Translating Virtual Prey-Predator Interaction to Real-World Robotic Environments: Enabling Multimodal Sensing and Evolutionary Dynamics
Abstract
:1. Introduction
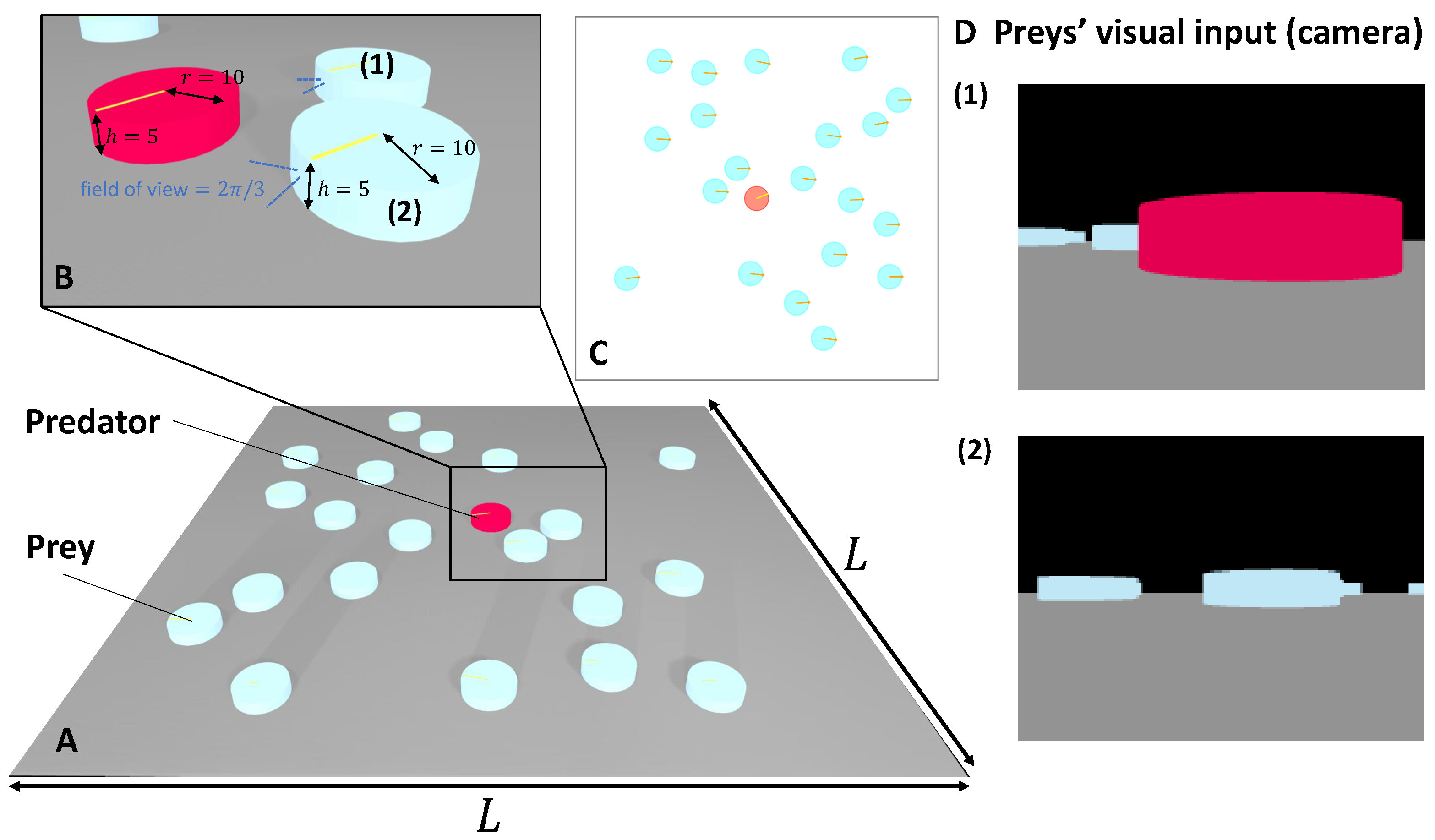
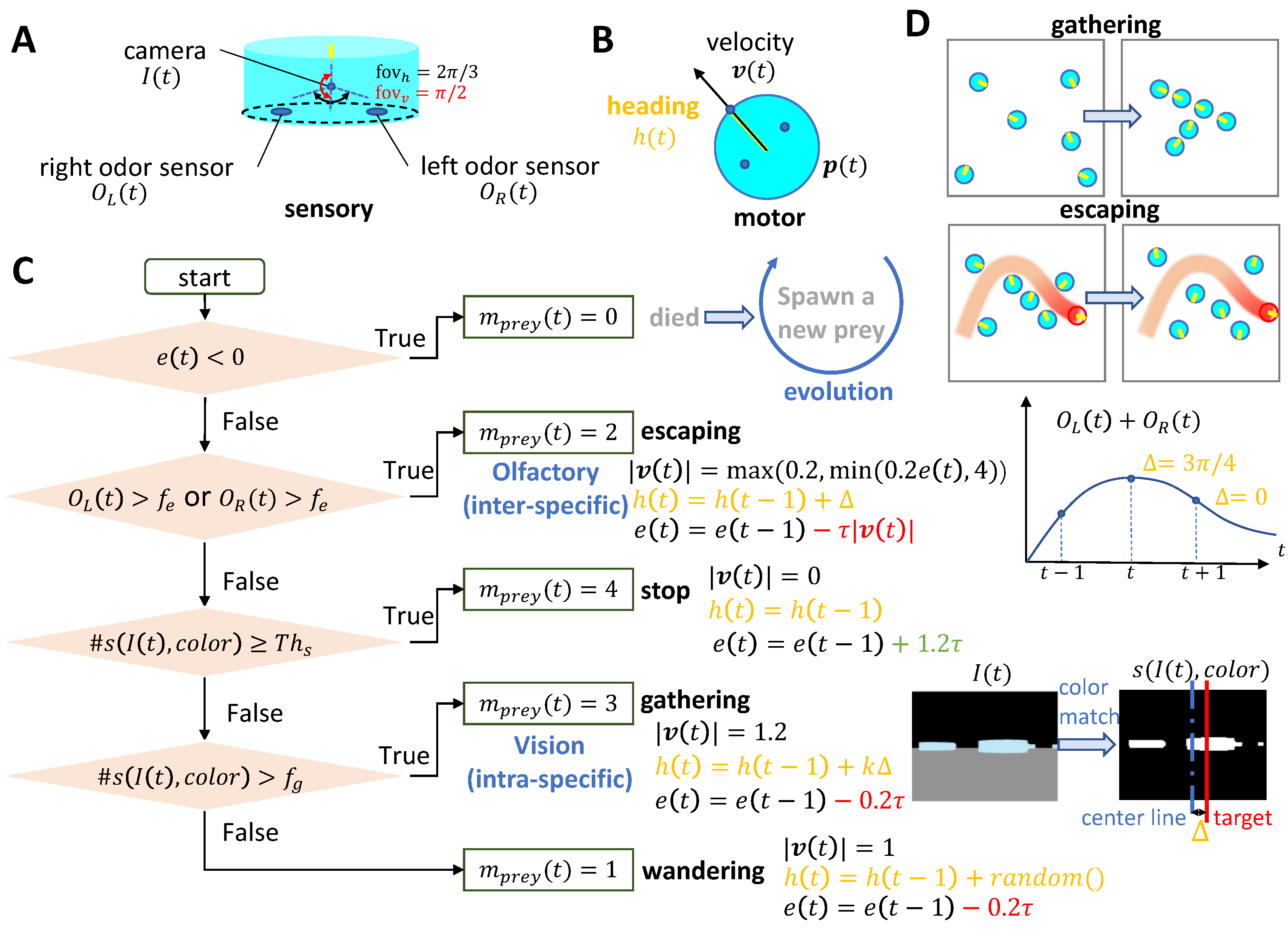
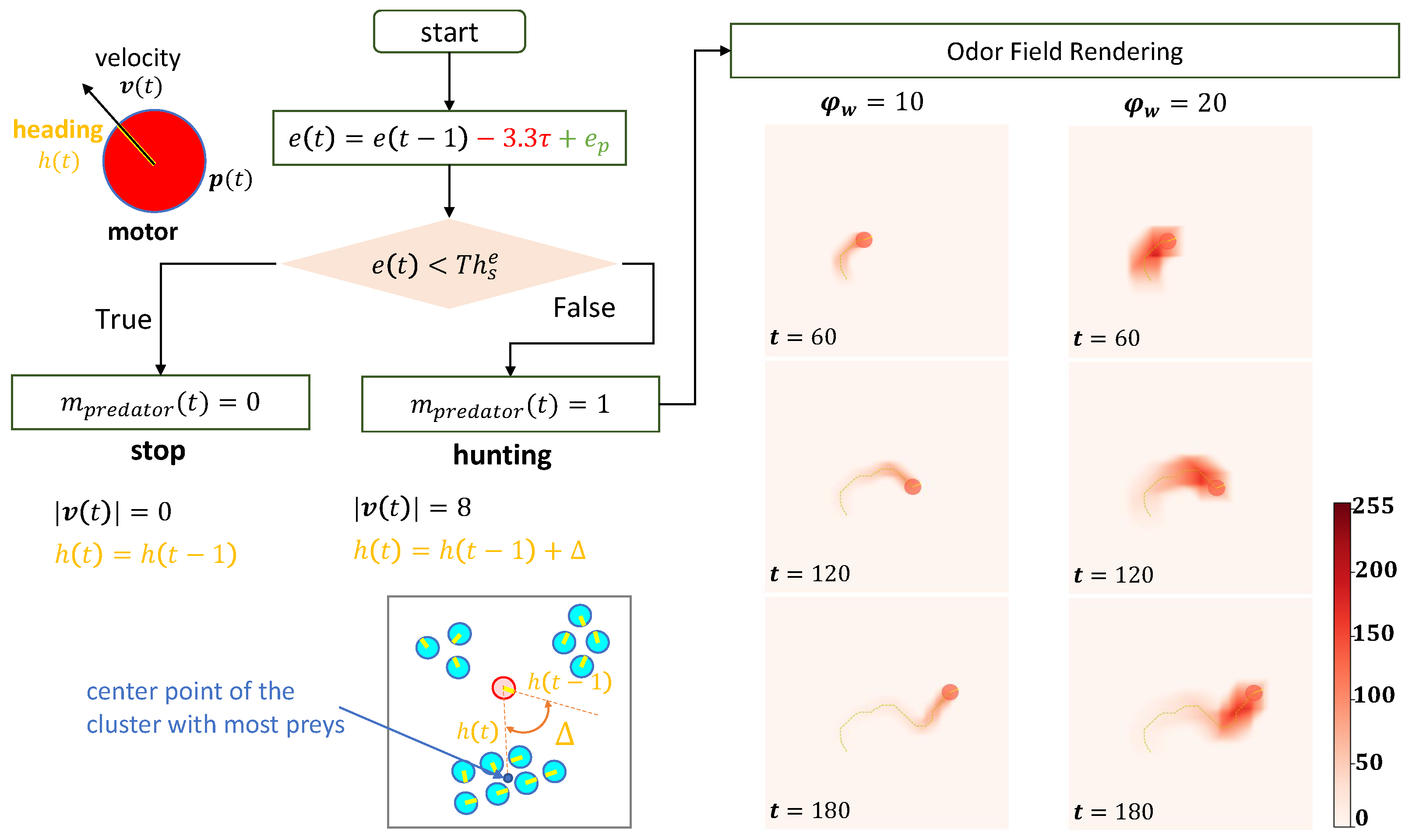
2. The Devised Prey-Predator Interaction with Multi-Modal and Evolutionary Processing
2.1. Computer Simulation
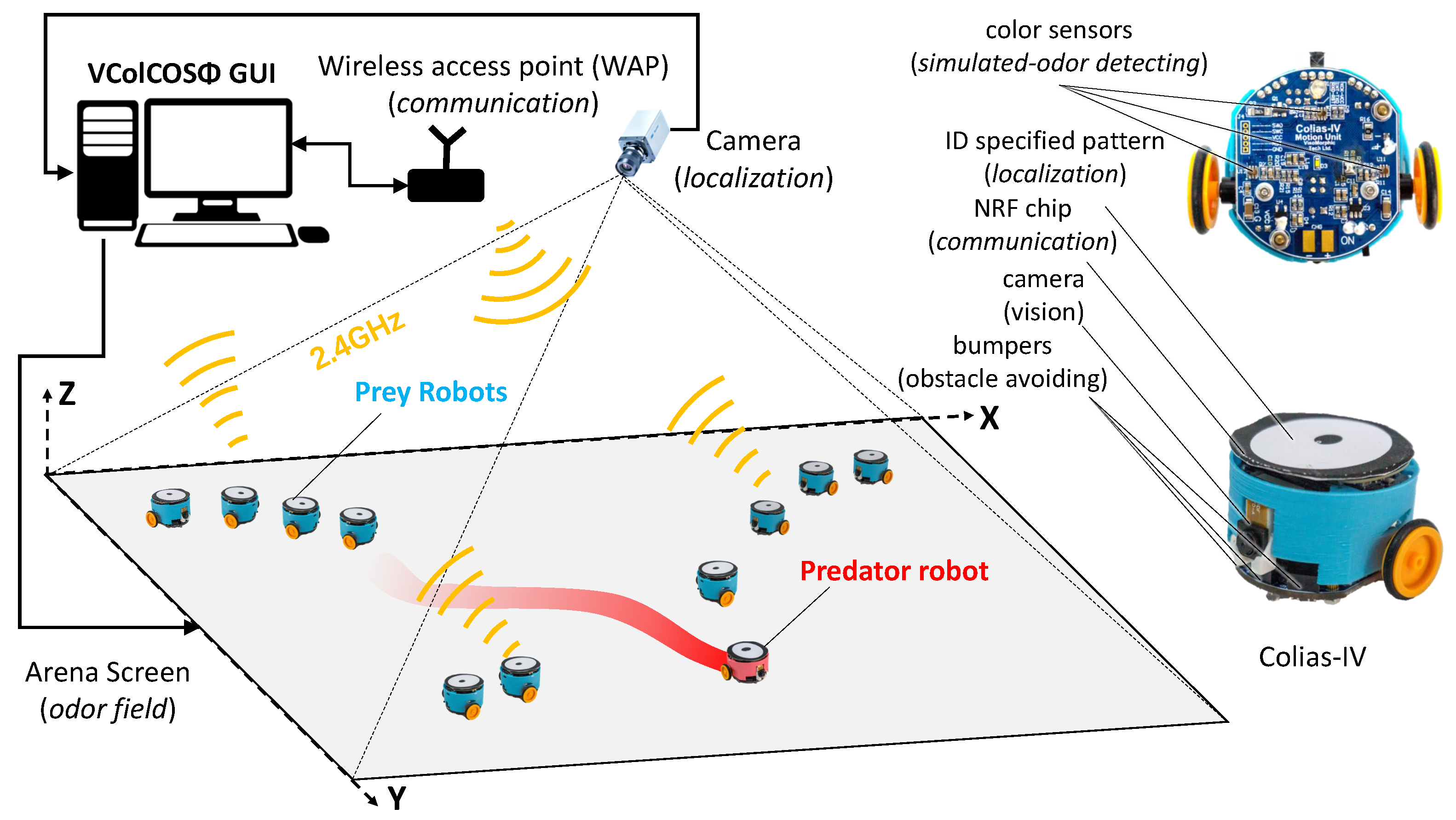
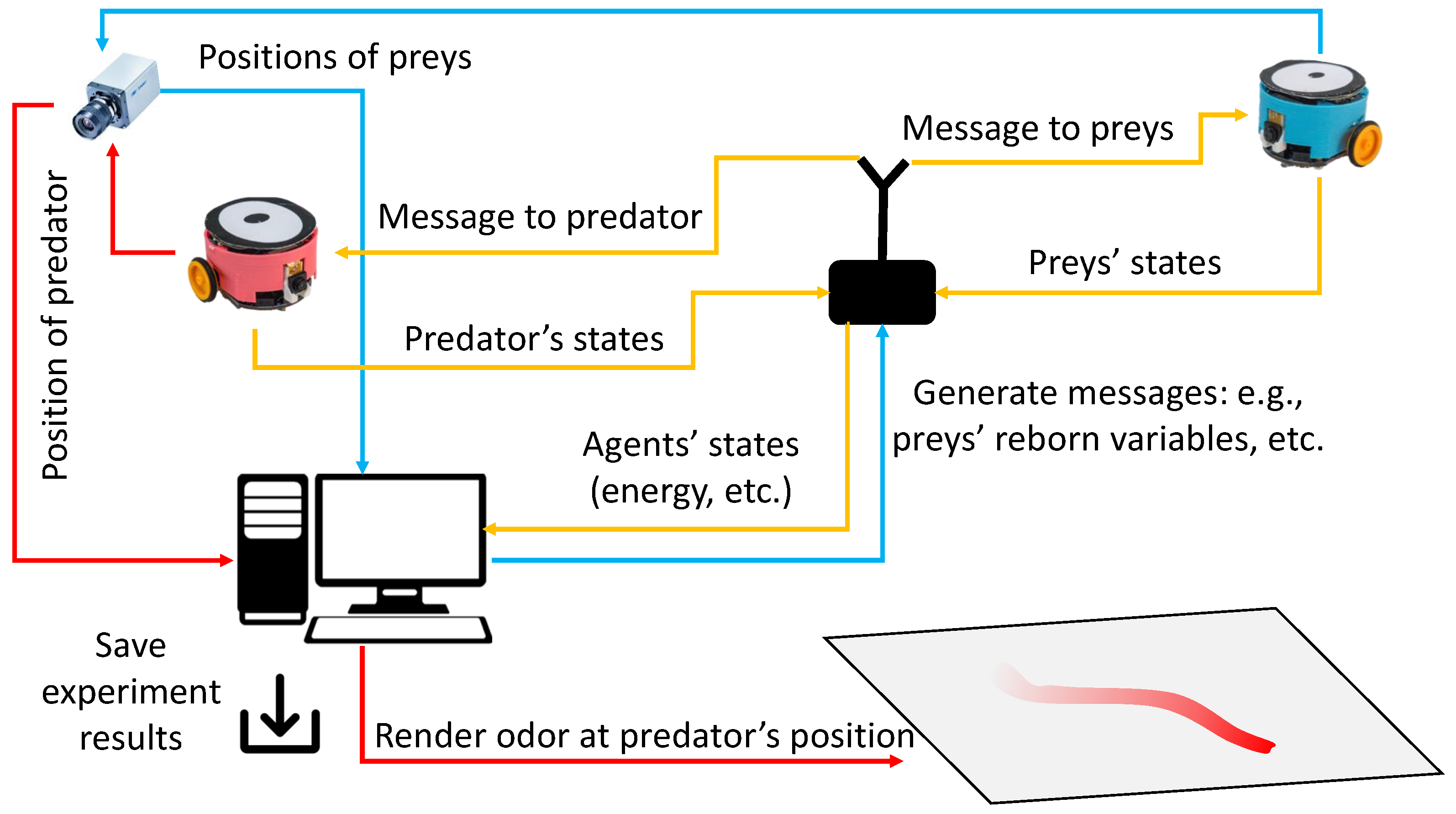
2.2. Real Robot Implementation
3. Results of Computer Simulation
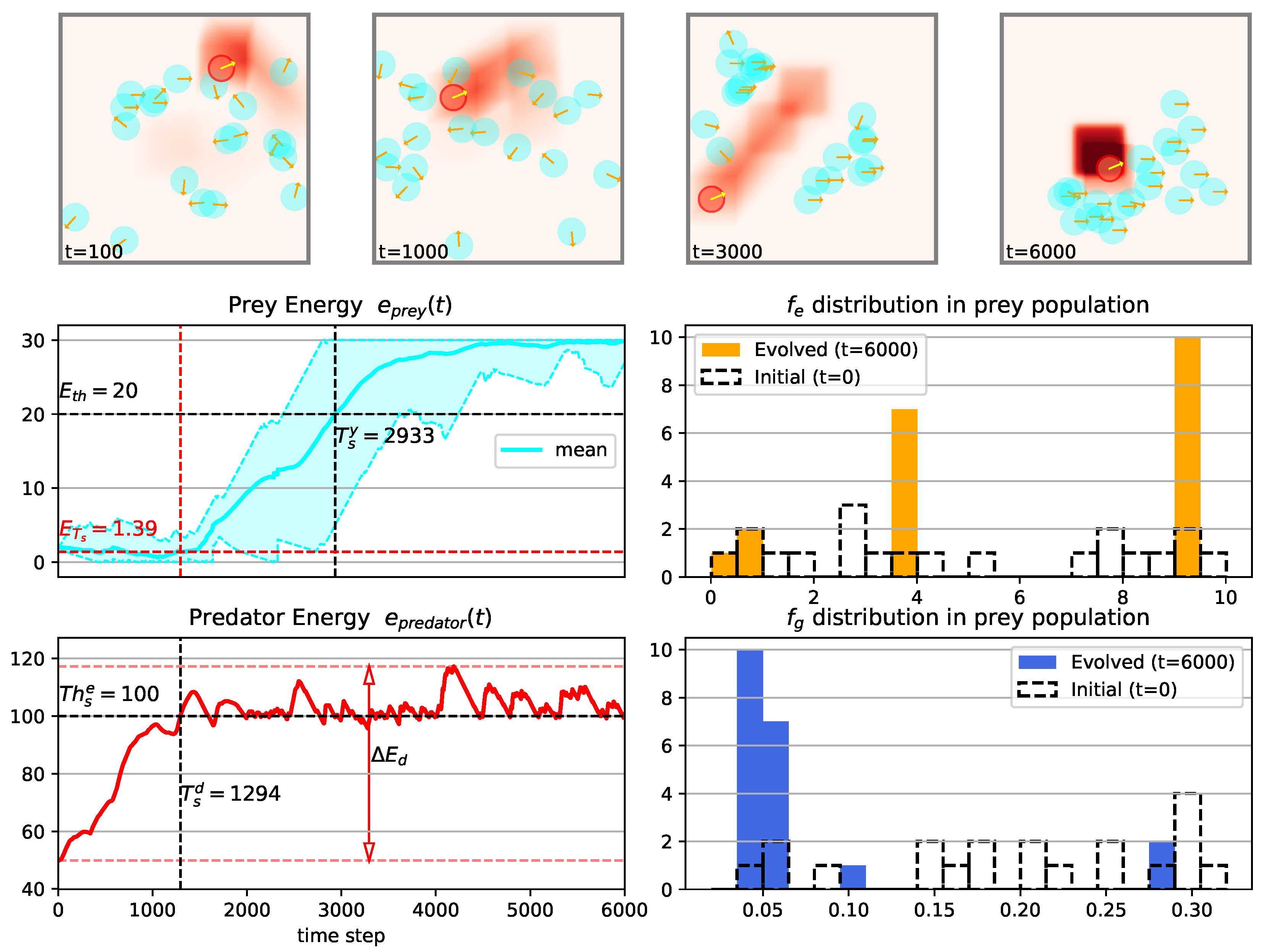
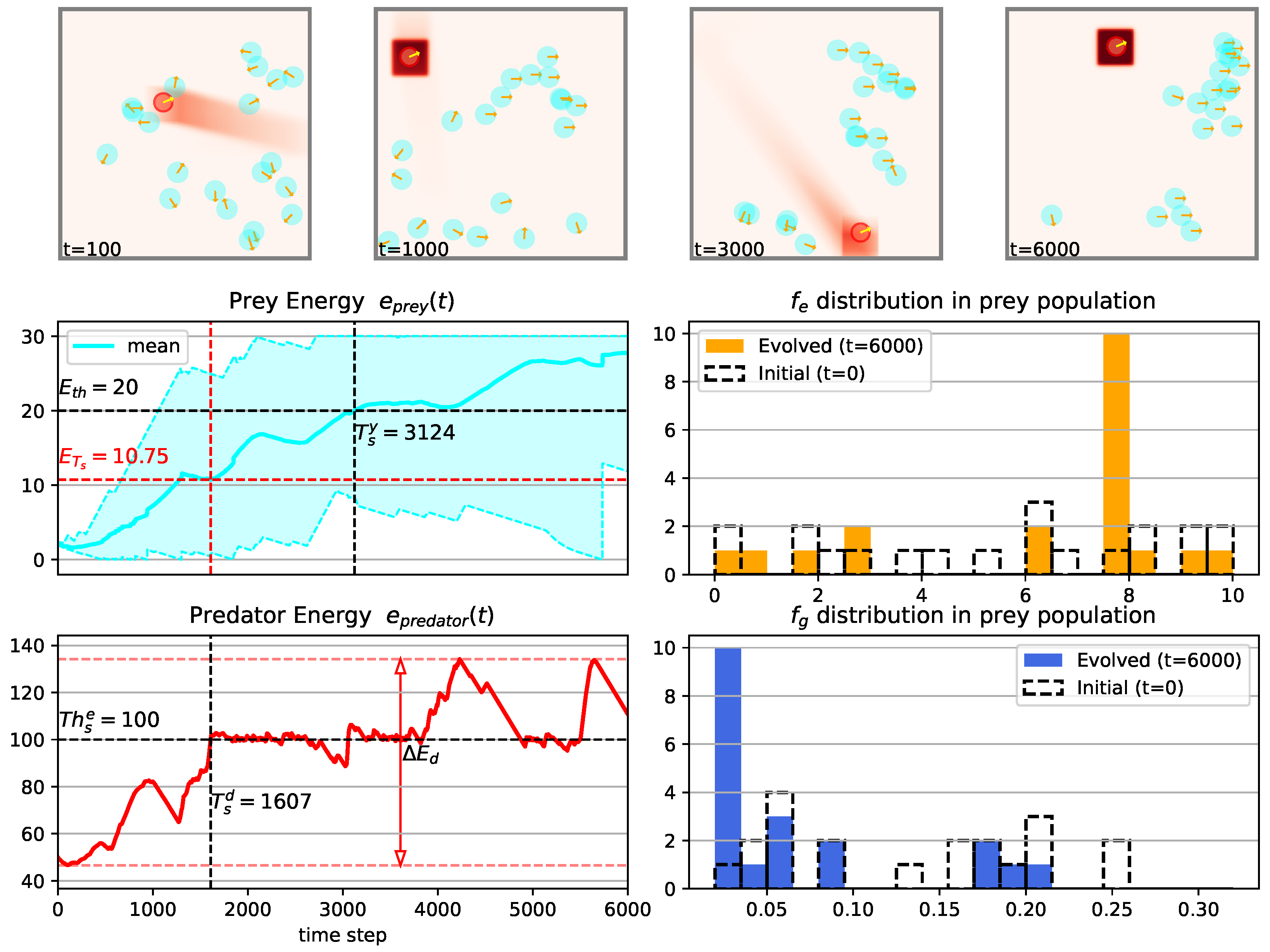
3.1. Temporal Dynamics of Population Energy
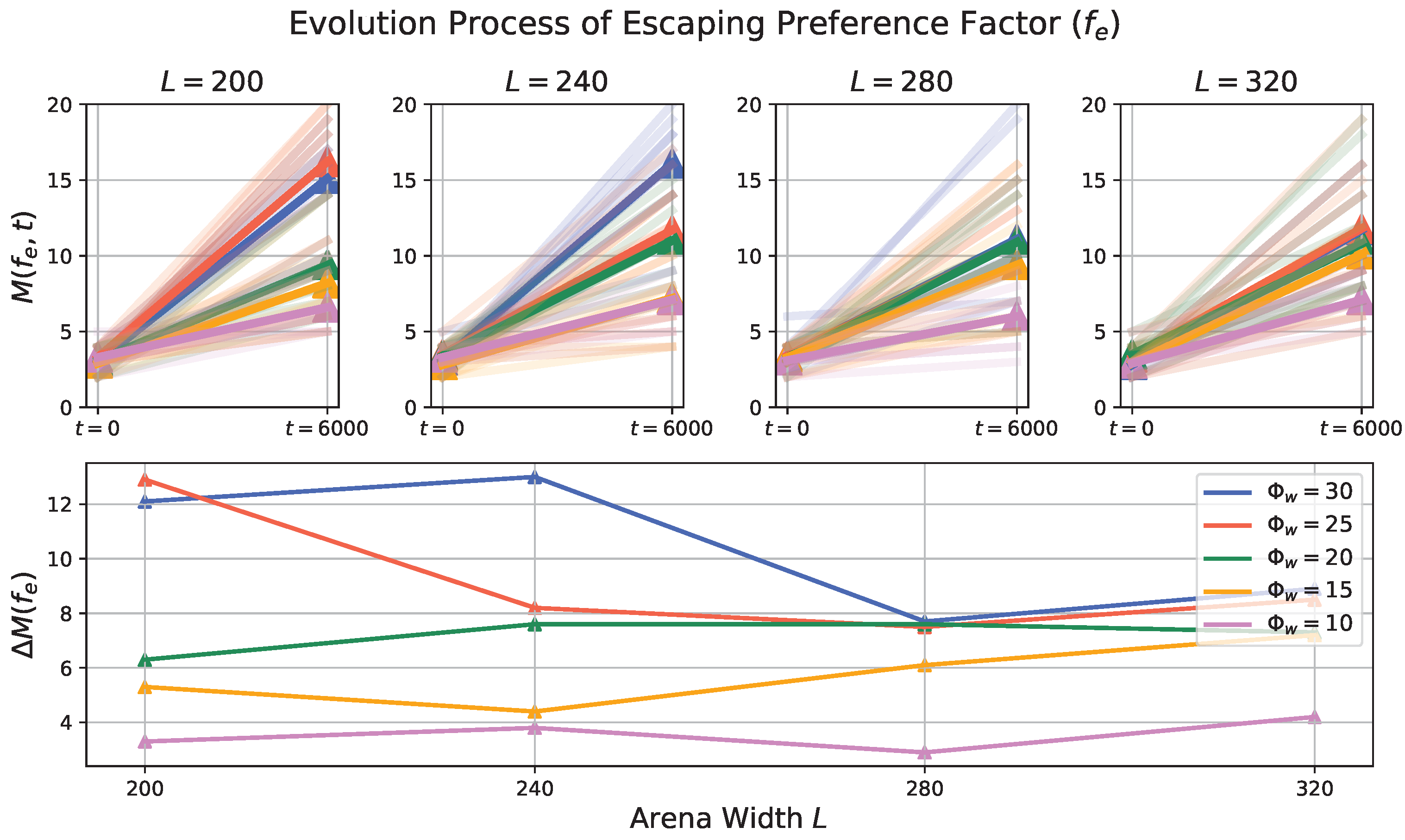
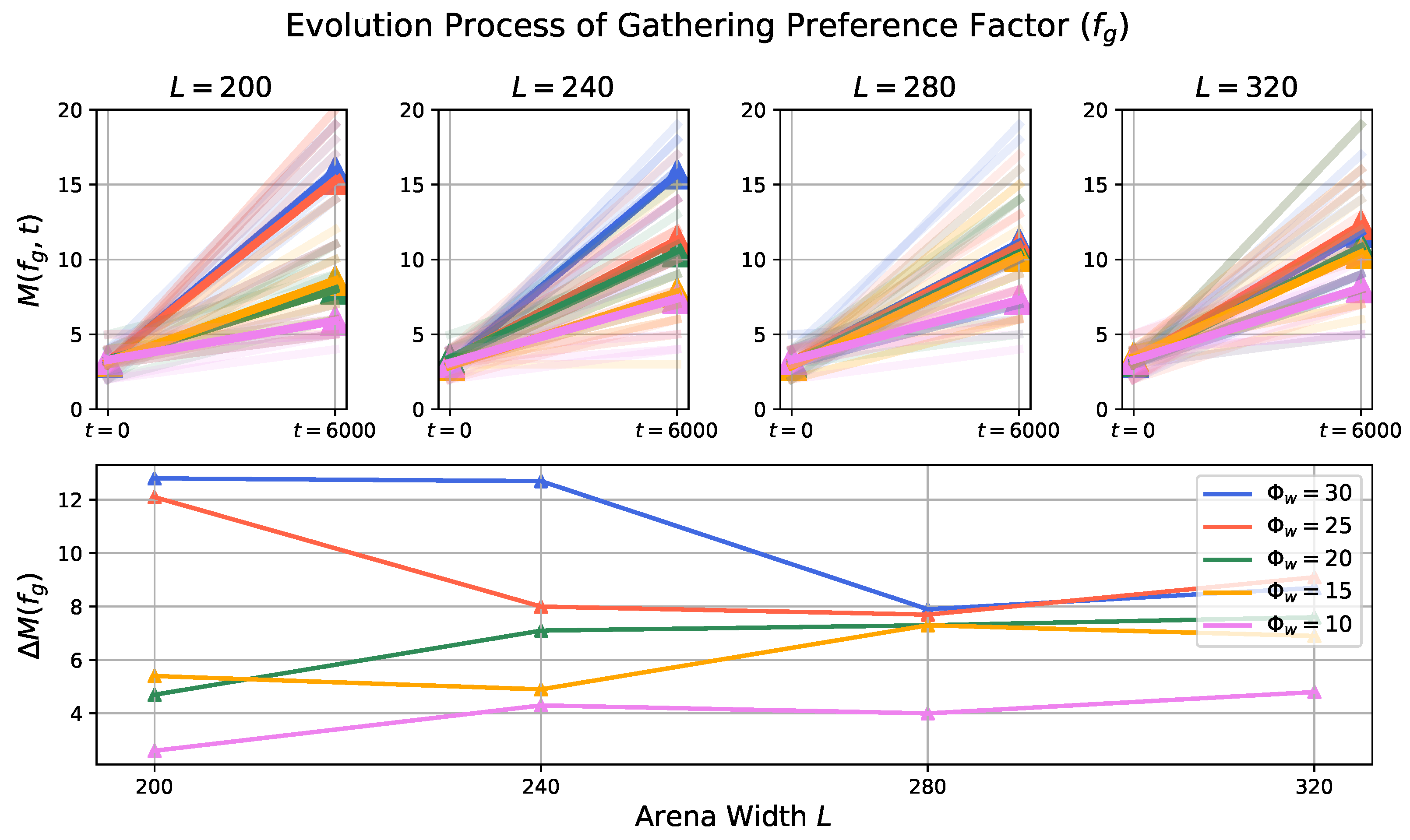
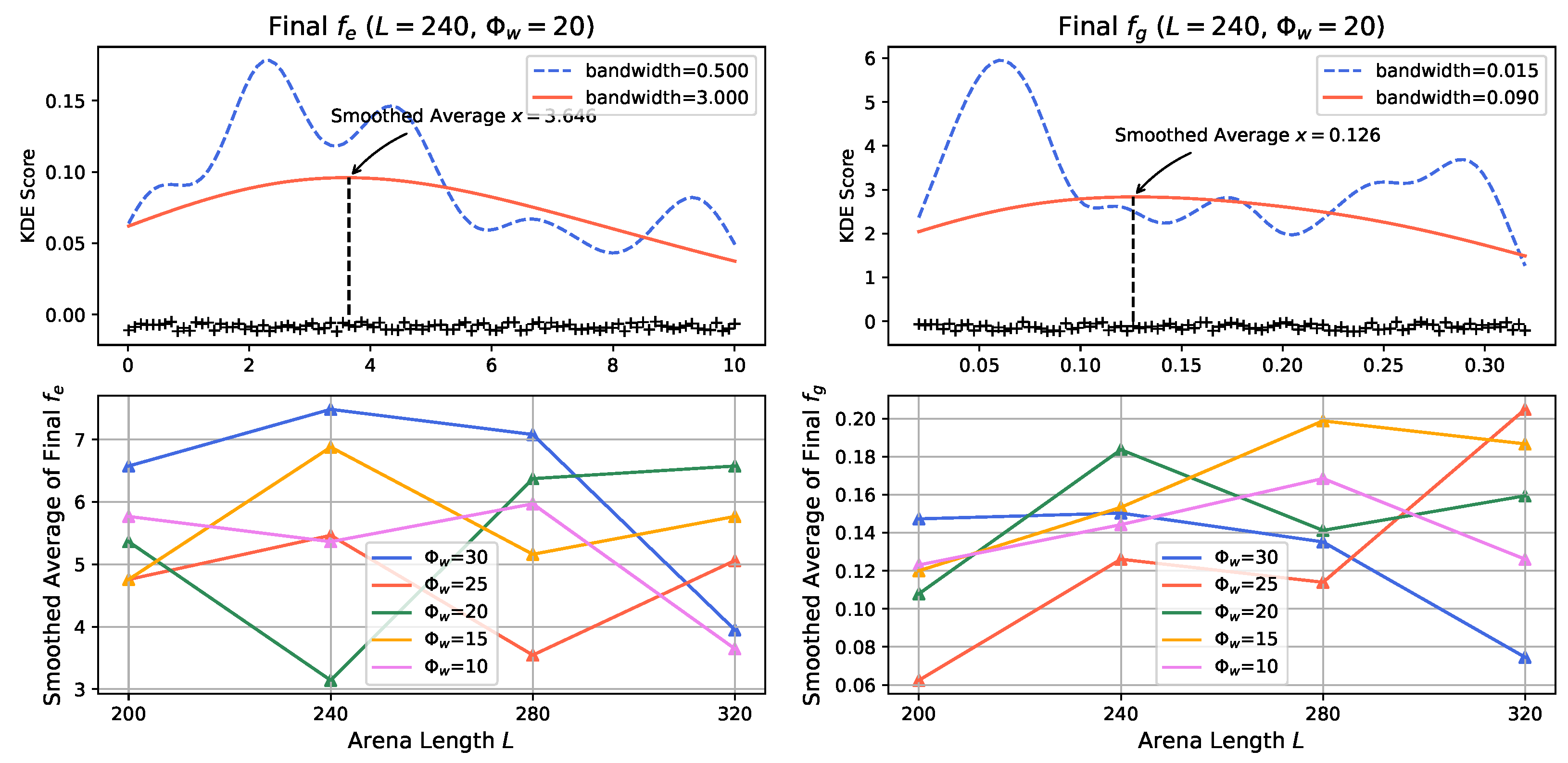
3.2. Evolution Process in Prey Population
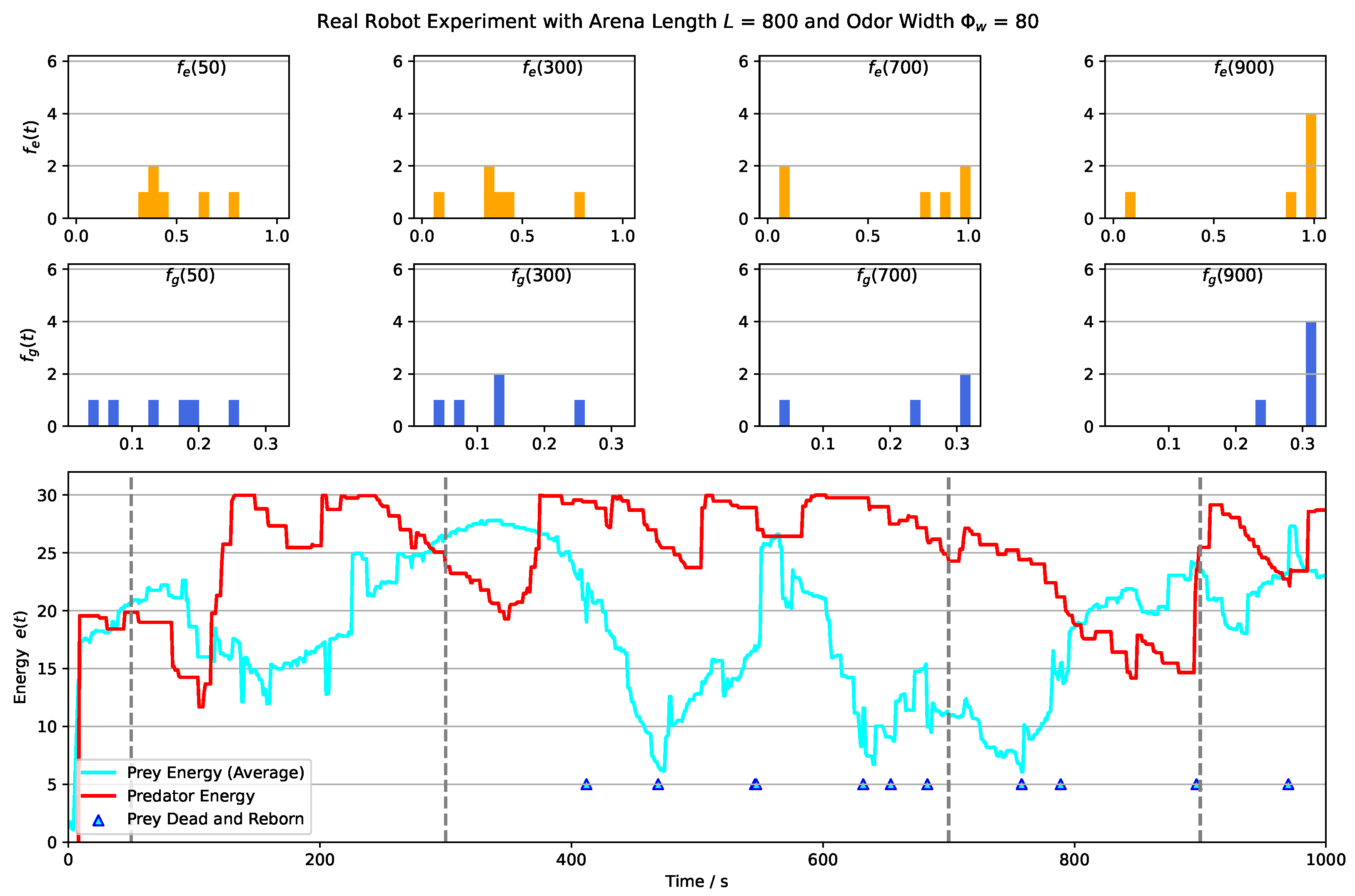
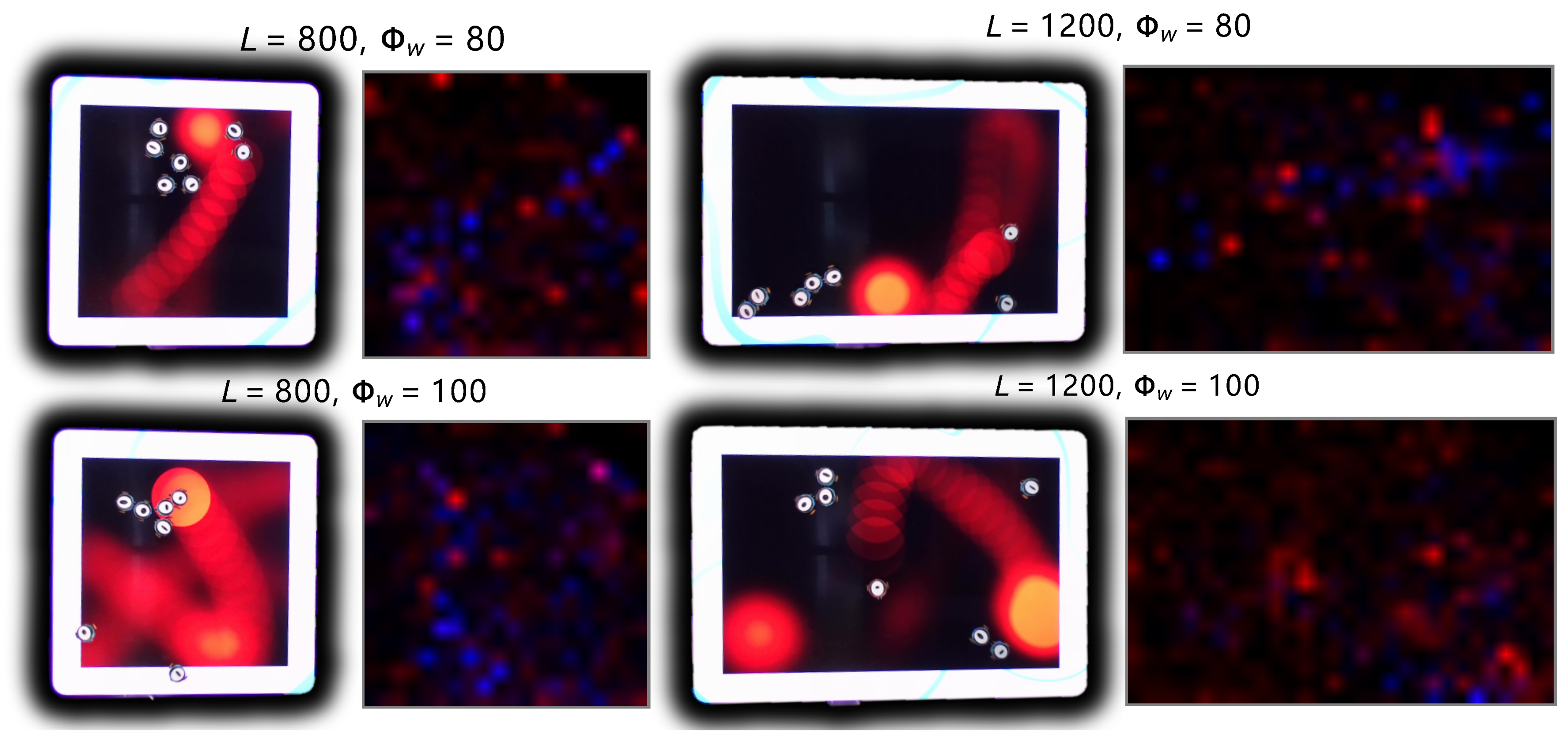
4. Robot Experiments of the Prey-Predator Interaction
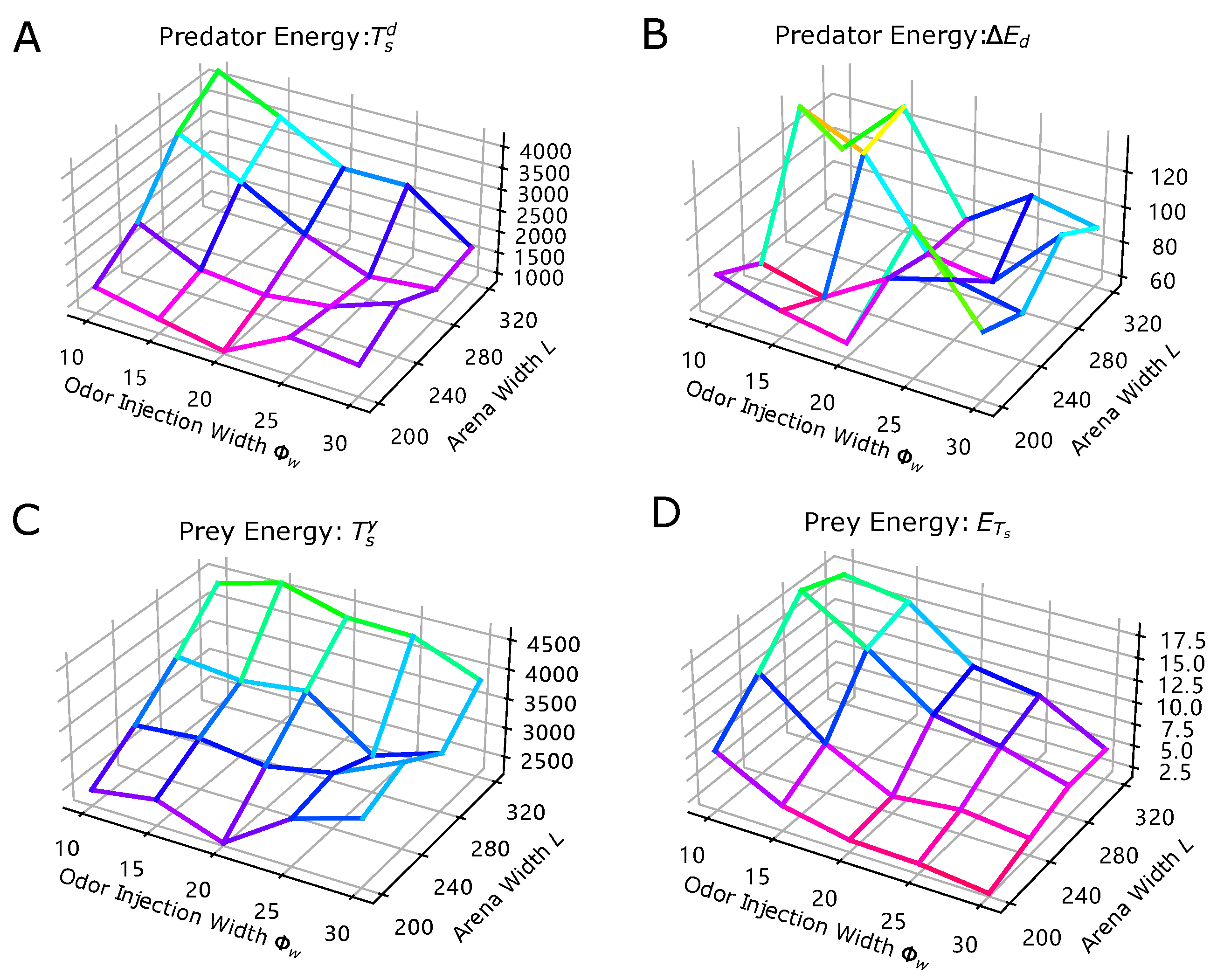
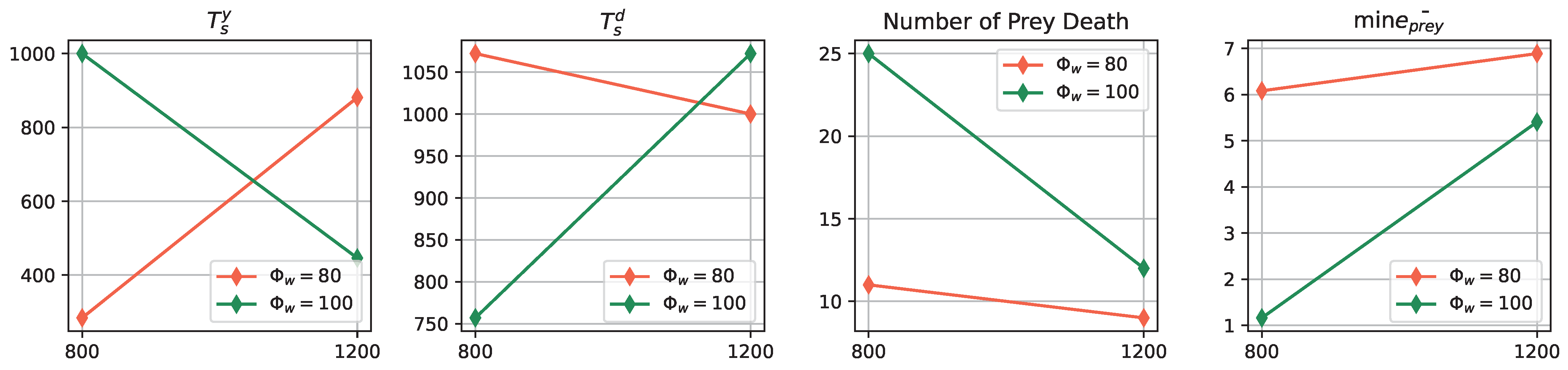
- Impact of Odor Width: The time taken for both the prey and predator populations to reach the defined energy levels displays varying trends based on different odor widths. This suggests that the odor width has a significant impact on the energy dynamics of the populations. The inconsistent tendencies observed could arise from factors such as the Allee effect [12], which pertains to population growth being negatively impacted at low densities. In this context, with a population of only 6 preys in the arena, the sparse distribution might lead to the observed dynamics.
- Effect of Environment on Prey Population: The number of prey deaths and the minimal prey energy levels show consistent trends across different environmental settings. Specifically, more challenging environments, such as smaller arenas with larger odor widths, lead to a greater number of prey deaths and lower minimal prey energy levels. This pattern indicates that more challenging conditions place higher selective pressure on the prey population, resulting in increased mortality and lower energy levels.
5. Conclusions and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Detailed Methods
Appendix A.1. Agents
Appendix A.2. Environment Settings in Simulation
Appendix A.2.1. Population Energy
Appendix A.2.2. Predator’s Odor Field
Appendix A.2.3. Prey’s Evolution
Appendix A.2.4. Parameters
| Category | Parameter | Value | Description |
|---|---|---|---|
| Environment | L | 200, 240, 280, 320 | environmental factor |
| Predator | (Figure 3) | 10, 15, 20, 25, 30 | prey-predator interaction factor |
| (Equation (A7)) | 100 | odor field | |
| (Equation (A9)) | 0.99 | ||
| (Equation (A11)) | 1000 | ||
| 1 | the number of preys is constant | ||
| (Figure 3) | 100 | threshold of energy for stop hunting | |
| 50 | initial energy of predator population | ||
| 8 | |||
| Prey | 20 | the number of preys is constant | |
| 2 | initial energy of prey population | ||
| initial value is randomly generated | |||
| Figure 2 | threshold (percent of image pixels) for stop gathering |
References
- Agrawal, A.A. Phenotypic plasticity in the interactions and evolution of species. Science 2001, 294, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Legreneur, P.; Laurin, M.; Bels, V. Predator–prey interactions paradigm: A new tool for artificial intelligence. Adapt. Behav. 2012, 20, 3–9. [Google Scholar] [CrossRef]
- Terborgh, J.; Estes, J.A. Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Abrams, P.A. The evolution of predator-prey interactions: Theory and evidence. Annu. Rev. Ecol. Syst. 2000, 31, 79–105. [Google Scholar] [CrossRef]
- Schmitz, O.J.; Grabowski, J.H.; Peckarsky, B.L.; Preisser, E.L.; Trussell, G.C.; Vonesh, J.R. From individuals to ecosystem function: Toward an integration of evolutionary and ecosystem ecology. Ecology 2008, 89, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, J.J.; Whitlatch, R.B.; Osman, R.W. Species diversity and invasion resistance in a marine ecosystem. Science 1999, 286, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Preisser, E.L.; Bolnick, D.I. The many faces of fear: Comparing the pathways and impacts of nonconsumptive predator effects on prey populations. PLoS ONE 2008, 3, e2465. [Google Scholar] [CrossRef]
- Ryberg, W.A.; Smith, K.G.; Chase, J.M. Predators alter the scaling of diversity in prey metacommunities. Oikos 2012, 121, 1995–2000. [Google Scholar] [CrossRef]
- Lotka, A.J. Elements of Physical Biology; Williams & Wilkins: Philadelphia, PA, USA, 1925. [Google Scholar]
- Wentz, R. Mathematical Models of Predator-Prey Interactions. Ph.D. Thesis, Millersville University of Pennsylvania, Mathematics Department, Millersville, PA, USA, 2002. [Google Scholar]
- Mondal, B.; Roy, S.; Ghosh, U.; Tiwari, P.K. A systematic study of autonomous and nonautonomous predator–prey models for the combined effects of fear, refuge, cooperation and harvesting. Eur. Phys. J. Plus 2022, 137, 724. [Google Scholar] [CrossRef]
- Kumbhakar, R.; Pal, S.; Pal, N.; Tiwari, P.K. Bistability and tristability in a predator–prey model with strong Allee effect in prey. J. Biol. Syst. 2023, 31, 215–243. [Google Scholar] [CrossRef]
- Maity, S.S.; Tiwari, P.K.; Shuai, Z.; Pal, S. Role of Space in an Eco-Epidemic Predator-Prey System with the Effect of Fear and Selective Predation. J. Biol. Syst. 2023, 31, 883–920. [Google Scholar] [CrossRef]
- Ito, T.; Pilat, M.L.; Suzuki, R.; Arita, T. Population and evolutionary dynamics based on predator–prey relationships in a 3d physical simulation. Artif. Life 2016, 22, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Baggio, J.A.; Salau, K.; Janssen, M.A.; Schoon, M.L.; Bodin, Ö. Landscape connectivity and predator–prey population dynamics. Landsc. Ecol. 2011, 26, 33–45. [Google Scholar] [CrossRef]
- Floreano, D.; Keller, L. Evolution of adaptive behaviour in robots by means of Darwinian selection. PLoS Biol. 2010, 8, e1000292. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.; Vorobyev, M. A review of the evolution of animal colour vision and visual communication signals. Vis. Res. 2008, 48, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Galizia, C.G.; Nouvian, M. Olfactory strategies in the defensive behaviour of insects. Insects 2022, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Brossette, L.; Lefloch, L.; Dupont, S.; Christidès, J.P.; Bagnères, A.G. When predator odour makes groups stronger: Effects on behavioural and chemical adaptations in two termite species. Ecol. Entomol. 2018, 43, 513–524. [Google Scholar] [CrossRef]
- Despland, E. Role of olfactory and visual cues in the attraction/repulsion responses to conspecifics by gregarious and solitarious desert locusts. J. Insect Behav. 2001, 14, 35–46. [Google Scholar] [CrossRef]
- Reisenman, C.E.; Lorenzo Figueiras, A.N.; Giurfa, M.; Lazzari, C.R. Interaction of visual and olfactory cues in the aggregation behaviour of the haematophagous bug Triatoma infestans. J. Comp. Physiol. A 2000, 186, 961–968. [Google Scholar] [CrossRef]
- Bouquet, F.; Chipeaux, S.; Lang, C.; Marilleau, N.; Nicod, J.M.; Taillandier, P. Introduction to the agent approach. In Agent-Based Spatial Simulation with NetLogo; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–28. [Google Scholar]
- Li, J.; Li, L.; Zhao, S. Predator–prey survival pressure is sufficient to evolve swarming behaviors. New J. Phys. 2023, 25, 092001. [Google Scholar] [CrossRef]
- Sun, X.; Liu, T.; Hu, C.; Fu, Q.; Yue, S. Colcos φ: A multiple pheromone communication system for swarm robotics and social insects research. In Proceedings of the 2019 IEEE 4th International Conference on Advanced Robotics and Mechatronics (ICARM), Toyonaka, Japan, 3–5 July 2019; pp. 59–66. [Google Scholar]
- Liu, T.; Sun, X.; Hu, C.; Fu, Q.; Yue, S. A multiple pheromone communication system for swarm intelligence. IEEE Access 2021, 9, 148721–148737. [Google Scholar] [CrossRef]
- Liu, T.; Sun, X.; Hu, C.; Fu, Q.; Yue, S. A Versatile Vision-Pheromone-Communication Platform for Swarm Robotics. In Proceedings of the 2021 IEEE International Conference on Robotics and Automation (ICRA), Xi’an, China, 30 May–5 June 2021; pp. 7261–7266. [Google Scholar]
- Das, A.K.; Fierro, R.; Kumar, V.; Ostrowski, J.P.; Spletzer, J.; Taylor, C.J. A vision-based formation control framework. IEEE Trans. Robot. Autom. 2002, 18, 813–825. [Google Scholar] [CrossRef]
- Hu, C.; Fu, Q.; Liu, T.; Yue, S. A hybrid visual-model based robot control strategy for micro ground robots. In Animals to Animats 15, Proceedings of the 15th International Conference on Simulation of Adaptive Behavior, SAB 2018, Frankfurt/Main, Germany, 14–17 August 2018; Proceedings 15; Springer: Berlin/Heidelberg, Germany, 2018; pp. 162–174. [Google Scholar]
- O’Bryne, C. Emotions, Motivation-Based Action Selection and Dynamic Environments; University of Hertfordshire: Hatfield, UK, 2013. [Google Scholar]
- Webb, B. Can robots make good models of biological behaviour? Behav. Brain Sci. 2001, 24, 1033–1050. [Google Scholar] [CrossRef] [PubMed]
- Gravish, N.; Lauder, G.V. Robotics-inspired biology. J. Exp. Biol. 2018, 221, jeb138438. [Google Scholar]
- Feynman, R.P. Simulating physics with computers. Int. J. Theor. Phys. 2018, 21. [Google Scholar]
- Krause, J.; Winfield, A.F.; Deneubourg, J.L. Interactive robots in experimental biology. Trends Ecol. Evol. 2011, 26, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, G.; van Laake, L.C.; Carissimo, C.; Miette, C.; Overvelde, J.T. Continuous learning of emergent behavior in robotic matter. Proc. Natl. Acad. Sci. USA 2021, 118, e2017015118. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Marin, A.; Zhang, Y. Emergent Behavior in Animal-Inspired Robotics. Front. Neurorobotics 2022, 16, 861831. [Google Scholar] [CrossRef]
- Duffy, B.R.; Joue, G. Intelligent Robots: The Question of Embodiment. 2000. Available online: https://www.researchgate.net/publication/228375813_Intelligent_robots_The_question_of_embodiment (accessed on 8 May 2023).
- Pfeifer, R.; Lungarella, M.; Iida, F. Self-Organization, Embodiment, and Biologically Inspired Robotics. Science 2007, 318, 1088–1093. [Google Scholar] [CrossRef]
- Webb, B. What does robotics offer animal behaviour? Anim. Behav. 2000, 60, 545–558. [Google Scholar] [CrossRef]
- Ramdya, P.; Ijspeert, A.J. The neuromechanics of animal locomotion: From biology to robotics and back. Sci. Robot. 2023, 8, eadg0279. [Google Scholar] [CrossRef]
- Ijspeert, A.J. Biorobotics: Using robots to emulate and investigate agile locomotion. Science 2014, 346, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ravi, S.; Wang, C. Editorial: Robotics to Understand Animal Behaviour. Front. Robot. AI 2022, 9, 963416. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Juusola, M. A biomimetic fly photoreceptor model elucidates how stochastic adaptive quantal sampling provides a large dynamic range. J. Physiol. 2017, 595, 5439–5456. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Cope, A.J.; Nikolaidis, E.; Marshall, J.A.; Sabo, C. ARK: Augmented reality for Kilobots. IEEE Robot. Autom. Lett. 2017, 2, 1755–1761. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Hu, C.; Liu, T.; Yue, S.; Peng, J.; Fu, Q. Translating Virtual Prey-Predator Interaction to Real-World Robotic Environments: Enabling Multimodal Sensing and Evolutionary Dynamics. Biomimetics 2023, 8, 580. https://doi.org/10.3390/biomimetics8080580
Sun X, Hu C, Liu T, Yue S, Peng J, Fu Q. Translating Virtual Prey-Predator Interaction to Real-World Robotic Environments: Enabling Multimodal Sensing and Evolutionary Dynamics. Biomimetics. 2023; 8(8):580. https://doi.org/10.3390/biomimetics8080580
Chicago/Turabian StyleSun, Xuelong, Cheng Hu, Tian Liu, Shigang Yue, Jigen Peng, and Qinbing Fu. 2023. "Translating Virtual Prey-Predator Interaction to Real-World Robotic Environments: Enabling Multimodal Sensing and Evolutionary Dynamics" Biomimetics 8, no. 8: 580. https://doi.org/10.3390/biomimetics8080580
APA StyleSun, X., Hu, C., Liu, T., Yue, S., Peng, J., & Fu, Q. (2023). Translating Virtual Prey-Predator Interaction to Real-World Robotic Environments: Enabling Multimodal Sensing and Evolutionary Dynamics. Biomimetics, 8(8), 580. https://doi.org/10.3390/biomimetics8080580






