Biomimetic Surface Engineering to Modulate the Coffee-Ring Effect for Amyloid-β Detection in Rat Brains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Gelsolin-Modified AuNPs (Gel-AuNPs)
2.2. Design of the Coffee-Ring-Effect-Based Assays
2.3. Animal Experiments
3. Results
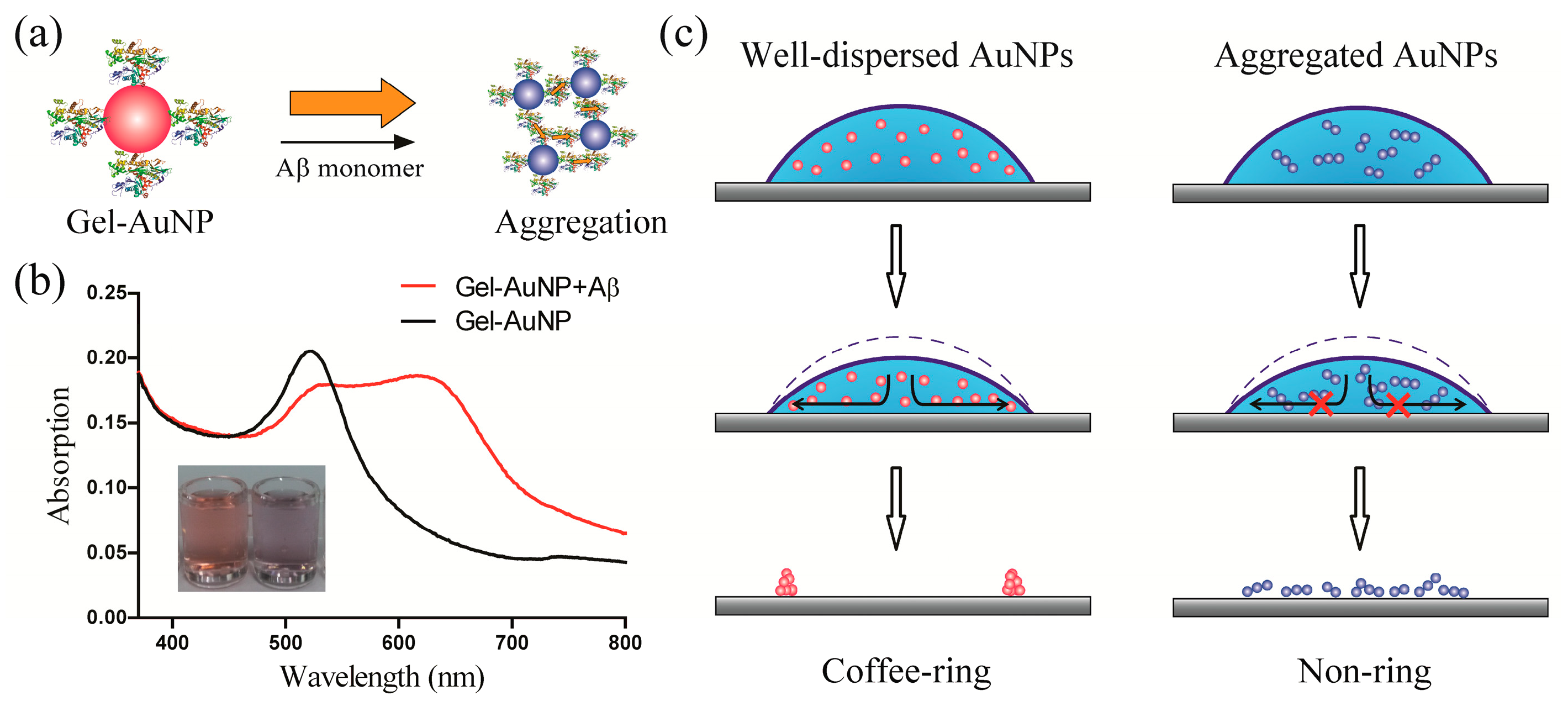
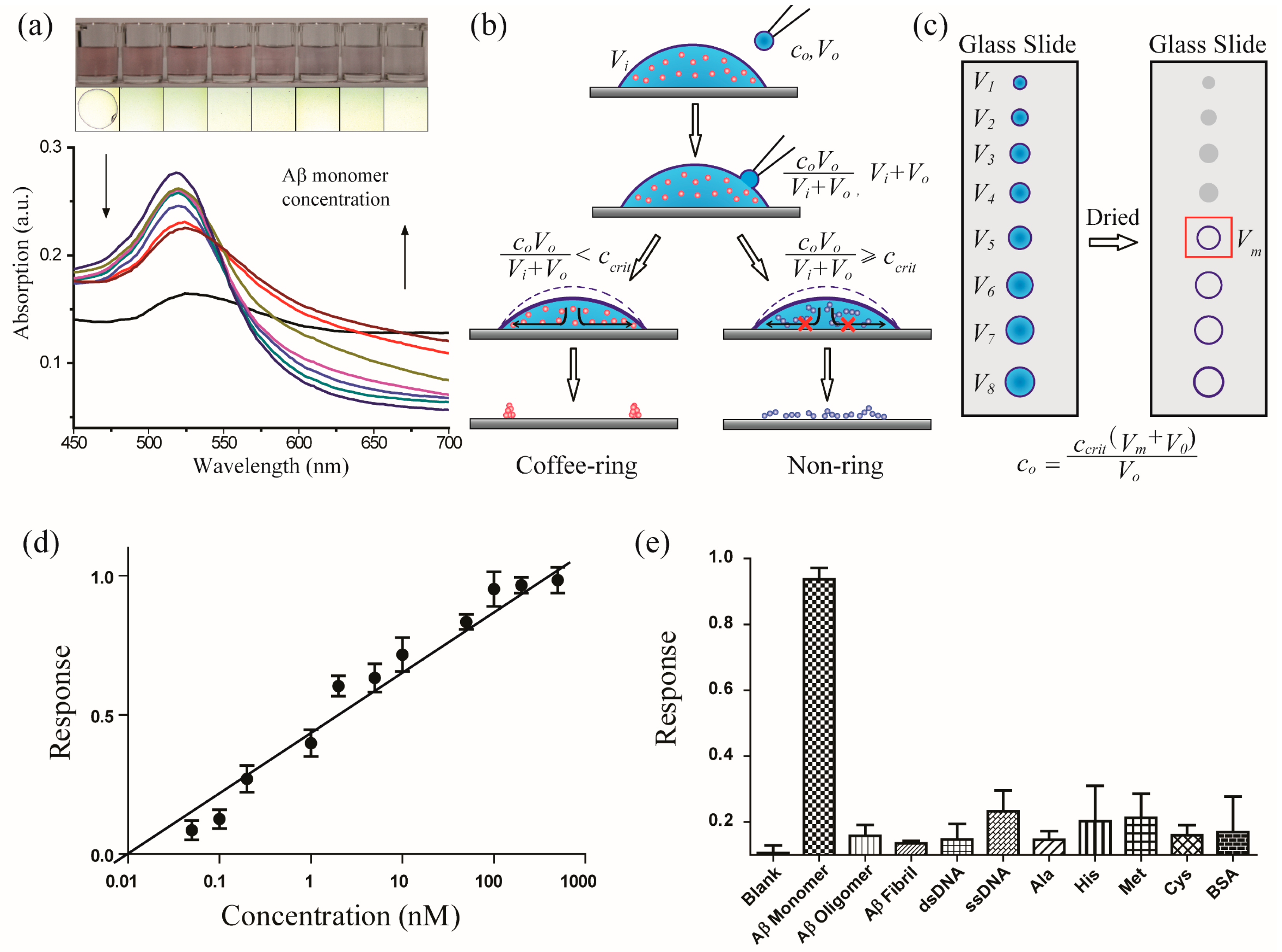
3.1. Design of the Coffee-Ring-Effect-Based Sensing Platform
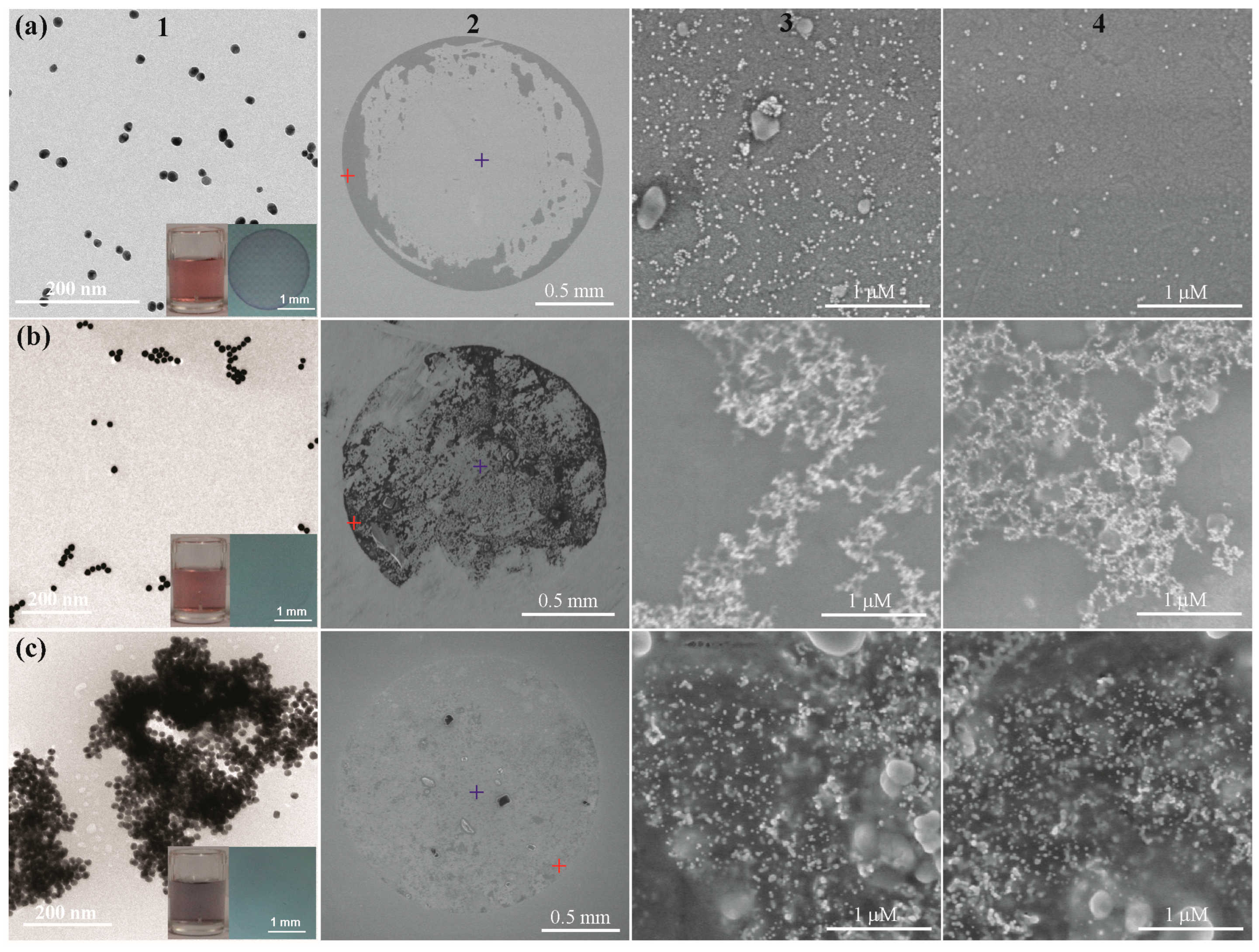
3.2. Mechanism of the Coffee-Ring Effect of AuNPs
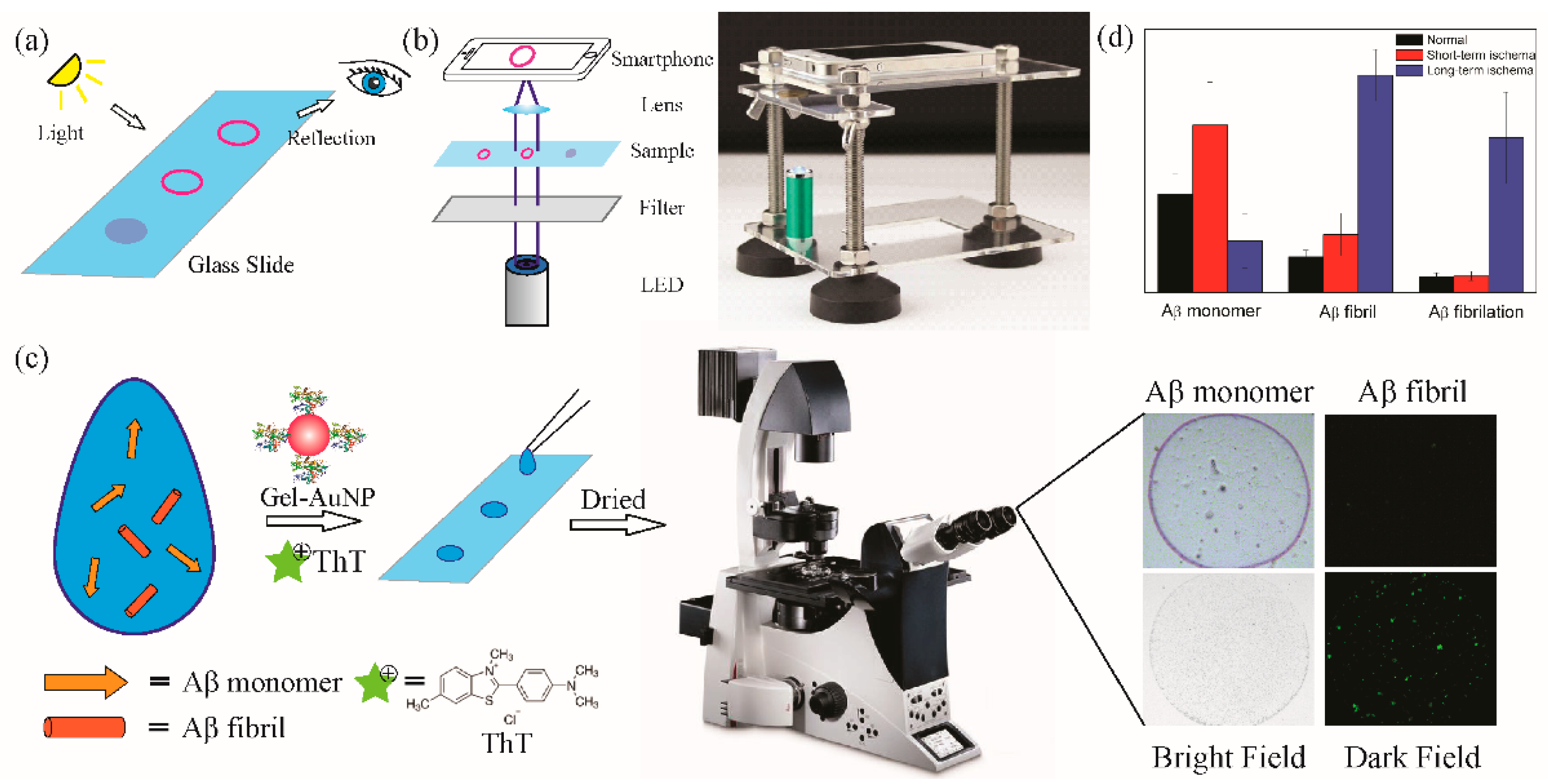
3.3. Animal Models and Real Sample Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the Coffee-Ring Effect by Shape-Dependent Capillary Interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Chen, T.H.; Shen, X.; Ho, C.M. Nanochromatography Driven by the Coffee Ring Effect. Anal. Chem. 2011, 83, 1871–1873. [Google Scholar] [CrossRef] [PubMed]
- Anyfantakis, M.; Baigl, D. Dynamic Photocontrol of the Coffee-Ring Effect with Optically Tunable Particle Stickiness. Angew. Chem. Int. Ed. 2014, 53, 14077–14081. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Stassi, S.S.; Pisano, A.P.A.P.; Zohdi, T.I.T.I. Coffee-Ring Effect-Based Three Dimensional Patterning of Micro/Nanoparticle Assembly with a Single Droplet. Langmuir 2010, 26, 11690–11698. [Google Scholar] [CrossRef] [PubMed]
- Soltman, D.; Subramanian, V. Inkjet-Printed Line Morphologies and Temperature Control of the Coffee Ring Effect. Langmuir 2008, 24, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Sun, D.-W.; Pu, H. SERS Detection of Urea and Ammonium Sulfate Adulterants in Milk with Coffee Ring Effect. Food Addit. Contam. Part A 2019, 36, 851–862. [Google Scholar] [CrossRef]
- Yukird, J.; Kaminsky, C.J.; Chailapakul, O.; Rodthongkum, N.; Vachet, R.W. Enhanced and Selective MALDI-MS Detection of Peptides via the Nanomaterial-Dependent Coffee Ring Effect. J. Am. Soc. Mass Spectrom. 2021, 32, 1780–1788. [Google Scholar] [CrossRef]
- Huang, C.; Wang, J.; Lv, X.; Liu, L.; Liang, L.; Hu, W.; Luo, C.; Wang, F.; Yuan, Q. Redefining Molecular Amphipathicity in Reversing the “Coffee-Ring Effect”: Implications for Single Base Mutation Detection. Langmuir 2018, 34, 6777–6783. [Google Scholar] [CrossRef]
- Ng, T.P.T.; Saltin, S.H.; Davies, M.S.; Johannesson, K.; Stafford, R.; Williams, G.A. Snails and Their Trails: The Multiple Functions of Trail-Following in Gastropods. Biol. Rev. 2013, 88, 683–700. [Google Scholar] [CrossRef]
- Qu, A.; Sun, M.; Xu, L.; Hao, C.; Wu, X.; Xu, C.; Kotov, N.A.; Kuang, H. Quantitative Zeptomolar Imaging of MiRNA Cancer Markers with Nanoparticle Assemblies. Proc. Natl. Acad. Sci. USA 2019, 116, 3391–3400. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; El-Sayed, M.A. Time Dependence and Signs of the Shift of the Surface Plasmon Resonance Frequency in Nanocages Elucidate the Nanocatalysis Mechanism in Hollow Nanoparticles. Nano Lett. 2011, 11, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.-X.; Qu, Z.; Wang, Q.-X.; Zhang, M.; Shi, G. Nanomolar Sensitive Colorimetric Assay for Mn2+ Using Cysteic Acid-Capped Silver Nanoparticles and Theoretical Investigation of Its Sensing Mechanism. Anal. Chim. Acta 2017, 980, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Gür, F.N.; Schwarz, F.W.; Ye, J.; Diez, S.; Schmidt, T.L. Toward Self-Assembled Plasmonic Devices: High-Yield Arrangement of Gold Nanoparticles on DNA Origami Templates. ACS Nano 2016, 10, 5374–5382. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Cukalevski, R.; Frohm, B.; Knowles, T.P.J.; Linse, S. Quantification of the Concentration of Aβ42 Propagons during the Lag Phase by an Amyloid Chain Reaction Assay. J. Am. Chem. Soc. 2014, 136, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Weon, B.M.; Je, J.H. Capillary Force Repels Coffee-Ring Effect. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2010, 82, 015305. [Google Scholar] [CrossRef]
- Ray, I.; Chauhan, A.; Wegiel, J.; Chauhan, V.P.S. Gelsolin Inhibits the Fibrillization of Amyloid Beta-Protein, and Also Defibrillizes Its Preformed Fibrils. Brain Res. 2000, 853, 344–351. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Li, C.; Sun, X.; Tang, D.; Shi, G. A Method for Evaluating the Level of Soluble β-Amyloid(1–40/1–42) in Alzheimer’s Disease Based on the Binding of Gelsolin to β-Amyloid Peptides. Angew. Chem. Int. Ed. 2014, 53, 12832–12835. [Google Scholar] [CrossRef]
- Xia, N.; Liu, L.; Harrington, M.G.; Wang, J.; Zhou, F. Regenerable and Simultaneous Surface Plasmon Resonance Detection of Aβ(1–40) and Aβ(1–42) Peptides in Cerebrospinal Fluids with Signal Amplification by Streptavidin Conjugated to an n-Terminus-Specific Antibody. Anal. Chem. 2010, 82, 10151–10157. [Google Scholar] [CrossRef]
- Alies, B.; Eury, H.; Essassi, E.M.; Pratviel, G.; Hureau, C.; Faller, P. Concept for Simultaneous and Specific in Situ Monitoring of Amyloid Oligomers and Fibrils via Förster Resonance Energy Transfer. Anal. Chem. 2014, 86, 11877–11882. [Google Scholar] [CrossRef]
- Tsukakoshi, K.; Abe, K.; Sode, K.; Ikebukuro, K. Selection of DNA Aptamers That Recognize Alpha-Synuclein Oligomers Using a Competitive Screening Method. Anal. Chem. 2012, 84, 5542–5547. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Ying, Y.L.; Li, Y.; Kraatz, H.B.; Long, Y.T. Nanopore Analysis of β-Amyloid Peptide Aggregation Transition Induced by Small Molecules. Anal. Chem. 2011, 83, 1746–1752. [Google Scholar] [CrossRef]
- Cui, M.; Ono, M.; Watanabe, H.; Kimura, H.; Liu, B.; Saji, H. Smart Near-Infrared Fluorescence Probes with Donor-Acceptor Structure for in Vivo Detection of Beta-Amyloid Deposits. J. Am. Chem. Soc. 2014, 136, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Z.; Lam, M.L.; Liu, W.; Yeung, P.P.; Chieng, C.C.; Chen, T.H. Hybridization-Induced Suppression of Coffee Ring Effect for Nucleic Acid Detection. Sens. Actuators B Chem. 2015, 206, 56–64. [Google Scholar] [CrossRef]
- Han, X.; Liu, Y.; Yin, Y. Colorimetric Stress Memory Sensor Based on Disassembly of Gold Nanoparticle Chains. Nano Lett. 2014, 14, 2466–2470. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Lam, S.J.; Ho, K.K.K.; Kumar, N.; Qiao, G.G.; Egan, S.; Boyer, C.; Wong, E.H.H. Rational Design of Single-Chain Polymeric Nanoparticles That Kill Planktonic and Biofilm Bacteria. ACS Infect. Dis. 2017, 3, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; Chen, W.Y.; Yen, Y.C.; Wang, C.W.; Chang, H.T.; Chen, C.F. Detection of Mercury(II) Ions Using Colorimetric Gold Nanoparticles on Paper-Based Analytical Devices. Anal. Chem. 2014, 86, 6843–6849. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Li, Z.; Tian, X.; Sun, H.; Liu, H.; Moore, A.; Ran, C. Design and synthesis of curcumin analogues for in vivo fluorescence imaging and inhibiting copper-induced cross-linking of amyloid beta species in Alzheimer's disease. J. Am. Chem. Soc. 2013, 135, 16397–16409. [Google Scholar] [CrossRef]
- Cassagnes, L.E.; Hervé, V.; Nepveu, F.; Hureau, C.; Faller, P.; Collin, F. The Catalytically Active Copper-Amyloid-Beta State: Coordination Site Responsible for Reactive Oxygen Species Production. Angew. Chem. Int. Ed. 2013, 52, 11110–11113. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Diabetes Mellitus and Risk of Alzheimer Disease and Decline in Cognitive Function. Arch. Neurol. 2004, 61, 661–666. [Google Scholar] [CrossRef]
- Mayeux, R.; Ottman, R.; Maestre, G.; Ngai, C.; Tang, M.X.; Ginsberg, H.; Chun, M.; Tycko, B.; Shelanski, M. Synergistic Effects of Traumatic Head Injury and Apolipoprotein-Epsilon4 in Patients with Alzheimer’s Disease. Neurology 1995, 45, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. The Role of Cerebral Ischemia in Alzheimer’s Disease. Neurobiol. Aging 2000, 21, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, G.; Di Lorenzo, G.; Ribersani, M.; Soldini, M.; Giancaspro, G.; Bellomo, G.; Belloni, G.; Grisorio, B.; Barbarini, G. Serum Ferritin and Hepatic Glutathione Concentrations in Chronic Hepatitis C Patients Related to the Hepatitis C Virus Genotype. J. Hepatol. 1999, 30, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yu, P.; Lin, Y.; Wang, Y.; Ohsaka, T.; Mao, L. Online Electrochemical Monitoring of Dynamic Change of Hippocampal Ascorbate: Toward a Platform for in Vivo Evaluation of Antioxidant Neuroprotective Efficiency against Cerebral Ischemia Injury. Anal. Chem. 2013, 85, 9947–9954. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, L.; Li, J.; Zhang, J.; Qu, Z.-B. Biomimetic Surface Engineering to Modulate the Coffee-Ring Effect for Amyloid-β Detection in Rat Brains. Biomimetics 2023, 8, 581. https://doi.org/10.3390/biomimetics8080581
Wang C, Li L, Li J, Zhang J, Qu Z-B. Biomimetic Surface Engineering to Modulate the Coffee-Ring Effect for Amyloid-β Detection in Rat Brains. Biomimetics. 2023; 8(8):581. https://doi.org/10.3390/biomimetics8080581
Chicago/Turabian StyleWang, Changxin, Lei Li, Jiaze Li, Jun Zhang, and Zhi-Bei Qu. 2023. "Biomimetic Surface Engineering to Modulate the Coffee-Ring Effect for Amyloid-β Detection in Rat Brains" Biomimetics 8, no. 8: 581. https://doi.org/10.3390/biomimetics8080581
APA StyleWang, C., Li, L., Li, J., Zhang, J., & Qu, Z.-B. (2023). Biomimetic Surface Engineering to Modulate the Coffee-Ring Effect for Amyloid-β Detection in Rat Brains. Biomimetics, 8(8), 581. https://doi.org/10.3390/biomimetics8080581





