In Vivo Analysis of the Dynamic Motion Stability Characteristics of Geese’s Neck
Abstract
1. Introduction
2. Materials and Methods
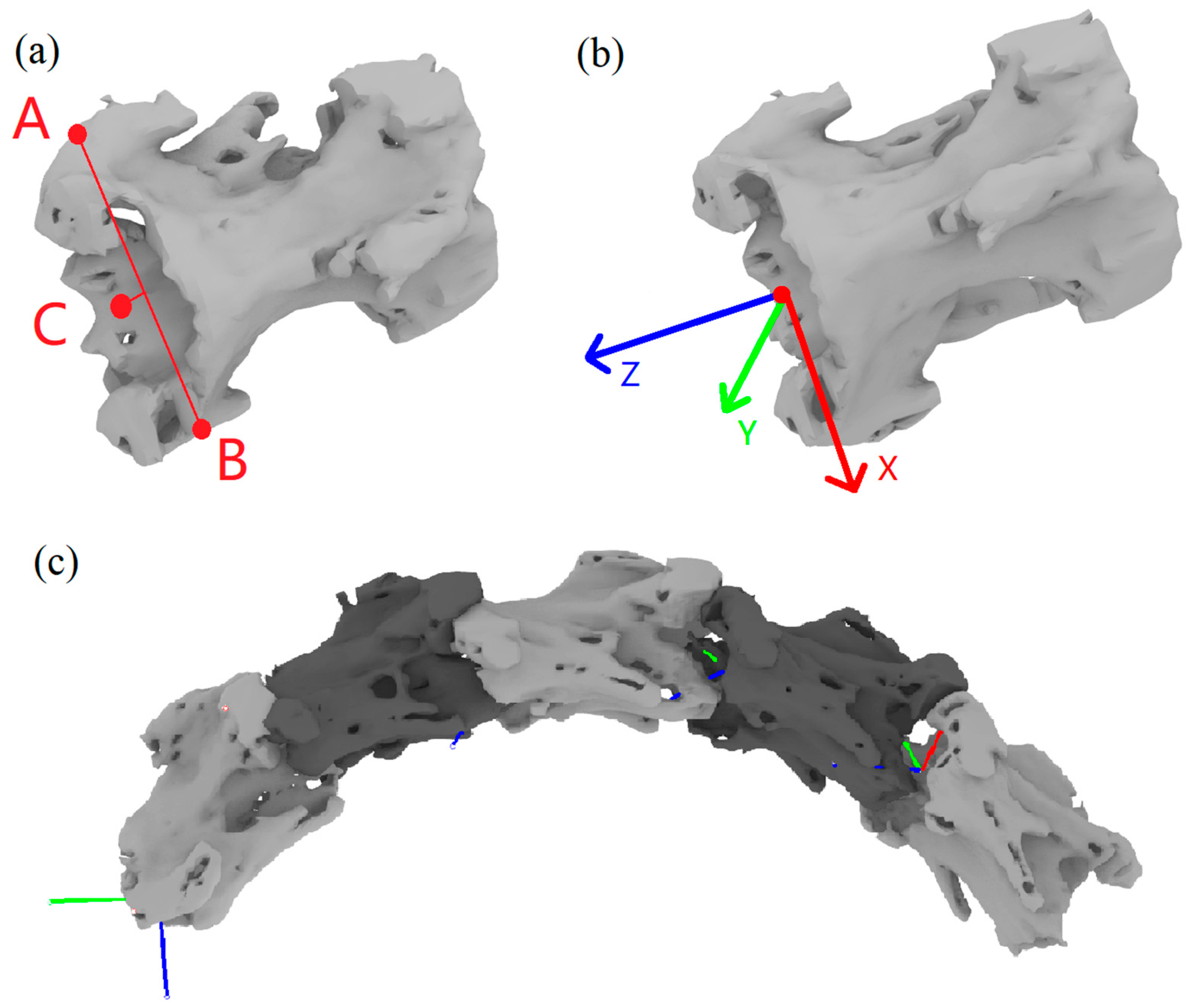
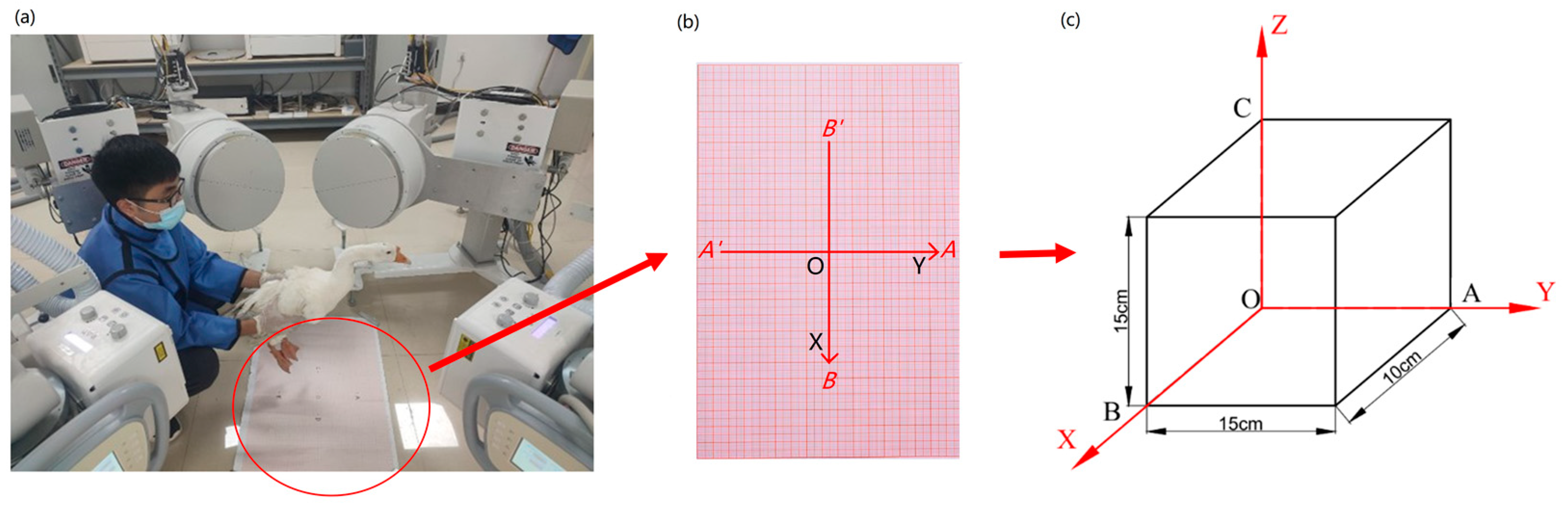
2.1. Cervical Spine 3D Structure Construction and Coordinate System Establishment
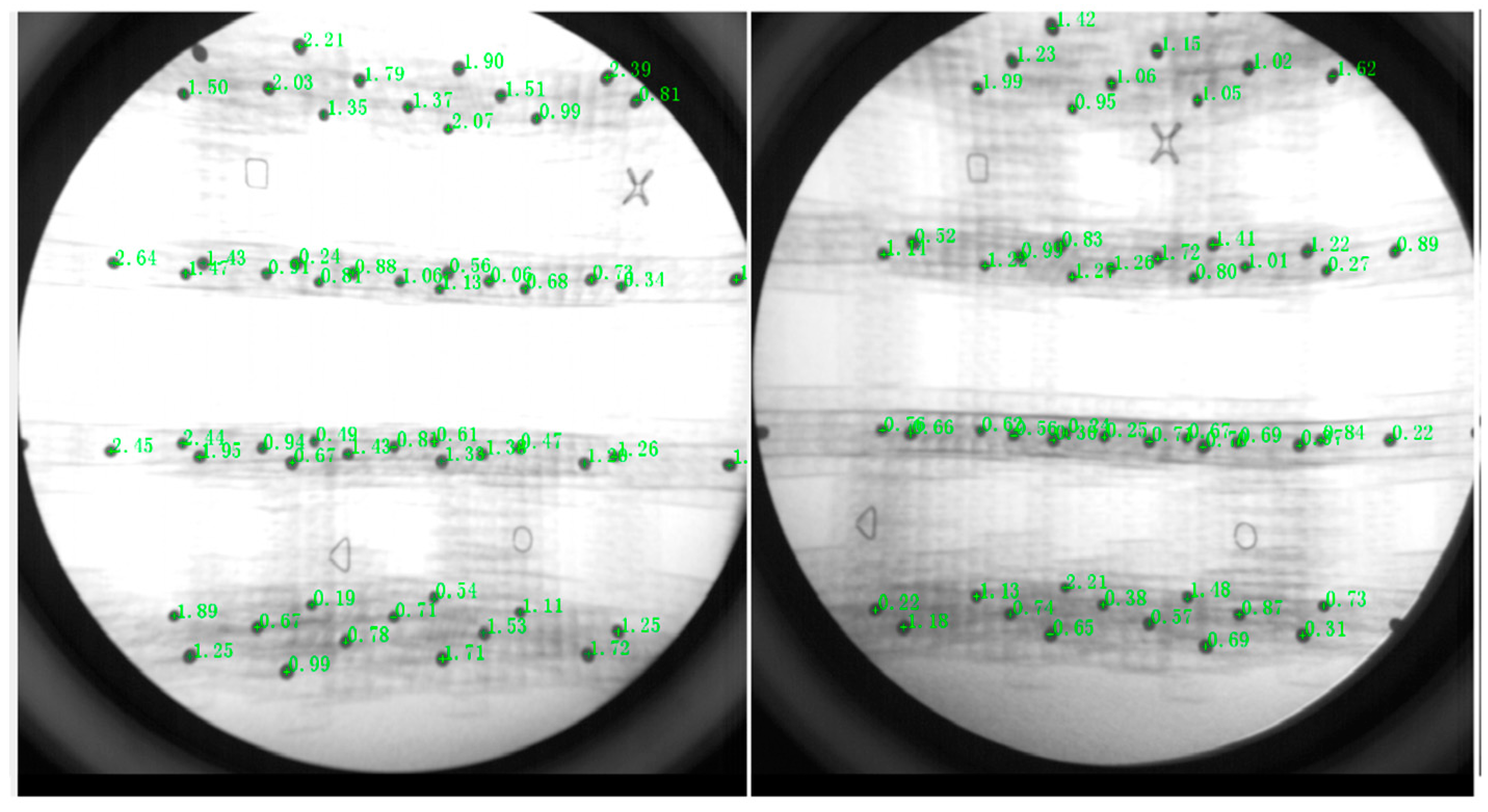
2.2. Biplane X-ray Image Acquisition
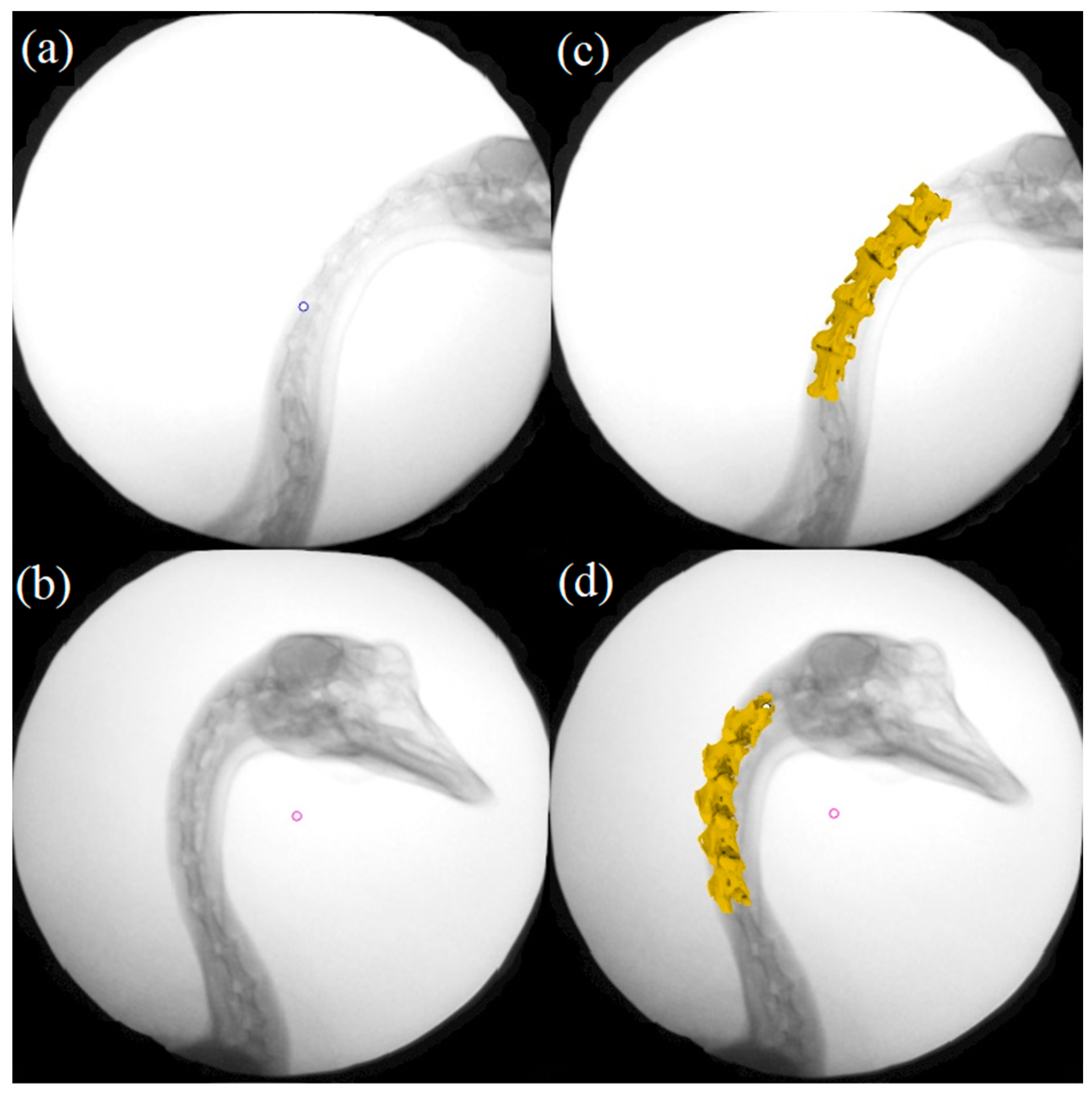
2.3. Biplane X-ray Data Processing
3. Results
3.1. Stability Analysis of Goose’s Neck When Moving the Body in Y-Direction
3.2. Stability Analysis of Goose’s Neck When Moving the Body in Z-Direction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galbusera, F.; Bassani, T. The Spine: A Strong, Stable, and Flexible Structure with Biomimetics Potential. Biomimetics 2019, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Kambic, R.E.; Biewener, A.A.; Pierce, S.E. Experimental determination of three-dimensional cervical joint mobility in the avian neck. Front. Zool. 2017, 14, 37. [Google Scholar] [CrossRef]
- Su, J.Y.; Ting, S.C.; Chang, Y.H.; Yang, J.T. Aerodynamic trick for visual stabilization during downstroke in a hovering bird. Phys. Rev. E 2011, 84, 012901. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Y.; Yang, J.T. Analysis of the aerodynamic force in an eye-stabilized flapping flyer. Bioinspiration Biomim. 2013, 8, 046010. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.B. Visual control of head movements during avian locomotion. Nature 1975, 255, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Troje, N.F.; Frost, B.J. Head-bobbing in Pigeons: How stable is the hold phase. J. Exp. Biol. 2000, 203, 935–940. [Google Scholar] [CrossRef]
- Frost, B.J. Bird head stabilization. Curr. Biol. 2009, 19, R315–R316. [Google Scholar] [CrossRef]
- Dunlap, K.; Mowrer, O.H. Head movements and eye functions of birds. J. Comp. Psychol. 1930, 11, 99–113. [Google Scholar] [CrossRef]
- Lovette, I.J.; Fitzpatrick, J.W. Handbook of Bird Biology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kisia, S.M. Vertebrates: Structures and Functions; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Dzemski, G.; Christian, A. Flexibility along the neck of the ostrich (Struthio camelus) and consequences for the reconstruction of dinosaurs with extreme neck length. J. Morphol. 2007, 268, 701–714. [Google Scholar] [CrossRef]
- Wang, L.Z.; Lu, S.; Liu, X.Y.; Niu, X.F.; Wang, C.; Ni, Y.K.; Zhao, M.Y.; Feng, C.L.; Zhang, M.; Fan, Y.B. Biomechanism of impact resistance in the woodpecker’s head and its application. Sci. China Life Sci. 2013, 56, 715–719. [Google Scholar] [CrossRef]
- Wang, L.Z.; Cheung, J.T.M.; Pu, F.; Li, D.; Zhang, M.; Fan, Y. Why do woodpeckers resist head impact injury: A biomechanical investigation. PLoS ONE 2011, 6, e26490. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Z.; Zhang, H.Q.; Fan, Y.B. Comparative study of the mechanical properties, micro-structure, and composition of the cranial and beak bones of the great spotted woodpecker and the lark bird. Sci. China Life Sci. 2011, 54, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Jia, W.F.; Zhang, F.; Ma, X.Q.; Qiu, Z.M.; Qian, Z.H.; Ren, L.Q.; Guo, Z.J.; Zhang, Y.K. Study on the structural characteristics of bird necks and their static motion features in the sagittal plane. Coatings 2021, 11, 1228. [Google Scholar] [CrossRef]
- Krings, M.; Nyakatura, J.A.; Fischer, M.S.; Wagner, H. The cervical spine of the American barn owl (Tyto furcata pratincola): I. Anatomy of the vertebrae and regionalization in their S-shaped arrangement. PLoS ONE 2014, 9, e91653. [Google Scholar] [CrossRef] [PubMed]
- Terray, L.; Plateau, O.; Abourachid, A.; Böhmer, C.; Delapré, A.; de la Bernardie, X.; Cornette, R. Modularity of the neck in birds (Aves). Evol. Biol. 2020, 47, 97–110. [Google Scholar] [CrossRef]
- Katzir, G.; Schechtman, E.; Carmi, N.; Weihs, D. Head stabilization in herons. J. Comp. Physiol. A 2001, 187, 423–432. [Google Scholar]
- Russell, A.P.; Bentley, A.D. Opisthotonic head displacement in the domestic chicken and its bearing on the ‘dead bird’ posture of non-avialan dinosaurs. J. Zool. 2016, 298, 20–29. [Google Scholar] [CrossRef]
- van der Leeuw, A.H.J.; Bout, R.G.; Zweers, G.A. Control of the Cranio-Cervical system during feeding in birds. Am. Zool. 2001, 41, 1352–1363. [Google Scholar] [CrossRef]
- Pete, A.E.; Kress, D.; Dimitrov, M.A.; Lentink, D. The role of passive avian head stabilization in flapping flight. J. R. Soc. Interface 2015, 12, 20150508. [Google Scholar] [CrossRef]
- Furet, M.; Abourachid, A.; Böhmer, C.; Chummun, V.; Chevallereau, C.; Cornette, R.; Bernardie, X.D.L.; Wenger, P. Estimating motion between avian vertebrae by contact modeling of joint surfaces. Comput. Methods Biomech. Biomed. Eng. 2022, 25, 123–131. [Google Scholar] [CrossRef]
- Krings, M.; Nyakatura, J.A.; Boumans, M.L.; Fischer, M.S.; Wagner, H. Barn owls maximize head rotations by a combination of yawing and rolling in functionally diverse regions of the neck. J. Anat. 2017, 231, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Grood, E.S.; Suntay, W.J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Gatesy, S.M.; Baier, D.B.; Jenkins, F.A.; Dial, K.P. Scientific rotoscoping: A morphology-based method of 3-D motion analysis and visualization. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2010, 313, 244–261. [Google Scholar] [CrossRef] [PubMed]
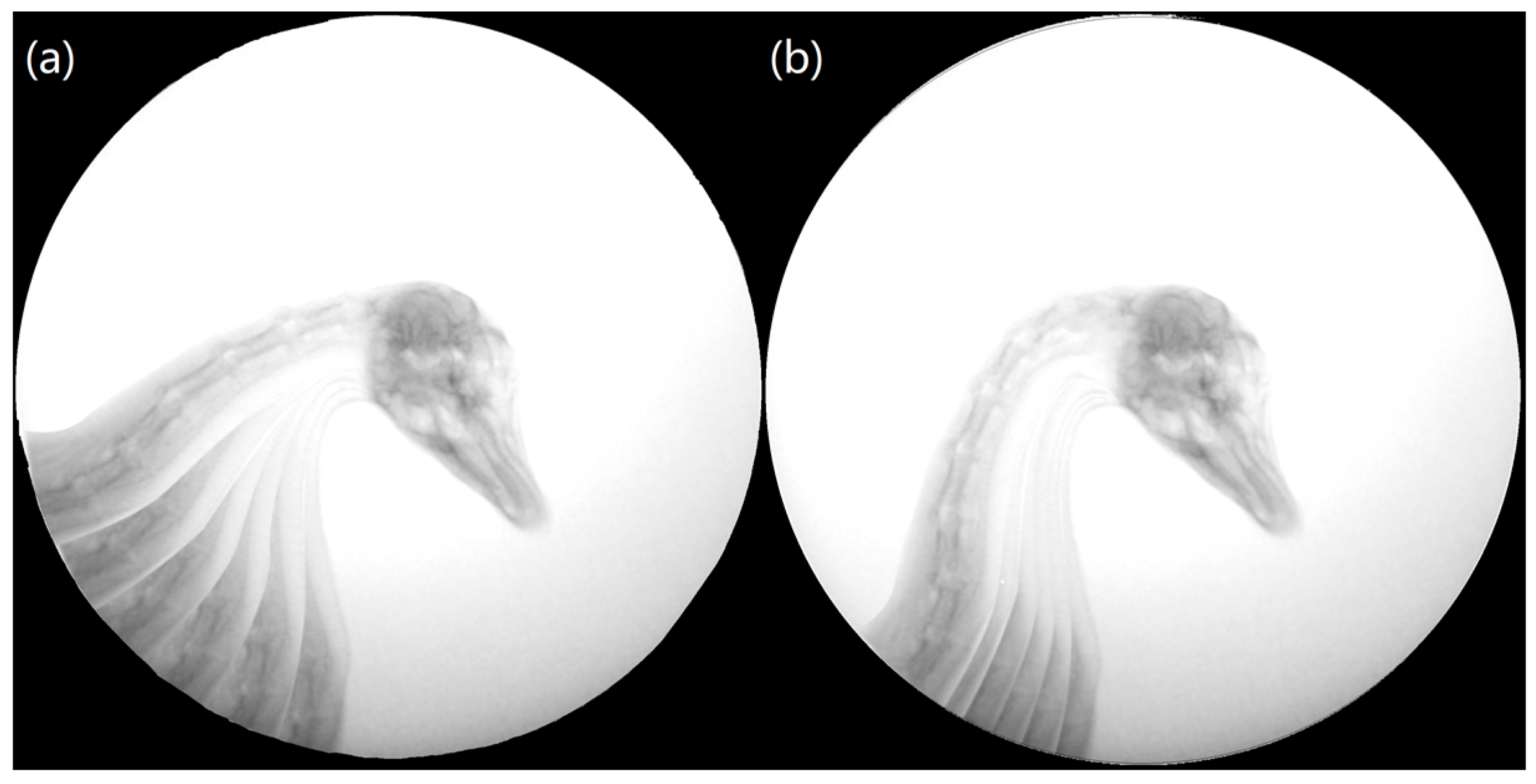

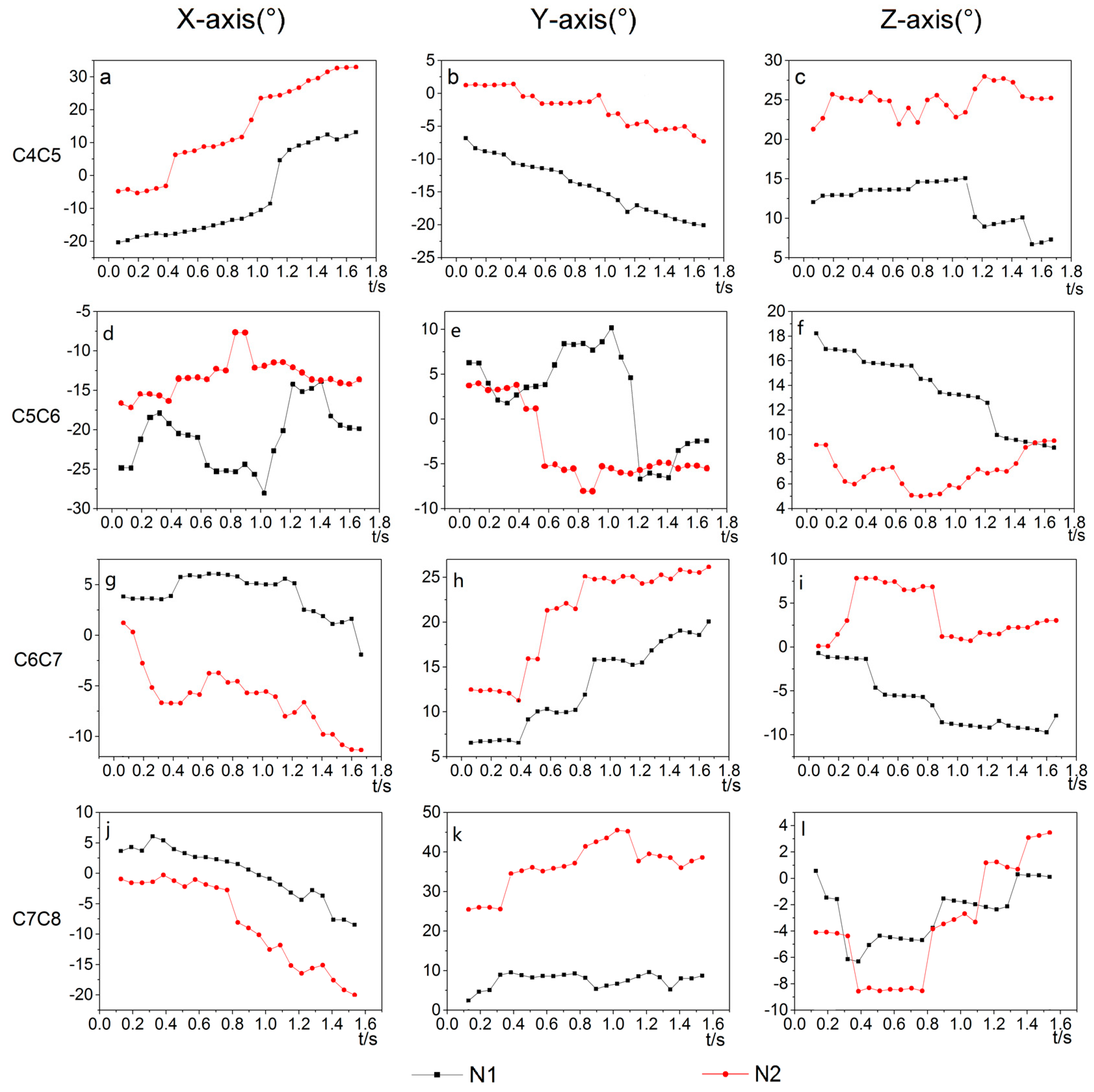
| Joint | Test Number | Coordinate Axes | Movement Data | |
|---|---|---|---|---|
| Range of Motion | Interval Size | |||
| C4C5 | N1 | X | 18.337°~29.262° | 10.925° |
| Y | −1.639°~2.746° | 4.385° | ||
| Z | 2.91°~5.98° | 3.07° | ||
| N2 | X | −4.482°~12.778° | 17.26° | |
| Y | −11.667°~−6.406° | 5.261° | ||
| Z | 10.46°~18.19° | 7.73° | ||
| C5C6 | N1 | X | 15.438°~6.482° | 21.92° |
| Y | −7.632°~4.413° | 12.045° | ||
| Z | 0.575°~18.421° | 17.85° | ||
| N2 | X | −25.115°~1.731° | 26.85° | |
| Y | −5.823°~7.701° | 13.524° | ||
| Z | 2.46°~10.49° | 8.03° | ||
| C6C7 | N1 | X | −29.916°~−4.211° | 25.705° |
| Y | 2.897°~28.16° | 25.263° | ||
| Z | 5.581°~29.447° | 23.867° | ||
| N2 | X | −34.271°~−9.146° | 25.125° | |
| Y | 7.024°~22.543° | 15.519° | ||
| Z | 5.55°~11.754° | 6.203° | ||
| C7C8 | N1 | X | −23.28°~−14.336° | 8.95° |
| Y | −0.83°~6.823° | 7.657° | ||
| Z | 14.738°~16.693° | 1.955° | ||
| N2 | X | −30.54°~−22.881° | 7.65° | |
| Y | 6.854°~13.183° | 6.329° | ||
| Z | 11.451°~13.819° | 2.368° | ||
| Joint | Test Number | Coordinate Axes | Movement Data | |
|---|---|---|---|---|
| Range of Motion | Interval Size | |||
| C4C5 | N1 | X | −20.374°~13.117° | 33.491° |
| Y | −20.068°~−6.821° | 13.246° | ||
| Z | 6.694°~15.07° | 8.376° | ||
| N2 | X | −5.316°~32.974° | 38.289° | |
| Y | −7.314°~1.411° | 8.725° | ||
| Z | 21.293°~27.98° | 6.687° | ||
| C5C6 | N1 | X | −28°~−13.852° | 14.152° |
| Y | −6.716°~10.203° | 16.92° | ||
| Z | 8.932°~18.209° | 9.276° | ||
| N2 | X | −17.187°~−7.694° | 9.49° | |
| Y | −8.064°~3.983° | 12.047° | ||
| Z | 5.026°~9.507° | 4.481° | ||
| C6C7 | N1 | X | −1.915°~6.064° | 7.979° |
| Y | 6.531°~20.064° | 13.532° | ||
| Z | −9.737°~−0.729° | 9° | ||
| N2 | X | −11.347°~1.21° | 12.558° | |
| Y | 11.268°~26.148° | 14.88° | ||
| Z | 0.111°~7.853° | 7.74° | ||
| C7C8 | N1 | X | −8.5°~6.056° | 14.556° |
| Y | 2.431°~9.581° | 7.15° | ||
| Z | −6.304°~0.566° | 6.87° | ||
| N2 | X | −20.019°~−0.276° | 19.743° | |
| Y | 25.438°~45.51° | 20.072° | ||
| Z | −8.56°~3.46° | 12.02° | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Sun, H.; Jia, W.; Zhang, F.; Qian, Z.; Cui, X.; Ren, L.; Ren, L. In Vivo Analysis of the Dynamic Motion Stability Characteristics of Geese’s Neck. Biomimetics 2022, 7, 160. https://doi.org/10.3390/biomimetics7040160
Wang J, Sun H, Jia W, Zhang F, Qian Z, Cui X, Ren L, Ren L. In Vivo Analysis of the Dynamic Motion Stability Characteristics of Geese’s Neck. Biomimetics. 2022; 7(4):160. https://doi.org/10.3390/biomimetics7040160
Chicago/Turabian StyleWang, Jiajia, Haoxuan Sun, Wenfeng Jia, Fu Zhang, Zhihui Qian, Xiahua Cui, Lei Ren, and Luquan Ren. 2022. "In Vivo Analysis of the Dynamic Motion Stability Characteristics of Geese’s Neck" Biomimetics 7, no. 4: 160. https://doi.org/10.3390/biomimetics7040160
APA StyleWang, J., Sun, H., Jia, W., Zhang, F., Qian, Z., Cui, X., Ren, L., & Ren, L. (2022). In Vivo Analysis of the Dynamic Motion Stability Characteristics of Geese’s Neck. Biomimetics, 7(4), 160. https://doi.org/10.3390/biomimetics7040160






