Dynamic Ocular Response to Mechanical Loading: The Role of Viscoelasticity in Energy Dissipation by the Cornea
Abstract
1. Introduction
2. Materials and Methods
2.1. VOCT
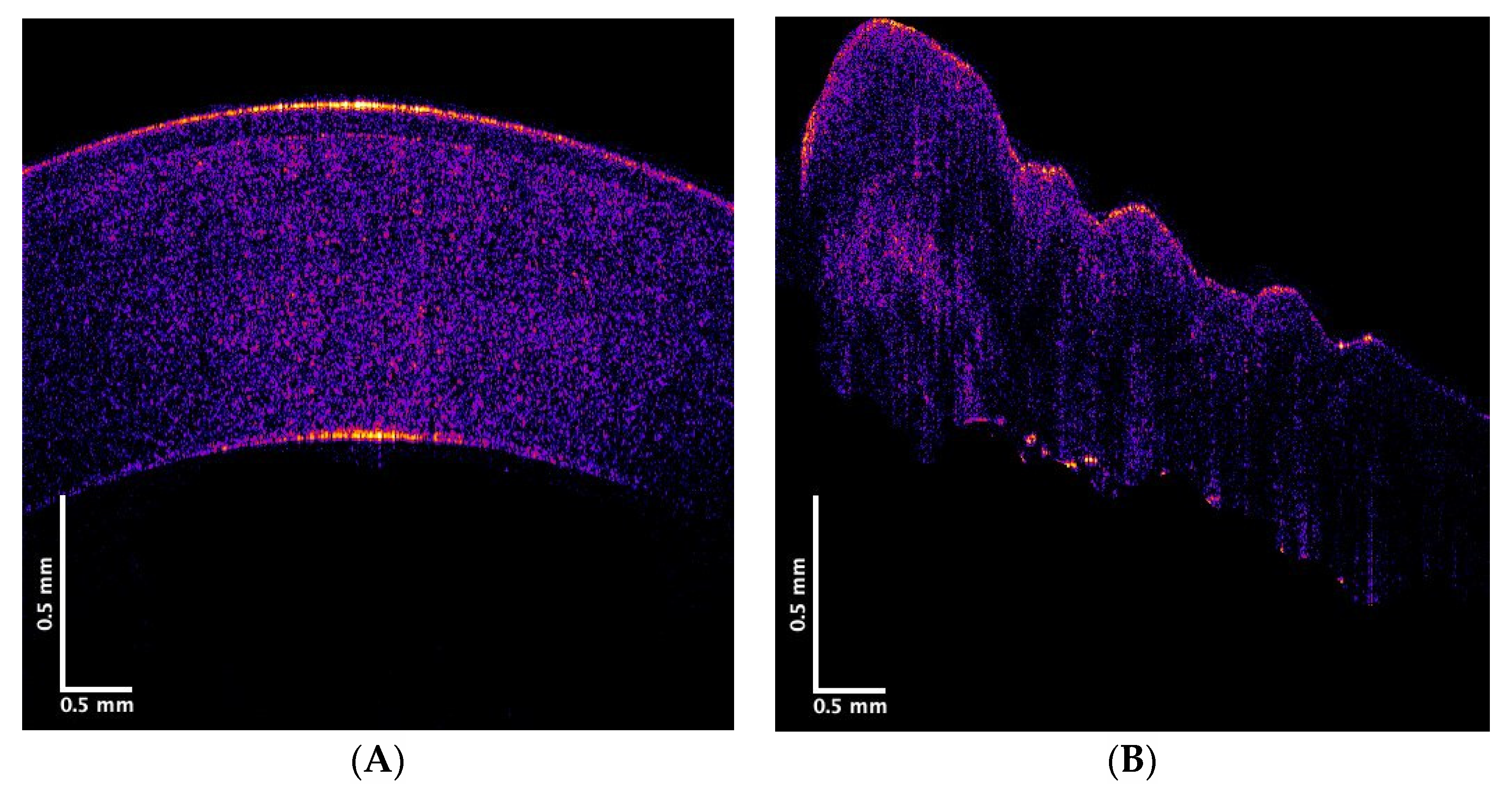
2.2. Pig Eyes and Excised Corneas
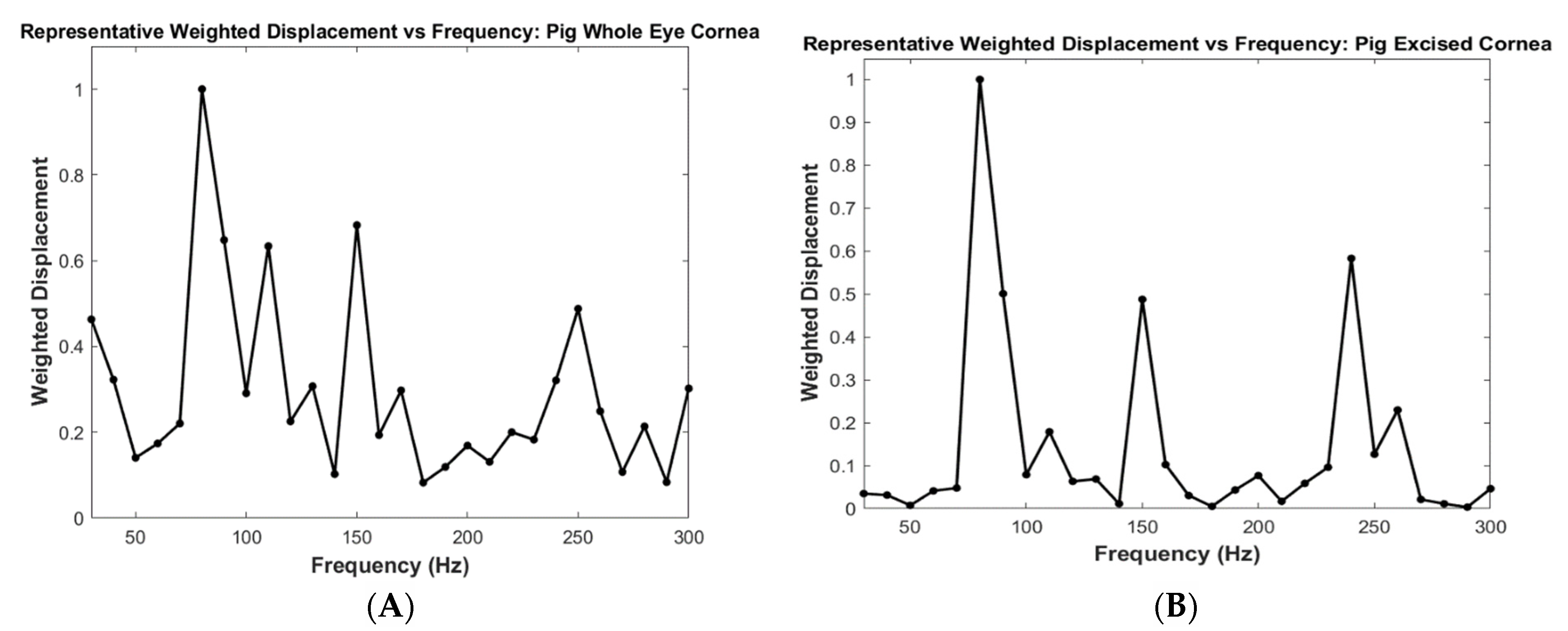
2.3. Measurement of Resonant Frequency and the Elastic Modulus
2.4. Statistics
2.5. Measurement of Loss Modulus
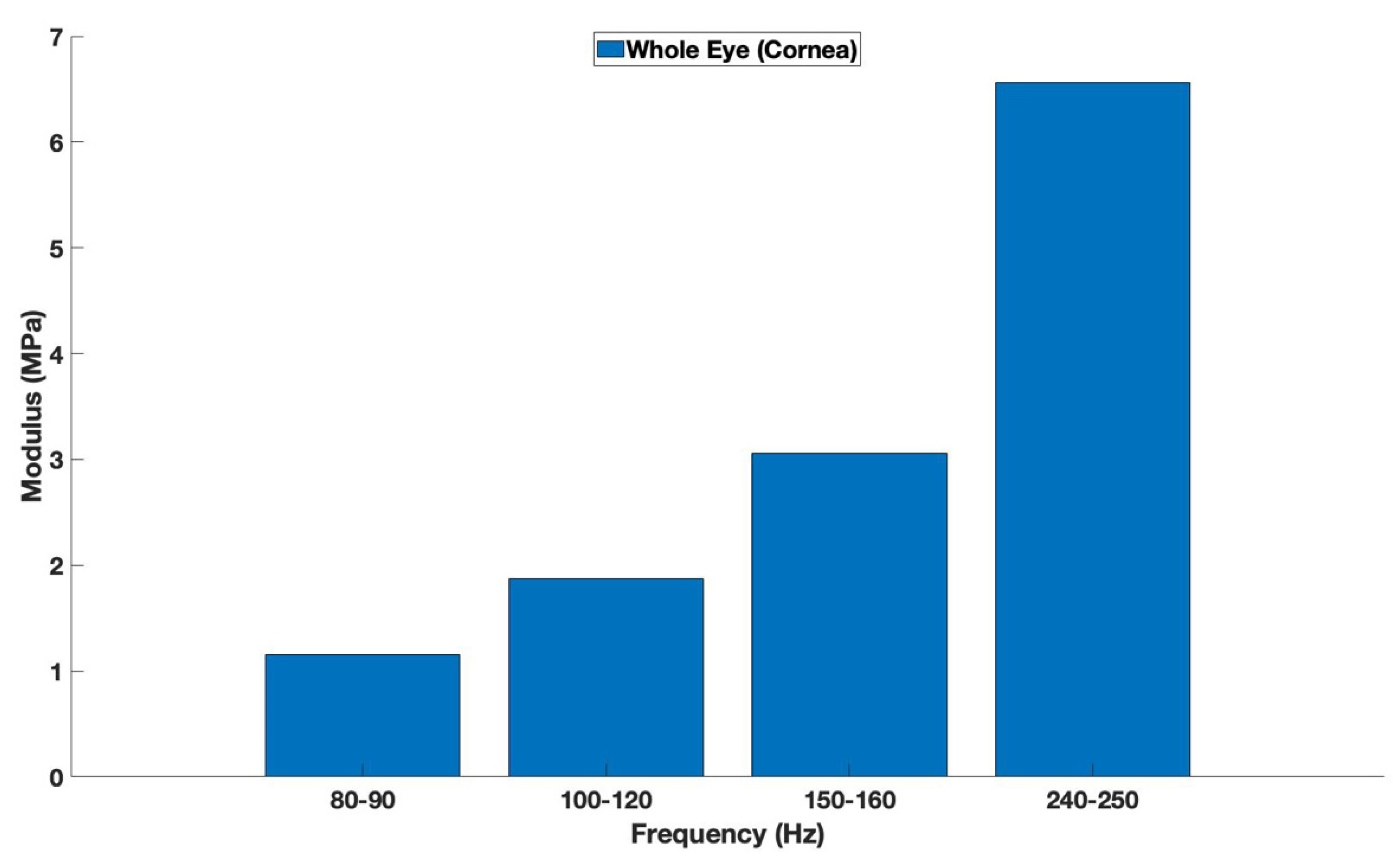
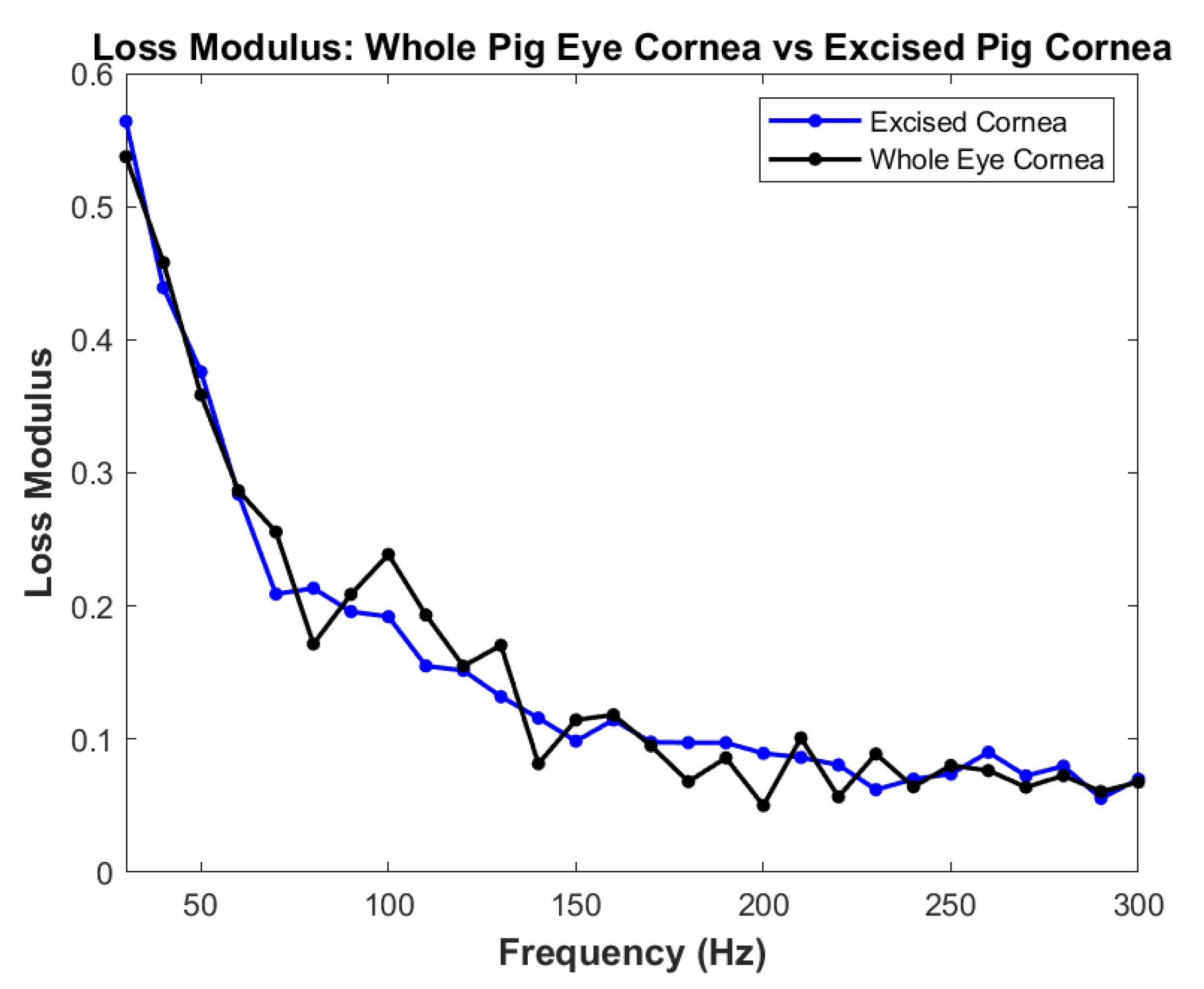
3. Results
4. Discussion
4.1. Viscoelasticity of Cornea
4.2. Young’s Modulus Versus Elastic Modulus
4.3. Defining Elastic Modulus of Different Parts of the Cornea Based on VOCT Measurements
4.4. Assumptions and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silver, F.H.; Bradica, G. Mechanobiology of cartilage: How do internal and external stresses affect mechanochemical transduction and elastic energy storage? Biomech. Model Mechanobiol. 2002, 1, 219–238. [Google Scholar] [CrossRef]
- Silver, F.H.; Siperko, L.M. Mechanosensing and Mechanochemical Transduction: How Is Mechanical Energy Sensed and Converted into Chemical Energy in an Extracellular Matrix? Crit. Rev. Biomed. Eng. 2003, 31, 255–331. [Google Scholar] [CrossRef]
- Silver, F.H.; Freeman, J.W.; Gurinder, G.P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003, 36, 1529–1553. [Google Scholar] [CrossRef]
- Horvath, I.; Foran, D.; Silver, F.H. Energy Analysis of Flow Induced Harmonic Motion in Blood Vessel Walls. Cardiovasc. Eng. 2005, 5, 21–28. [Google Scholar] [CrossRef]
- Silver, F.H.; Kelkar, N.; Deshmukh, T. Molecular Basis for Mechanical Properties of ECMs: Proposed Role of Fibrillar Collagen and Proteoglycans in Tissue Biomechanics. Biomolecules 2021, 11, 1018. [Google Scholar] [CrossRef]
- Silver, F.H.; Deshmukh, T.; Benedetto, D.; Gonzalez-Mercedes, M.; Mesica, A. Measurement of the Elastic Modulus of Cornea, Sclera and Limbus: The Importance of the Corneal-Limbus-Scleral Biomechanical Unit. Front. Biosci. 2022, 14, 30. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, H. Corneal and scleral biomechanics in ophthalmic diseases: An updated review. Med. Nov. Technol. Devices 2022, 15, 100140. [Google Scholar] [CrossRef]
- Maczynska, E.; Karnowski, K.; Szulzycki, K.; Malinowska, M.; Dolezycazek, H.; Cichanski, A.; Wojtkowski, M.; Malinoska, M.; Kaluzny, B.; Grukowski, I. Assessment of the influence of viscoelasticity of cornea in animal ex vivo model using air-puff optical coherence tomography and corneal hysteresis. J. Biophotonics 2019, 12, e201800154. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Wilson, A. A review of corneal biomechanics: Mechanisms for measurement and the implications for refractive surgery. Indian J. Ophthalmol. 2020, 68, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Ruberti, J.W.; Roy, A.S.; Roberts, C.J. Corneal biomechanics and biomaterials. Annu. Rev. Biomed. Eng. 2011, 13, 269–954. [Google Scholar] [CrossRef]
- Pinero, D.P.; Alcon, N. Corneal biomechanics: A review. Clin. Exp. Optom. 2015, 98, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.; Pye, D. A Clinical Description of Ocular Response Analyzer Measurements. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Ramier, A.; Eltoiny, A.M.; Chen, Y.T.; Clouser, F.; Birkenfeld, J.S.; Watt, R.; Yum, S.-H. In vivo measurement of shear modulus of the human cornea using optical coherence elastography. Nat. Res. Sci. Rep. 2020, 10, 17366. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.; Pinero, D.G.P.; Paredes, M.; Alio, J.L.; Caves, F. Iterative methods for biomechanical evaluation of corneal biomechanical evaluation of corneal response. Appl. Sci. 2021, 11, 10819. [Google Scholar] [CrossRef]
- Lopes, B.T.; Bao, F.; Wang, J.; Liu, X.; Wang, L.; Abass, A.; Eliasy, A.; Elsheikh, A. Review of in-vivo characterisation of corneal biomechanics. Med. Nov. Technol. Devices 2021, 11, 100073. [Google Scholar] [CrossRef]
- Crespo, M.A.; Jimenez, H.J.; Deshmukh, T.; Pulido, J.S.; Saad, A.S.; Silver, F.H.; Benedetto, D.A.; Rapuano, C.J.; Syed, Z.A. In Vivo Determination of the Human Corneal Elastic Modulus Using Vibrational Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2022, 11, 11. [Google Scholar] [CrossRef]
- Silver, F.H.; Deshmukh, T.; Ryan, N.; Romm, A.; Nadiminti, H. “Fingerprinting” Benign and Cancerous Skin Lesions Using Vibrational Optical Coherence Tomography: Differentiation among Cancerous Lesion Types Based on the Presence of New Cells, Blood Vessels, and Fibrosis. Biomolecules 2022, 12, 1332. [Google Scholar] [CrossRef]
- Silver, F.H.; Gonzalez-Mercedes, M.; Mesica, A. A Rapid Method to Noninvasively Measure the Viscoelastic Properties of Synthetic Polymers Using Mechanical Vibrations and Photonics. Photonics 2022, 9, 925. [Google Scholar] [CrossRef]
- Tirella, A.; Mattei, G.; Ahluwalia, A. Strain rate viscoelastic analysis of soft and highly hydrated biomaterials. J. Biomed Mater. Res. Part A 2014, 102A, 3352–3360. [Google Scholar] [CrossRef]
- Zhu, F.; Yasin, S.; Hussain, M. Dynamic oscillatory shear testing is used to investigate polymeric viscoelastic behavior. Viscoelastic Rheological Behaviors of Polypropylene and LMPP Blends. Polymers 2021, 13, 3485. [Google Scholar] [CrossRef]
- Yamashita, J.; Li, X.; Furman, B.; Rawis, H.R.; Wang, X.; Agrawal, C.M. Collagen and bone viscoelasticity: A dynamic mechanical analysis. J. Biomed. Mater. Res. (Appl. Biomater.) 2002, 63, 31–36. [Google Scholar] [CrossRef]
- Chan, R.W. Nonlinear viscoelastic characterization of human vocal fold tissues under large-amplitude oscillatory shear (LAOS). J. Rheol. 2018, 62, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Desrochers, J.M.W.; Amrein, M.W.; Matyas, J.R.; Desrochers, J. Viscoelasticity of the articular cartilage surface in early osteoarthritis. Osteoarthr. Cartil. 2012, 20, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.J.; Vicari, J.; Buckley, M.R.; Silverberg, J.L.; Cohen, I.; Bonassar, L.J. Effects of Enzymatic Treatments on the Depth-Dependent Viscoelastic Shear Properties of Articular Cartilage. J. Orthop. Res. 2014, 32, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Taylor, Z.D.; Garritano, J.; Sung, S.; Bajwa, N.; Bennett, D.B.; Nowroozi, B.; Tewari, P.; Sayre, J.; Hubschman, J.-P.; Deng, S.; et al. THz and mm-Wave Sensing of Corneal Tissue Water Content: Electromagnetic Modeling and Analysis. IEEE Trans. Terahertz Sci. Technol. 2015, 5, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Jung, D.W.; Mohammad-Sedighi, H.; Żur, K.K.; Habibi, M.; Safa, M. Stability and dynamics of viscoelastic moving rayleigh beams with an asymmetrical distribution of material parameters. Symmetry 2020, 12, 586. [Google Scholar] [CrossRef]
- Rizov, V. Effects of Periodic Loading on Longitudinal Fracture in Viscoelastic Functionally Graded Beam Structures. J. Appl. Comput. Mech. 2022, 8, 370–378. [Google Scholar]
- Michelacci, Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res. 2003, 36, 1037–1046. [Google Scholar] [CrossRef]
- Bishop, P.N.; Holmes, D.F.; Kalder, K.E.; McLeod, D.; Bos, K.J. Age-Related Changes on the Surface of Vitreous Collagen Fibrils. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1041–1046. [Google Scholar] [CrossRef]
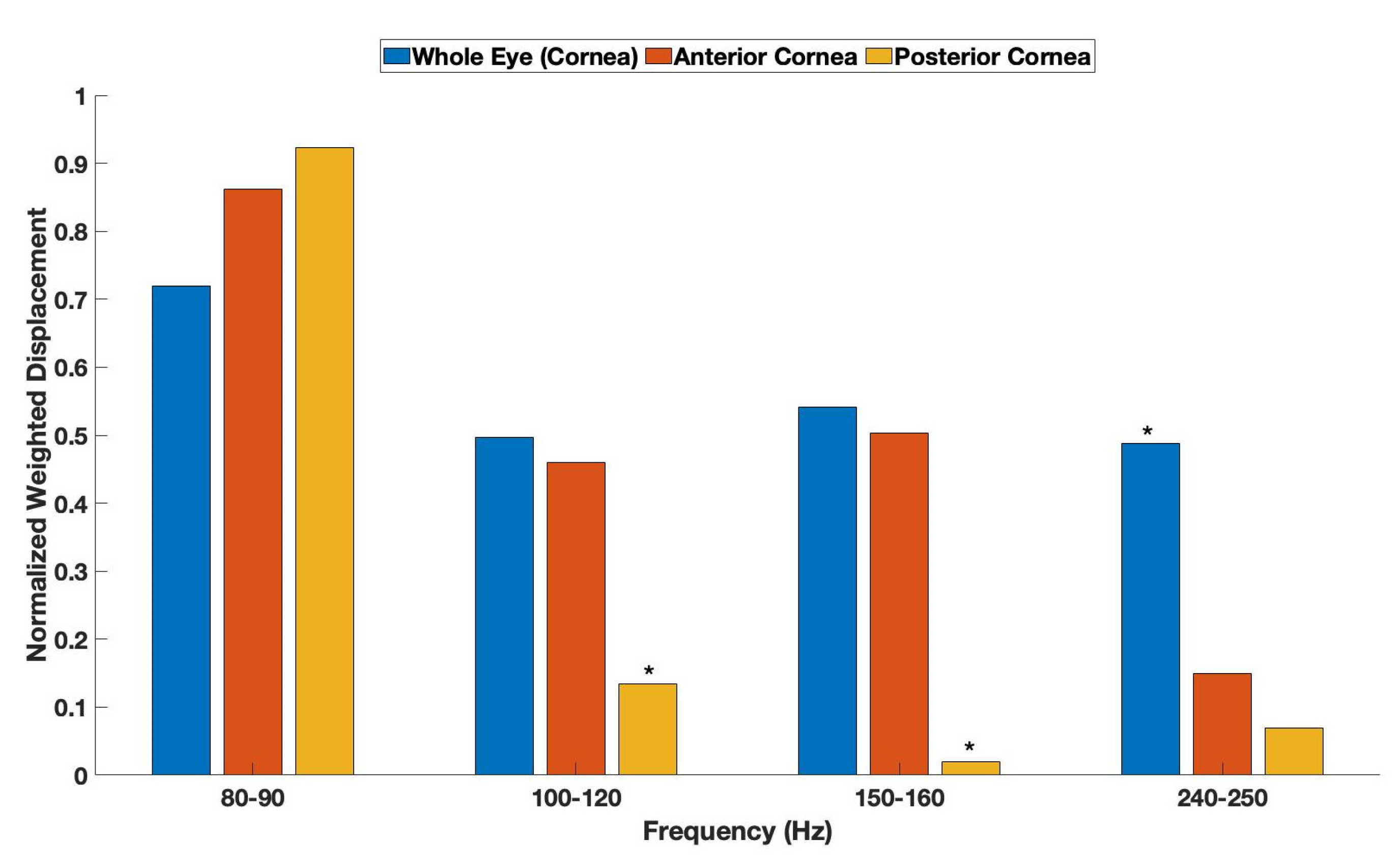
| Anterior Cornea | Posterior Cornea | |
|---|---|---|
| 80–90 Hz | ||
| Whole Eye Cornea | 0.03 | 0.018 |
| Anterior Cornea | - | 0.43 |
| 100–120 Hz | ||
| Whole Eye Cornea | 0.45 | 0.008 |
| Anterior Cornea | - | 0.009 |
| 150–160 Hz | ||
| Whole Eye Cornea | 0.43 | 0.004 |
| Anterior Cornea | - | 0.006 |
| 240–250 Hz | ||
| Whole Eye Cornea | 0.0016 | 0.0017 |
| Anterior Cornea | - | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silver, F.H.; Deshmukh, T.; Benedetto, D.; Gonzalez-Mercedes, M. Dynamic Ocular Response to Mechanical Loading: The Role of Viscoelasticity in Energy Dissipation by the Cornea. Biomimetics 2023, 8, 63. https://doi.org/10.3390/biomimetics8010063
Silver FH, Deshmukh T, Benedetto D, Gonzalez-Mercedes M. Dynamic Ocular Response to Mechanical Loading: The Role of Viscoelasticity in Energy Dissipation by the Cornea. Biomimetics. 2023; 8(1):63. https://doi.org/10.3390/biomimetics8010063
Chicago/Turabian StyleSilver, Frederick H., Tanmay Deshmukh, Dominick Benedetto, and Michael Gonzalez-Mercedes. 2023. "Dynamic Ocular Response to Mechanical Loading: The Role of Viscoelasticity in Energy Dissipation by the Cornea" Biomimetics 8, no. 1: 63. https://doi.org/10.3390/biomimetics8010063
APA StyleSilver, F. H., Deshmukh, T., Benedetto, D., & Gonzalez-Mercedes, M. (2023). Dynamic Ocular Response to Mechanical Loading: The Role of Viscoelasticity in Energy Dissipation by the Cornea. Biomimetics, 8(1), 63. https://doi.org/10.3390/biomimetics8010063






