentrations of SA and CaCl2
2.1.5. Evaluation of Fungal Growth Using Circumference of Fungal Colonies
2.1.6. Hyphae Growth Speed Observed Using Confocal Microscope
2.2. Results and Discussion
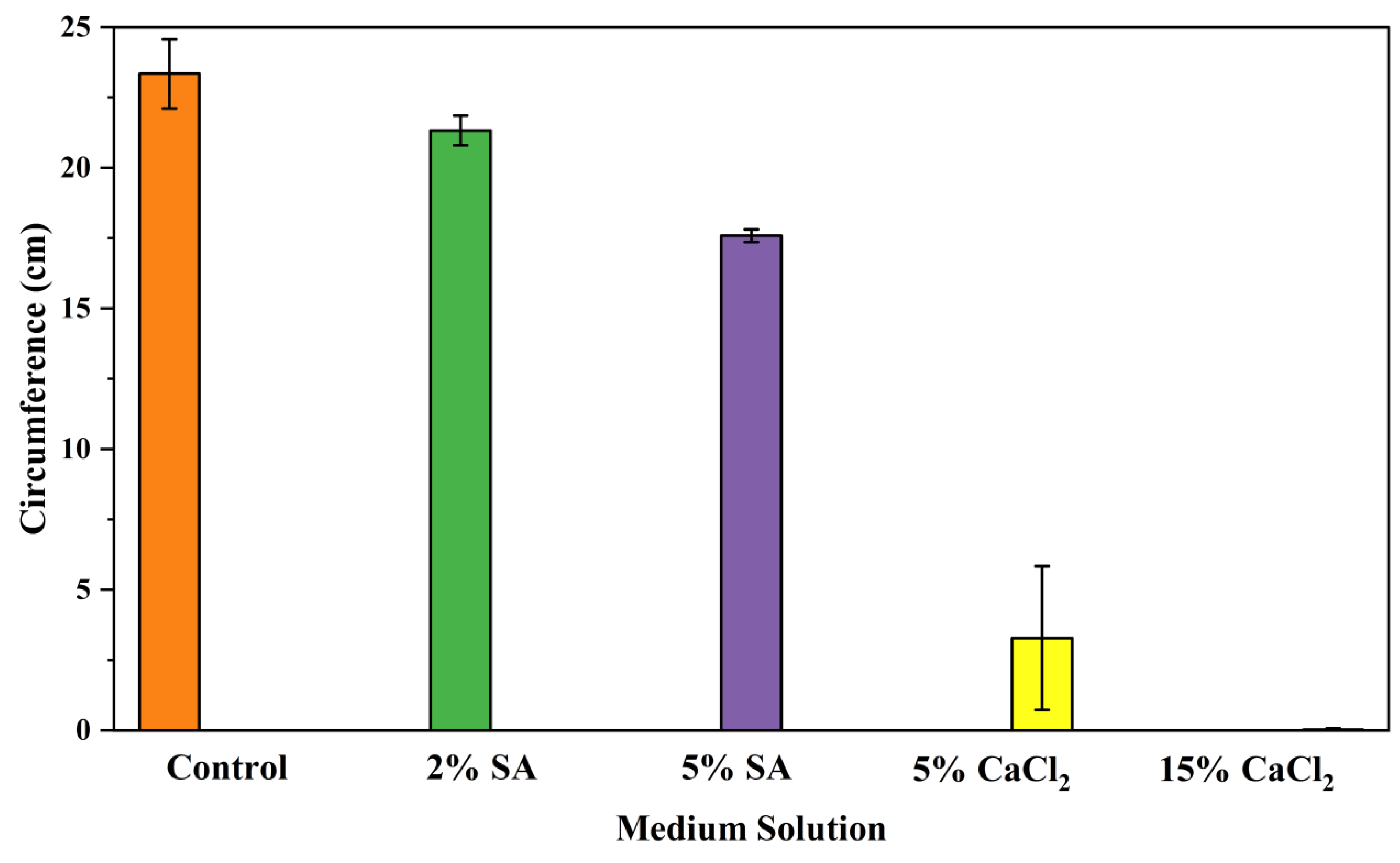
2.2.1. Effects on Fungal Growth Measured by Circumference of Fungal Colonies
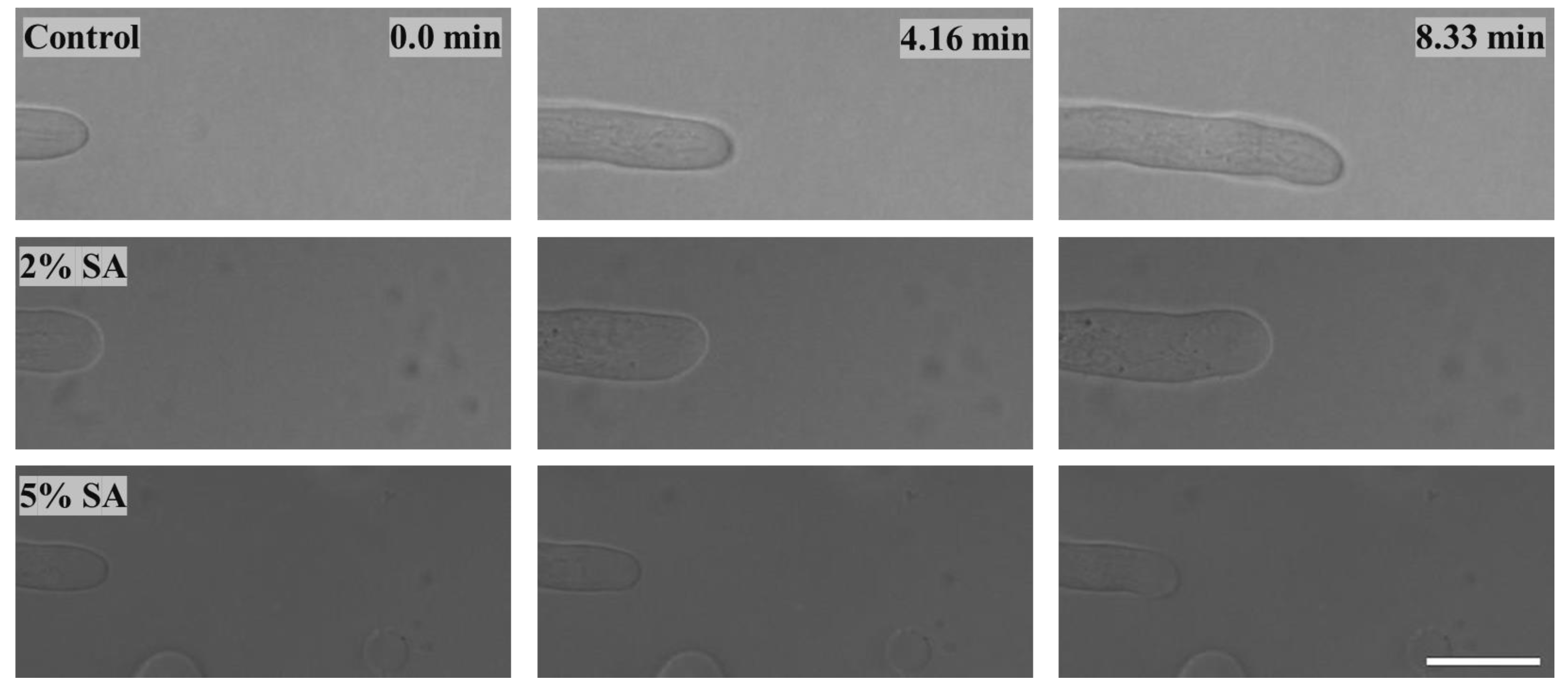
2.2.2. Effect on Fungal Growth Observed under the Confocal Microscope
3. Effects on Fungal Viability in Biomass-Fungi Mixture
3.1. Experimental Procedure
3.1.1. Procurement of Materials
3.1.2. Preparation of Primary Colonized Material
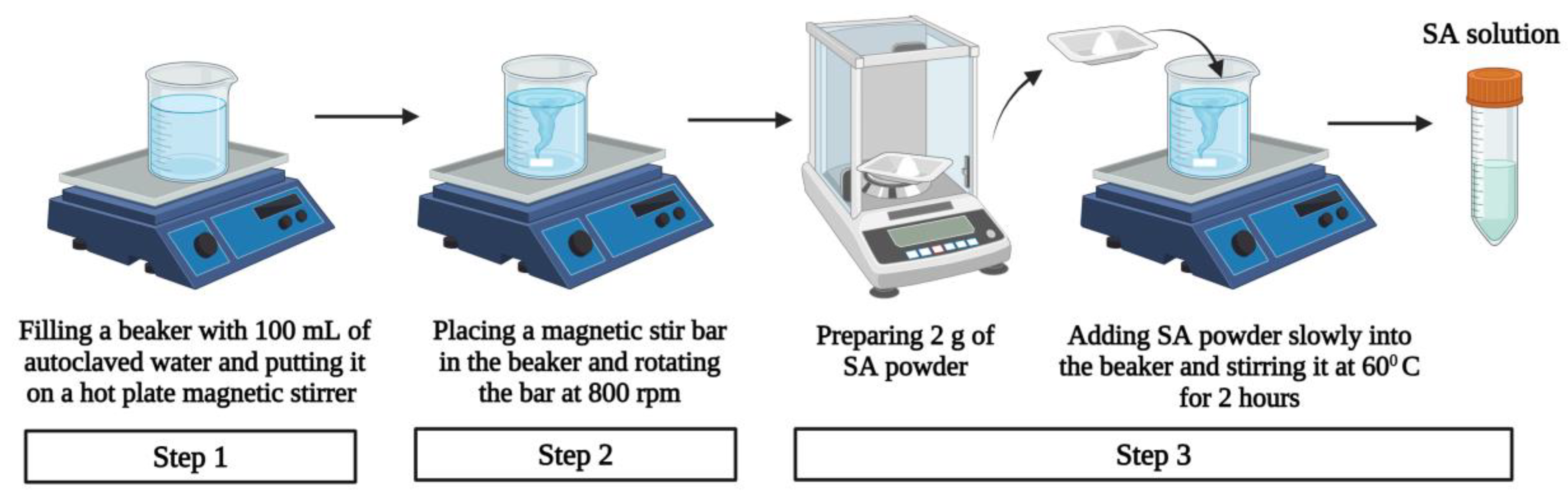
3.1.3. Preparation of Sodium Alginate Solution
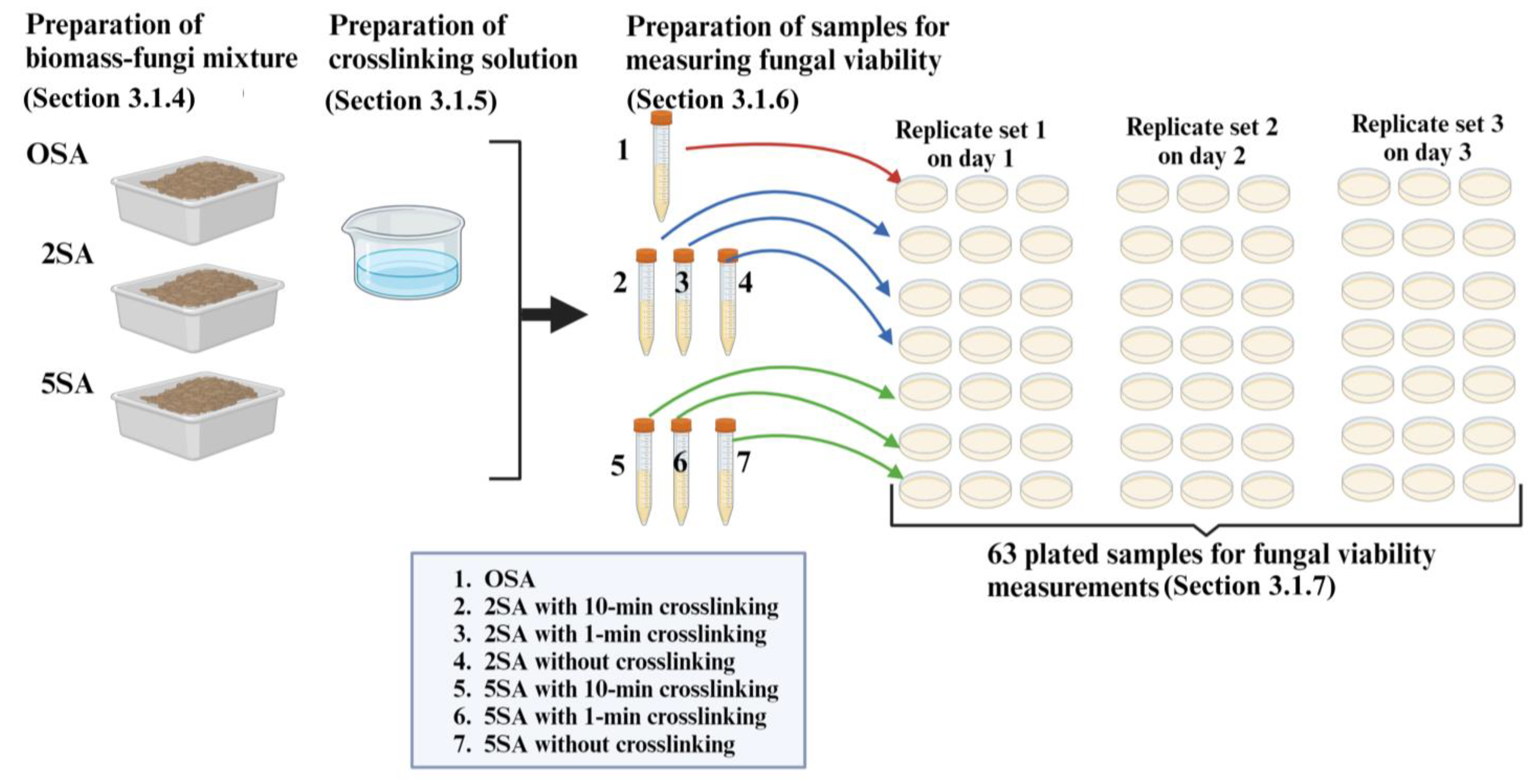
3.1.4. Preparation of Biomass-Fungi Mixtures with Different SA Concentrations
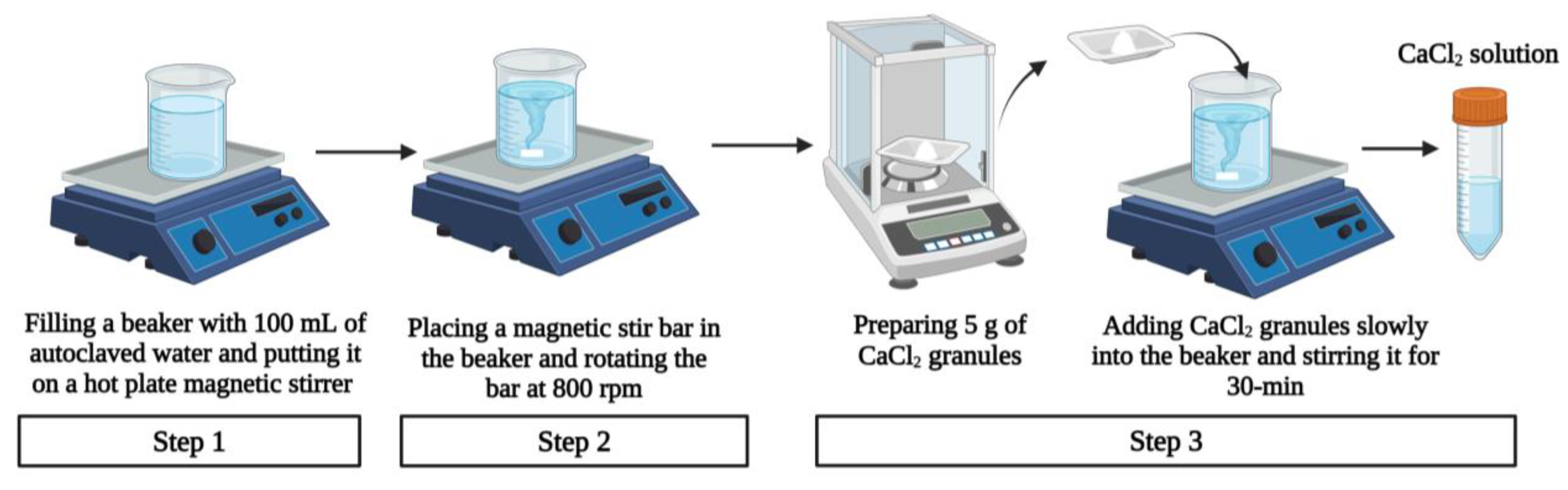
3.1.5. Preparation of Crosslinking Solution
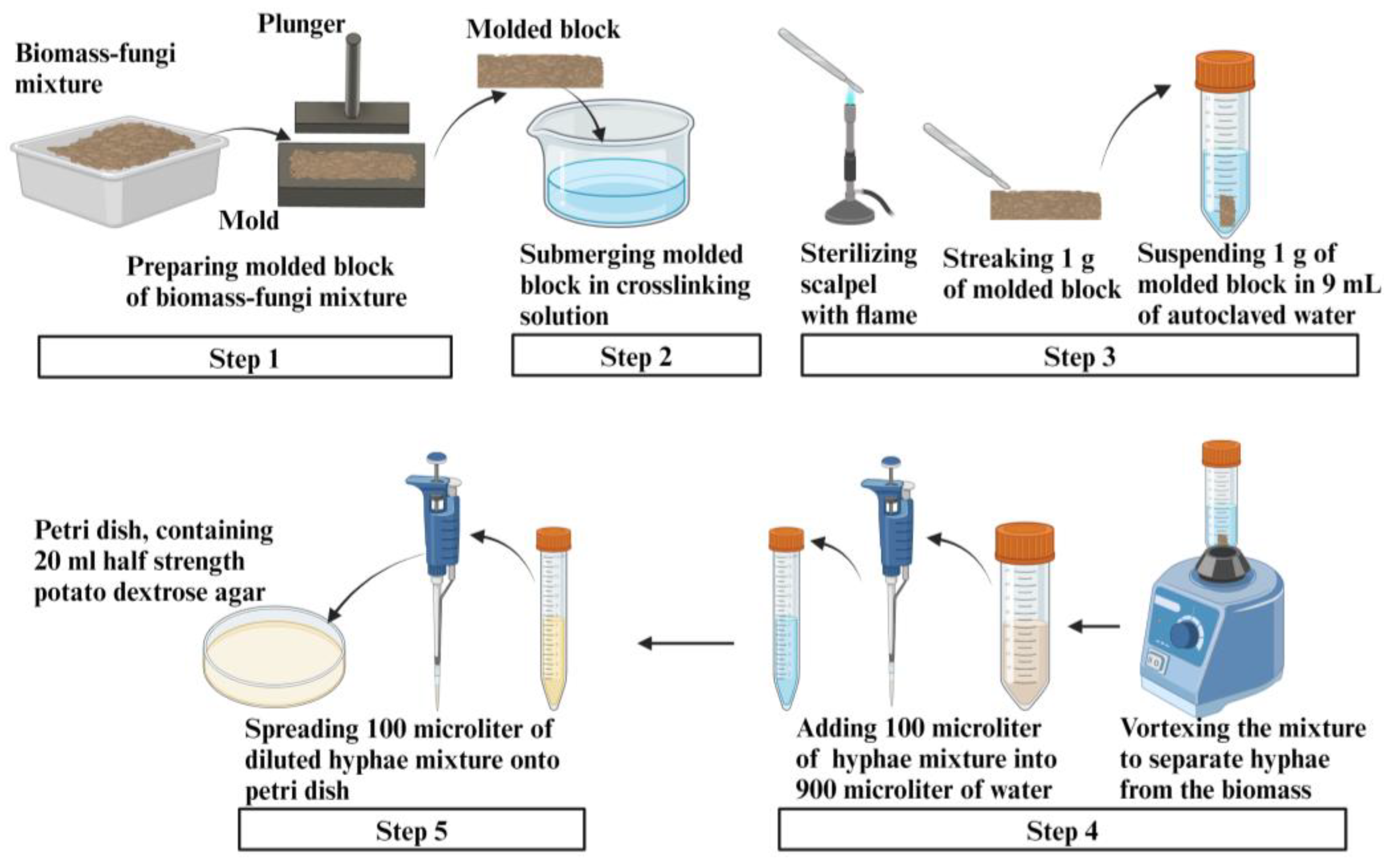
3.1.6. Preparation of Samples for Measuring Fungal Viability in Biomass-Fungi Mixtures
3.1.7. Evaluation of Fungal Viability by Counting Colony Forming Units
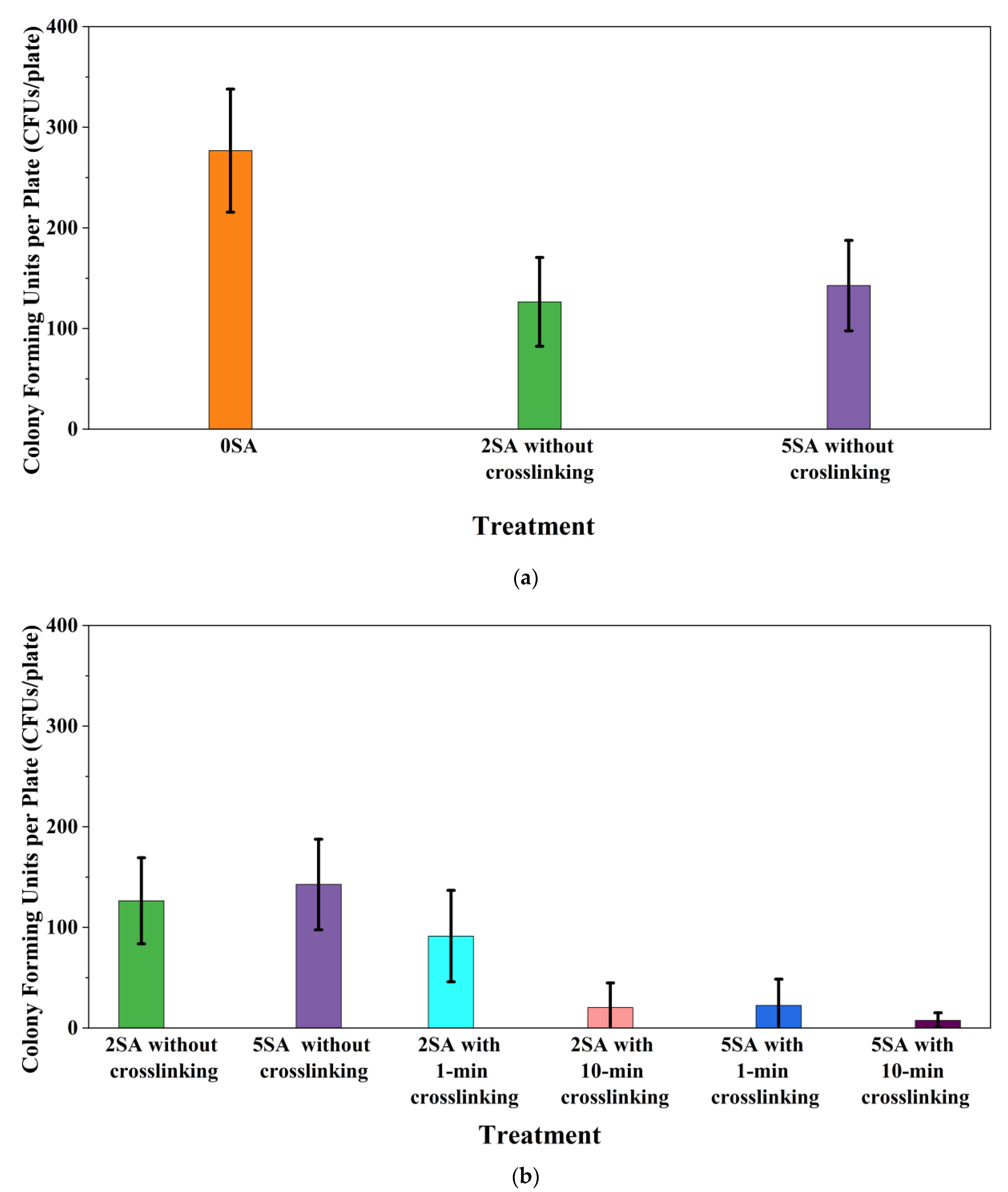
3.2. Results and Discussion
4. Concluding Remarks
- Five different types of Petri dishes with different concentrations of SA and CaCl2 were prepared. The control Petri dishes had the highest fungal growth (circumference = 23.34 cm). The 2% and 5% SA Petri dishes had significantly reduced fungal growth compared with the control Petri dishes.
- Based on results from the effects of different concentrations of SA and CaCl2 on fungal growth, only control, 2%, and 5% SA Petri dishes were used for confocal microscopy observations. The results showed that control Petri dishes had a higher rate of hyphae growth than the 2% SA and 5% SA Petri dishes.
- In the set of experiments using plated samples, biomass-fungi mixtures were treated with different concentrations of SA and exposure times in the crosslinking solution. Fungal viability was measured by counting colony-forming units. Among the tested concentrations, the 0SA plated samples had the highest fungal viability, and the addition of SA into biomass-fungi mixtures reduced fungal viability, but a change in concentration of SA did not make any significant difference in fungal viability. The results also showed that 2SA with a 1 min crosslinking treatment can be used to prepare mixtures with biomass-fungi composite materials for 3D printing. Crosslinking might improve the print quality and the mechanical properties of the 3D printed parts using biomass-fungi composite materials.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soh, E.; Chew, Z.Y.; Saeidi, N.; Javadian, A.; Hebel, D.; Le Ferrand, H. Development of an extrudable paste to build mycelium-bound composites. Mater. Des. 2020, 195, 109058. [Google Scholar] [CrossRef]
- Ecovative Design. Available online: https://grow.bio/pages/grow-it-yourself-education-and-instruction-docs (accessed on 2 July 2023).
- Abhijith, R.; Ashok, A.; Rejeesh, C. Sustainable packaging applications from mycelium to substitute polystyrene: A review. Mater. Today Proc. 2018, 5, 2139–2145. [Google Scholar] [CrossRef]
- Holt, G.A.; Mcintyre, G.; Flagg, D.; Bayer, E.; Wanjura, J.; Pelletier, M. Fungal mycelium and cotton plant materials in the manufacture of biodegradable molded packaging material: Evaluation study of select blends of cotton byproducts. J. Biobased Mater. Bioenergy 2012, 6, 431–439. [Google Scholar] [CrossRef]
- Rahman, A.M.; Rahman, T.T.; Pei, Z.; Ufodike, C.O.; Lee, J.; Elwany, A. Additive Manufacturing Using Agriculturally Derived Biowastes: A Systematic Literature Review. Bioengineering 2023, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A comprehensive framework for the production of mycelium-based lignocellulosic composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef] [PubMed]
- Ecovative at MoMA PS1. Available online: https://www.bfi.org/2014/07/28/ecovative-at-moma-ps1/ (accessed on 28 July 2014).
- Gandia, A.; van den Brandhof, J.G.; Appels, F.V.; Jones, M.P. Flexible fungal materials: Shaping the future. Trends Biotechnol. 2021, 39, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Pelletier, M.; Holt, G.; Wanjura, J.; Lara, A.; Tapia-Carillo, A.; McIntyre, G.; Bayer, E. An evaluation study of pressure-compressed acoustic absorbers grown on agricultural by-products. Ind. Crops Prod. 2017, 95, 342–347. [Google Scholar] [CrossRef]
- Rahmani, A.M.; Gahlot, P.; Moustakas, K.; Kazmi, A.; Ojha, C.S.P.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Vasselli, J.; Lucht, M.; Pei, Z.; Shaw, B.; Grasley, Z.; Wei, X.; Zou, N. 3D Printing of Biomass-Fungi Composite Material: A Preliminary Study. Manuf. Lett. 2020, 24, 96–99. [Google Scholar] [CrossRef]
- Zheng, J.; Rehmann, L. Extrusion pretreatment of lignocellulosic biomass: A review. Int. J. Mol. Sci. 2014, 15, 18967–18984. [Google Scholar] [CrossRef] [PubMed]
- Sydor, M.; Bonenberg, A.; Doczekalska, B.; Cofta, G. Mycelium-based composites in art, architecture, and interior design: A review. Polymers 2021, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Elsacker, E.; Peeters, E.; De Laet, L. Large-scale robotic extrusion-based additive manufacturing with living mycelium materials. Sustain. Futures 2022, 4, 100085. [Google Scholar] [CrossRef]
- Rahman, A.M.; Bhardwaj, A.; Vasselli, J.G.; Pei, Z.; Shaw, B.D. Three-Dimensional Printing of Biomass–Fungi Biocomposite Materials: The Effects of Mixing and Printing Parameters on Fungal Growth. J. Manuf. Mater. Process. 2024, 8, 2. [Google Scholar] [CrossRef]
- Mohseni, A.; Vieira, F.R.; Pecchia, J.A.; Gürsoy, B. Three-Dimensional Printing of Living Mycelium-Based Composites: Material Compositions, Workflows, and Ways to Mitigate Contamination. Biomimetics 2023, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Soh, E.; Teoh, J.H.; Leong, B.; Xing, T.; Le Ferrand, H. 3D printing of mycelium engineered living materials using a waste-based ink and non-sterile conditions. Mater. Des. 2023, 236, 112481. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Rahman, A.M.; Wei, X.; Pei, Z.; Truong, D.; Lucht, M.; Zou, N. 3d printing of biomass–fungi composite material: Effects of mixture composition on print quality. J. Manuf. Mater. Process. 2021, 5, 112. [Google Scholar] [CrossRef]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef]
- Panchal, N.; Patel, D.; Shah, N. Synthesis of hydrogels. In Proceedings of the 4th International Conference on Multidisciplinary Research & Practice (4ICMRP-2017), Ahmedabad, India, 22 December 2017. [Google Scholar]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Chen, H.; Abdullayev, A.; Bekheet, M.F.; Schmidt, B.; Regler, I.; Pohl, C.; Vakifahmetoglu, C.; Czasny, M.; Kamm, P.H.; Meyer, V.; et al. Extrusion-based additive manufacturing of fungal-based composite materials using the tinder fungus Fomes fomentarius. Fungal Biol. Biotechnol. 2021, 8, 21. [Google Scholar] [CrossRef]
- Liu, S.; Bastola, A.K.; Li, L. A 3D Printable and Mechanically Robust Hydrogel Based on Alginate and Graphene Oxide. ACS Appl. Mater. Interfaces 2017, 9, 41473–41481. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Singh, A.; Sudha; Sahay, N.S.; Sharma, J.; Roy, A.; Kumari, M.; Rana, D.; Thakran, S.; Deka, D.; et al. Piriformospora indica: An Axenically Culturable Mycorrhiza-Like Endosymbiotic Fungus. In Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 125–150. [Google Scholar]
- Moghadam, H.; Samimi, M.; Samimi, A.; Khorram, M. Electro-spray of high viscous liquids for producing mono-sized spherical alginate beads. Particuology 2008, 6, 271–275. [Google Scholar] [CrossRef]
- Rahman, T.T.; Wood, N.; Rahman, A.M.; Pei, Z.; Qin, H. Applying In-situ Ionic Crosslinking in Bioprinting Using Algae Cells. J. Manuf. Sci. Eng. 2023, 146, 034501. [Google Scholar] [CrossRef]
- Wang, L.; Shelton, R.; Cooper, P.; Lawson, M.; Triffitt, J.; Barralet, J. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 2003, 24, 3475–3481. [Google Scholar] [CrossRef]
- Suvarna, S.; Dsouza, J.; Ragavan, M.L.; Das, N. Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition. Food Sci. Biotechnol. 2018, 27, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, P.; Tabandeh, F.; Alemzadeh, I.; Soltani, M. Effects of Sucrose, Skim Milk and Yeast Powder on Survival of Lactobacillus rhamnosus GG Encapsulated with Alginate during One-week Storage at room Conditions. Appl. Food Biotechnol. 2022, 9, 251–259. [Google Scholar]
- Abdel-Raouf, N.; Al-Homaidan, A.; Ibraheem, I. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Kokova, V.; Lukova, P.; Baldzhieva, A.; Katsarov, P.; Delattre, C.; Molinié, R.; Petit, E.; Elboutachfaiti, R.; Murdjeva, M.; Apostolova, E. Extraction, Structural Characterization, and In Vivo Anti-Inflammatory Effect of Alginate from Cystoseira crinita (Desf.) Borry Harvested in the Bulgarian Black Sea. Mar. Drugs 2023, 21, 245. [Google Scholar] [CrossRef]
- Henry, P.; Halbus, A.F.; Athab, Z.H.; Paunov, V.N. Enhanced Antimould Action of Surface Modified Copper Oxide Nanoparticles with Phenylboronic Acid Surface Functionality. Biomimetics 2021, 6, 19. [Google Scholar] [CrossRef]
- Boh, B.; Berovic, M.; Zhang, J.; Lin, Z.-B. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [PubMed]
- Buchalo, A.; Zakordonec, O.; Šašek, V. Scanning electron microscopic study of clamp connections in higher Basidiomycetes. Folia Microbiol. 1983, 28, 420–423. [Google Scholar] [CrossRef]
- Seo, G.S.; Shin, G.C.; Otani, H.; Kodama, M.; Kohmoto, K. Formation of atypical fruiting structures in Ganoderma lucidum isolates on a nutrient agar medium. Mycoscience 1995, 36, 1–7. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Hocking, A.D.; Fleet, G.H. Comparison of hyphal length, ergosterol, mycelium dry weight, and colony diameter for quantifying growth of fungi from foods. In Advances in Food Mycology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 49–67. [Google Scholar]
- Hickey, P.C.; Read, N.D. Imaging living cells of Aspergillus in vitro. Med. Mycol. 2009, 47 (Suppl. S1), S110–S119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richard, E.; Chang, T.-H.; Sutherland, J.W. Statistical Quality Design and Control; Prentice Hall: Upper Saddle River, NJ, USA, 1992. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S. Experimental Designs Using ANOVA; Thomson/Brooks/Cole: Belmont, CA, USA, 2007; Volume 724. [Google Scholar]
- Jung, J.; Li, L.; Yeh, C.-K.; Ren, X.; Sun, Y. Amphiphilic quaternary ammonium chitosan/sodium alginate multilayer coatings kill fungal cells and inhibit fungal biofilm on dental biomaterials. Mater. Sci. Eng. C 2019, 104, 109961. [Google Scholar] [CrossRef] [PubMed]
- Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Powell, L.C.; Pritchard, M.F.; Khan, S.; Craine, K.M.; Onsøyen, E.; Rye, P.D.; et al. Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp. PLoS ONE 2014, 9, e112518. [Google Scholar] [CrossRef] [PubMed]
- Boumaaza, B.; Benkhelifa, M.; Belkhoudja, M. Effects of two salts compounds on mycelial growth, sporulation, and spore germination of six isolates of Botrytis cinerea in the western north of Algeria. Int. J. Microbiol. 2015, 2015, 572626. [Google Scholar] [CrossRef] [PubMed]
- Maouni, A.; Lamarti, A.; Aidoun, A.; Khaddor, M.; Badoc, A. Effect of benzimidazole fungicides and calcium chloride on Alternaria alternata and Penicillium expansum rot during storage of pears. Afr. J. Biotechnol. 2007, 6, 1289–1292. [Google Scholar]
- Tian, S.; Fan, Q.; Xu, Y.; Jiang, A. Effects of calcium on biocontrol activity of yeast antagonists against the postharvest fungal pathogen Rhizopus stolonifer. Plant Pathol. 2002, 51, 352–358. [Google Scholar] [CrossRef]
- Juvvadi, P.R.; Lamoth, F.; Steinbach, W.J. Calcineurin as a multifunctional regulator: Unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol. Rev. 2014, 28, 56–69. [Google Scholar] [CrossRef]
- Ohsumi, Y.; Anraku, Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J. Biol. Chem. 1983, 258, 5614–5617. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.W.; Fink, G.R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 1994, 124, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Ohya, Y.; Ohsumi, Y.; Anraku, Y. Isolation and characterization of Ca2+-sensitive mutants of Saccharomyces cerevisiae. Microbiology 1986, 132, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E. Intracellular calcium homeostasis. Annu. Rev. Biochem. 1987, 56, 395–433. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.; Rasmussen, J.E. Calcium as intracellular messenger: From simplicity to complexity. Curr. Top. Cell. Regul. 1990, 31, 1–109. [Google Scholar] [PubMed]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, B.; Sharma, S. Hydrogels for potential food application: Effect of sodium alginate and calcium chloride on physical and morphological properties. Pharma Innov. J. 2018, 7, 142–148. [Google Scholar]
- Díez-García, I.; de Costa Lemma, M.R.; Barud, H.S.; Eceiza, A.; Tercjak, A. Hydrogels based on waterborne poly (urethane-urea) s by physically cross-linking with sodium alginate and calcium chloride. Carbohydr. Polym. 2020, 250, 116940. [Google Scholar] [CrossRef] [PubMed]
- Khot, P.D.; Suci, P.A.; Tyler, B.J. Candida albicans viability after exposure to amphotericin B: Assessment using metabolic assays and colony forming units. J. Microbiol. Methods 2008, 72, 268–274. [Google Scholar] [CrossRef]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium bio-composites in industrial design and architecture: Comparative review and experimental analysis. J. Clean. Prod. 2020, 246, 119037. [Google Scholar] [CrossRef]
| Concentration (%) | Number of Samples | |
|---|---|---|
| Control (No SA or CaCl2) | 0 | 4 |
| SA | 2 | 4 |
| 5 | 4 | |
| CaCl2 | 5 | 4 |
| 15 | 4 |
| Petri Dish | Day 0 | Day 3 | Day 5 |
|---|---|---|---|
| Control |  | 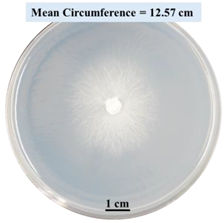 | 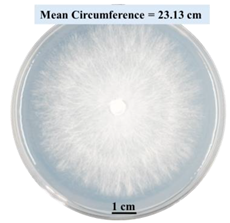 |
| 2% SA | 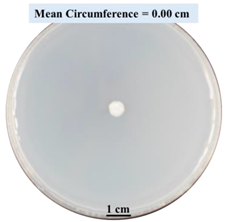 | 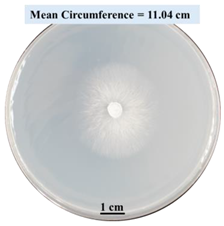 | 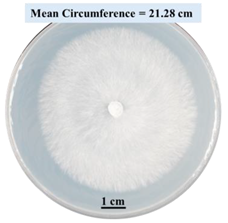 |
| 5% SA |  |  | 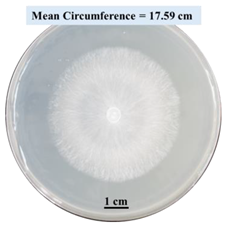 |
| 5% CaCl2 |  |  |  |
| 15% CaCl2 | 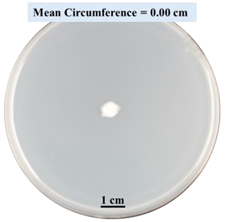 | 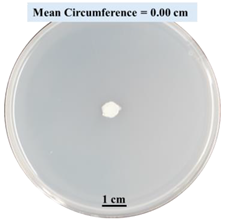 | 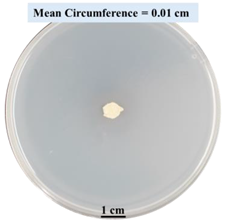 |
| Crosslinking Exposure Time (min) | Total Number of Plated Samples | Treatment | |
|---|---|---|---|
| 0SA | No crosslinking | 9 | 0SA |
| 2SA | 0 | 9 | 2SA without crosslinking |
| 1 | 9 | 2SA with 1 min crosslinking | |
| 10 | 9 | 2SA with 10 min crosslinking | |
| 5SA | 0 | 9 | 5SA without crosslinking |
| 1 | 9 | 5SA with 1 min crosslinking | |
| 10 | 9 | 5SA with 10 min crosslinking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.M.; Bedsole, C.O.; Akib, Y.M.; Hamilton, J.; Rahman, T.T.; Shaw, B.D.; Pei, Z. Effects of Sodium Alginate and Calcium Chloride on Fungal Growth and Viability in Biomass-Fungi Composite Materials Used for 3D Printing. Biomimetics 2024, 9, 251. https://doi.org/10.3390/biomimetics9040251
Rahman AM, Bedsole CO, Akib YM, Hamilton J, Rahman TT, Shaw BD, Pei Z. Effects of Sodium Alginate and Calcium Chloride on Fungal Growth and Viability in Biomass-Fungi Composite Materials Used for 3D Printing. Biomimetics. 2024; 9(4):251. https://doi.org/10.3390/biomimetics9040251
Chicago/Turabian StyleRahman, Al Mazedur, Caleb Oliver Bedsole, Yeasir Mohammad Akib, Jillian Hamilton, Taieba Tuba Rahman, Brian D. Shaw, and Zhijian Pei. 2024. "Effects of Sodium Alginate and Calcium Chloride on Fungal Growth and Viability in Biomass-Fungi Composite Materials Used for 3D Printing" Biomimetics 9, no. 4: 251. https://doi.org/10.3390/biomimetics9040251
APA StyleRahman, A. M., Bedsole, C. O., Akib, Y. M., Hamilton, J., Rahman, T. T., Shaw, B. D., & Pei, Z. (2024). Effects of Sodium Alginate and Calcium Chloride on Fungal Growth and Viability in Biomass-Fungi Composite Materials Used for 3D Printing. Biomimetics, 9(4), 251. https://doi.org/10.3390/biomimetics9040251








