Biomimetic Construction of Artificial Selenoenzymes
Abstract
1. Introduction
2. Natural Selenoenzymes
3. Artificial Selenoenzymes
3.1. Strategies for Constructing Artificial Selenoenzymes
3.1.1. Molecularly Imprinted Selenoenzymes
3.1.2. Antibody with Selenoenzyme Activity
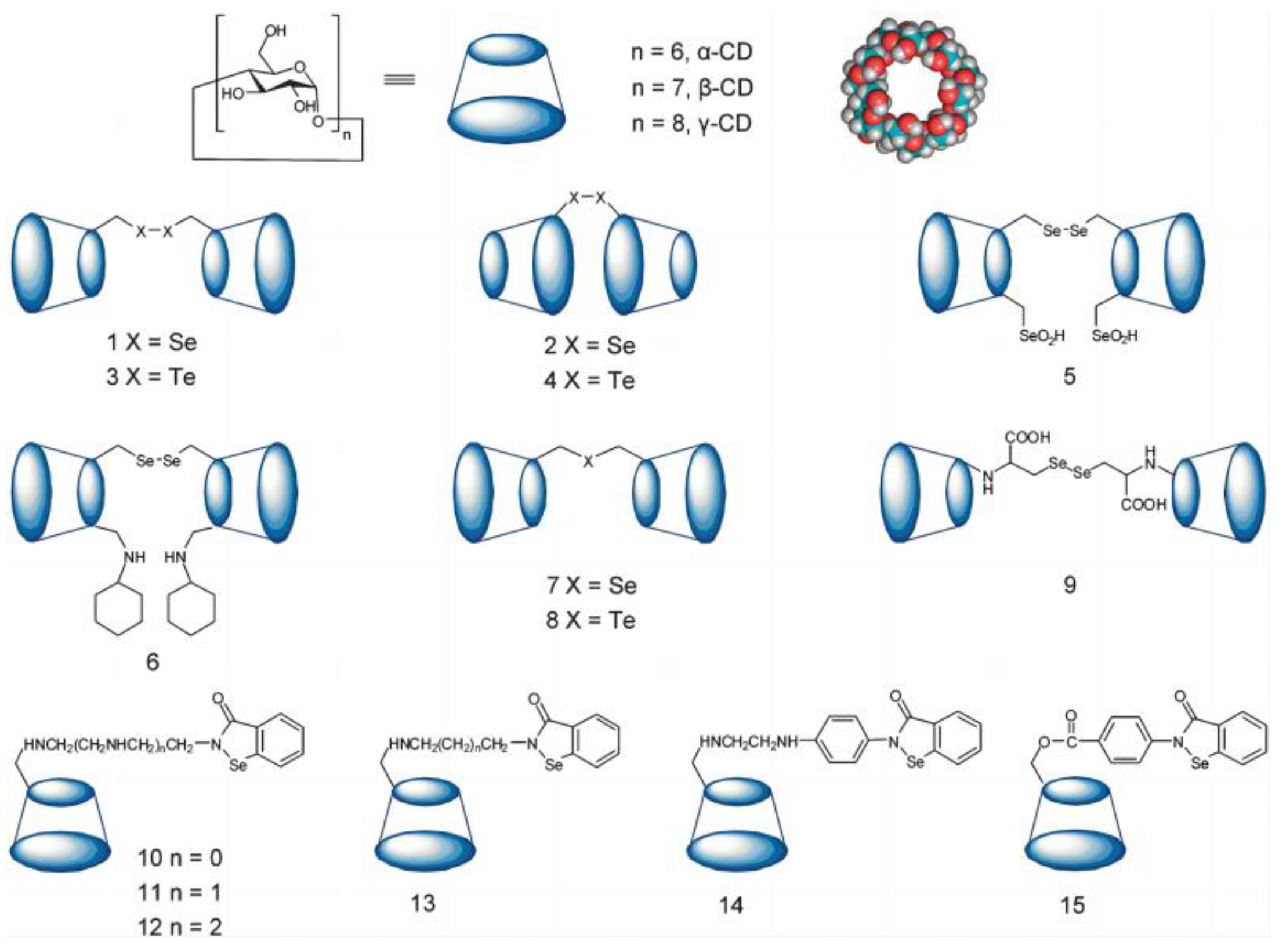
3.1.3. Cyclodextrin as Host of Artificial Selenonzyme
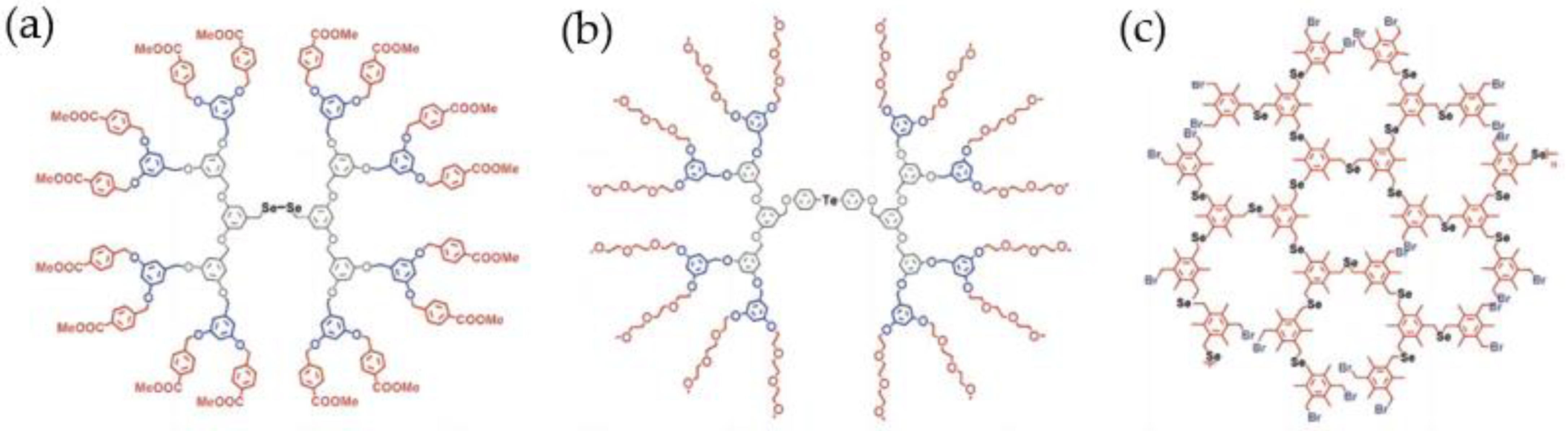
3.1.4. Dendrimers and Hyperbranched Polymers as Hosts of Artificial Selenonzymes
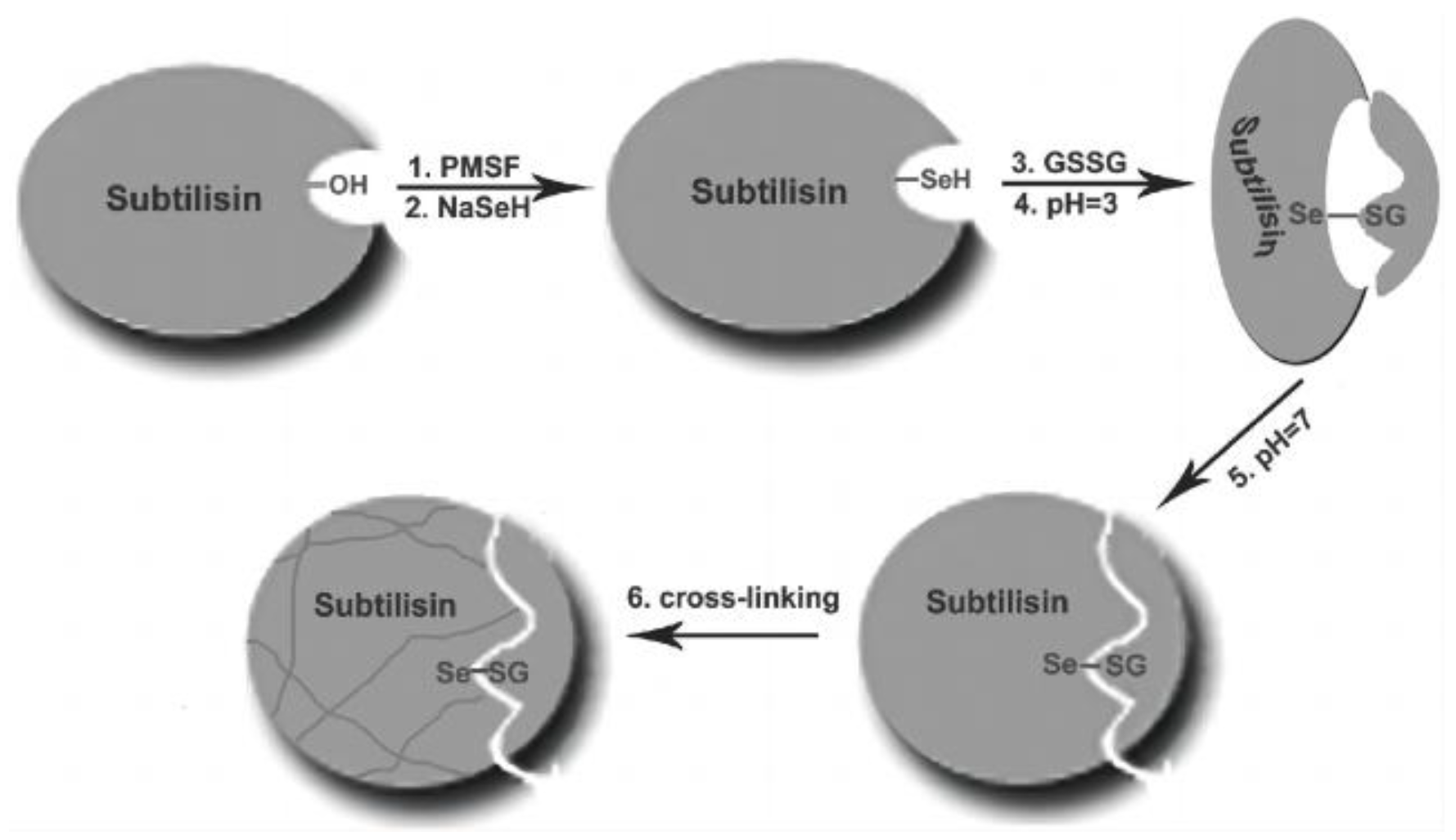
3.1.5. Semisynthetic Selenoenzyme by Chemical Mutation
3.1.6. Selenoenzyme Design by Genetic Engineering
3.2. Multiple Assembly of Artificial Selenoenzymes
3.2.1. Assembly of Artificial Selenoenzymes by Electrostatic Interaction
3.2.2. Assembly of Artificial Selenoenzymes by Metal Chelation Force-Coordination
3.2.3. Assembly of Artificial Selenoenzymes by Host–Guest Interaction
3.3. Multi-Enzyme Cascade Nanozyme Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction with Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Li, W.-L.; Head-Gordon, T. Catalytic Principles from Natural Enzymes and Translational Design Strategies for Synthetic Catalysts. ACS Cent. Sci. 2020, 7, 72–80. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Fan, K. Nanozymes Inspired by Natural Enzymes. Acc. Mater. Res. 2021, 2, 534–547. [Google Scholar] [CrossRef]
- Kaushal, N.; Hegde, S.; Lumadue, J.; Paulson, R.F.; Prabhu, K.S. The Regulation of Erythropoiesis by Selenium in Mice. Antioxid. Redox Signal. 2011, 14, 1403–1412. [Google Scholar] [CrossRef]
- Ahmed, A.; Abagana, A.; Cui, D.; Zhao, M. De Novo Iron Oxide Hydroxide, Ferrihydrite Produced by Comamonas Testosteroni Exhibiting Intrinsic Peroxidase-like Activity and Their Analytical Applications. BioMed Res. Int. 2019, 2019, 7127869. [Google Scholar] [CrossRef]
- Liu, L.; Lai, Y.; Cao, J.; Peng, Y.; Tian, T.; Fu, W. Exploring the Antibacterial and Biosensing Applications of Peroxidase-Mimetic Ni0.1Cu0.9S Nanoflower. Biosensors 2022, 12, 874. [Google Scholar] [CrossRef]
- Feldbauer, R.; Gosch, L.; Lüftinger, L.; Hyden, P.; Flexer, A.; Rattei, T. DeepNOG: Fast and Accurate Protein Orthologous Group Assignment. Bioinformatics 2020, 36, 5304–5312. [Google Scholar] [CrossRef]
- Hong, S.; Zhang, Q.-L.; Zheng, D.-W.; Zhang, C.; Zhang, Y.; Ye, J.-J.; Cheng, H.; Zhang, X.-Z. Enzyme Mimicking Based on the Natural Melanin Particles from Human Hair. iScience 2020, 23, 100778. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, D.; Cho, A.; Weon, S.; Lee, S.; Lee, J.; Han, J.W.; Kim, D.-P.; Choi, W. Modified Carbon Nitride Nanozyme as Bifunctional Glucose Oxidase-Peroxidase for Metal-Free Bioinspired Cascade Photocatalysis. Nat. Commun. 2019, 10, 940. [Google Scholar] [CrossRef]
- Huang, X.; Liu, X.; Luo, Q.; Liu, J.; Shen, J. Artificial Selenoenzymes: Designed and Redesigned. Chem. Soc. Rev. 2011, 40, 1171–1184. [Google Scholar] [CrossRef]
- Ishihara, Y.; Takemoto, T.; Ishida, A.; Yamazaki, T. Protective Actions of 17β-Estradiol and Progesterone on Oxidative Neuronal Injury Induced by Organometallic Compounds. Oxidative Med. Cell. Longev. 2015, 2015, 343706. [Google Scholar] [CrossRef]
- Ingold, I.; Aichler, M.; Yefremova, E.; Roveri, A.; Buday, K.; Doll, S.; Tasdemir, A.; Hoffard, N.; Wurst, W.; Walch, A.; et al. Expression of a Catalytically Inactive Mutant Form of Glutathione Peroxidase 4 (Gpx4) Confers a Dominant-Negative Effect in Male Fertility. J. Biol. Chem. 2015, 290, 14668–14678. [Google Scholar] [CrossRef]
- Subermaniam, K.; Yow, Y.Y.; Lim, S.H.; Koh, O.H.; Wong, K.H. Malaysian Macroalga Padina Australis Hauck Attenuates High Dose Corticosterone-Mediated Oxidative Damage in PC12 Cells Mimicking the Effects of Depression. Saudi J. Biol. Sci. 2020, 27, 1435–1445. [Google Scholar] [CrossRef]
- Shen, J.; Yang, D.; Zhou, X.; Wang, Y.; Tang, S.; Yin, H.; Wang, J.; Chen, R.; Chen, J. Role of Autophagy in Zinc Oxide Nanoparticles-Induced Apoptosis of Mouse LEYDIG Cells. Int. J. Mol. Sci. 2019, 20, 4042. [Google Scholar] [CrossRef]
- Ren, T.-B.; Zhang, Q.-L.; Su, D.; Zhang, X.-X.; Yuan, L.; Zhang, X.-B. Detection of Analytes in Mitochondria without Interference from Other Sites Based on an Innovative Ratiometric Fluorophore. Chem. Sci. 2018, 9, 5461–5466. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Liu, Y.; Disasa, D.; Akira, M.; Xiang, L.; Qi, J. A New Geniposidic Acid Derivative Exerts Antiaging Effects through Antioxidative Stress and Autophagy Induction. Antioxidants 2021, 10, 987. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef]
- Alharby, H.; Abdelati, T.; Rizk, M.; Youssef, E.; Moghazy, K.; Gaber, N.; Yafei, S. Association of Lipid Peroxidation and Interleukin-6 with Carotid Atherosclerosis in Type 2 Diabetes. Cardiovasc. Endocrinol. Metab. 2019, 8, 73–76. [Google Scholar] [CrossRef]
- Moosmayer, D.; Hilpmann, A.; Hoffmann, J.; Schnirch, L.; Zimmermann, K.; Badock, V.; Furst, L.; Eaton, J.K.; Viswanathan, V.S.; Schreiber, S.L.; et al. Crystal Structures of the Selenoprotein Glutathione Peroxidase 4 in Its Apo Form and in Complex with the Covalently Bound Inhibitor ML162. Acta Crystallogr. Sect. D Struct. Biol. 2021, 77, 237–248. [Google Scholar] [CrossRef]
- Limaye, A.; Yu, R.-C.; Chou, C.-C.; Liu, J.-R.; Cheng, K.-C. Protective and Detoxifying Effects Conferred by Dietary Selenium and Curcumin against AFB1-Mediated Toxicity in Livestock: A Review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef]
- Dong, C.; Shi, H.; Han, Y.; Yang, Y.; Wang, R.; Men, J. Molecularly Imprinted Polymers by the Surface Imprinting Technique. Eur. Polym. J. 2021, 145, 110231. [Google Scholar] [CrossRef]
- Pajewska-Szmyt, M.; Biniewska, E.; Buszewski, B.; Gadzała-Kopciuch, R. Synthesis of Magnetic Molecularly Imprinted Polymer Sorbents for Isolation of Parabens from Breast Milk. Materials 2020, 13, 4328. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yin, Y.; Liu, Y.; Bai, X.; Zhang, Z.; Xu, J.; Shen, J.; Liu, J. Incorporation of Glutathione Peroxidase Active Site into Polymer Based on Imprinting Strategy. Biosens. Bioelectron. 2009, 25, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Liang, K.; Tang, Y.; Liu, J. Construction of the Active Site of Glutathione Peroxidase on Polymer-Based Nanoparticles. Biomacromolecules 2008, 9, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mao, S.; Liu, X.; Huang, X.; Xu, J.; Liu, J.; Luo, G.; Shen, J. Functional Mimicry of the Active Site of Glutathione Peroxidase by Glutathione Imprinted Selenium-Containing Protein. Biomacromolecules 2007, 9, 363–368. [Google Scholar] [CrossRef]
- Bergwerff, A.A.; Debast, S.B. Modernization of Control of Pathogenic Micro-Organisms in the Food-Chain Requires a Durable Role for Immunoaffinity-Based Detection Methodology—A Review. Foods 2021, 10, 832. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Enzyme Models—From Catalysis to Prodrugs. Molecules 2021, 26, 3248. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Ding, L.; Luo, G.M.; Liu, Z.; Sun, Q.A.; Yang, T.S.; Shen, J.C. Some Physicochemical and Enzymatic Properties of Selenium-Containing Abzyme. Biochem. Biophys. Res. Commun. 1994, 202, 1645–1650. [Google Scholar] [CrossRef]
- Su, D.; Ren, X.; You, D.; Li, D.; Mu, Y.; Yan, G.; Zhang, Y.; Luo, Y.; Xue, Y.; Shen, J.; et al. Generation of Three Selenium-Containing Catalytic Antibodies with High Catalytic Efficiency Using a Novel Hapten Design Method. Arch. Biochem. Biophys. 2001, 395, 177–184. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Gao, S.-J.; Luo, G.-M.; Yan, G.-L.; Shen, J.-C. Artificial Imitation of Glutathione Peroxidase with 6-Selenium-Bridged β-Cyclodextrin. Biochem. Biophys. Res. Commun. 1998, 247, 397–400. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Huang, X.; Mao, S.-Z.; Liang, K.; Liu, J.-Q.; Luo, G.-M.; Shen, J.-C. Cyclodextrin-Derived Mimic of Glutathione Peroxidase Exhibiting Enzymatic Specificity and High Catalytic Efficiency. Chem. A Eur. J. 2006, 12, 3575–3579. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, M.; Engman, L.; Birmingham, A.; Powis, G.; Cotgreave, I.A. Cyclodextrin-Derived Diorganyl Tellurides as Glutathione Peroxidase Mimics and Inhibitors of Thioredoxin Reductase and Cancer Cell Growth. J. Med. Chem. 2003, 47, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ren, X.; Liu, J.; Mu, Y.; Shen, J. Towards More Efficient Glutathione Peroxidase Mimics: Substrate Recognition and Catalytic Group Assembly. Curr. Med. Chem. 2003, 10, 1151–1183. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, R.; Izadyar, M. Computational Modeling of the Kinetics and Mechanism of Tellurium-Based Glutathione Peroxidase Mimic. Int. J. Quantum Chem. 2020, 120, e26201. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Wang, L.; Li, S.; Chu, S.; Wang, J.; Li, Y.; Hou, J.; Luo, Q.; Liu, J. Design of Cyclodextrin-Based Functional Systems for Biomedical Applications. Front. Chem. 2021, 9, 635507. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qu, X.; Xie, Y.; Lv, S. Study of 8 Types of Glutathione Peroxidase Mimics Based on β-Cyclodextrin. Catalysts 2017, 7, 289. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Smart Stimuli-Responsive Drug Delivery Systems Based on Cyclodextrin: A Review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef]
- Marasini, N.; Fu, C.; Fletcher, N.L.; Subasic, C.; Er, G.; Mardon, K.; Thurecht, K.J.; Whittaker, A.K.; Kaminskas, L.M. The Impact of Polymer Size and Cleavability on the Intravenous Pharmacokinetics of PEG-Based Hyperbranched Polymers in Rats. Nanomaterials 2020, 10, 2452. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Li, P. Current Advances in Development of New Docetaxel Formulations. Expert Opin. Drug Deliv. 2019, 16, 301–312. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-Based Drug Delivery Systems: History, Challenges, and Latest Developments. J. Biol. Eng. 2022, 16. [Google Scholar] [CrossRef] [PubMed]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-Based Drug and Imaging Conjugates: Design Considerations for Nanomedical Applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Dwivedi, D.K.; Singh, A.K. Dendrimers: A novel carrier system for drug delivery. J. Drug Deliv. Ther. 2014, 4. [Google Scholar] [CrossRef]
- Matveev, V.V.; Markelov, D.A.; Dvinskikh, S.V.; Shishkin, A.N.; Tyutyukin, K.V.; Penkova, A.V.; Tatarinova, E.A.; Ignat’eva, G.M.; Milenin, S.A. Investigation of Melts of Polybutylcarbosilane Dendrimers by 1H NMR Spectroscopy. Sci. Rep. 2017, 7, 13710. [Google Scholar] [CrossRef] [PubMed]
- Kurtoglu, Y.E.; Mishra, M.K.; Kannan, S.; Kannan, R.M. Drug Release Characteristics of PAMAM Dendrimer–Drug Conjugates with Different Linkers. Int. J. Pharm. 2010, 384, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Nayak, S. Dendrimers: A Review on Synthetic Approaches. J. Appl. Pharm. Sci. 2015, 5, 117–122. [Google Scholar] [CrossRef]
- Liu, J.; Gray, W.D.; Davis, M.E.; Luo, Y. Peptide- and Saccharide-Conjugated Dendrimers for Targeted Drug Delivery: A Concise Review. Interface Focus 2012, 2, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Morshed, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Chen, G.; Nierstrasz, V. Surface Modification of Polyester Fabric Using Plasma-Dendrimer for Robust Immobilization of Glucose Oxidase Enzyme. Sci. Rep. 2019, 9, 15730. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Advances in Development of the Dendrimers Having Natural Saccharides in Their Structure for Efficient and Controlled Drug Delivery Applications. Eur. Polym. J. 2021, 148, 110356. [Google Scholar] [CrossRef]
- Lee, S.-R.; Kim, Y.-J. Hydrophilic Chlorin E6-Poly(Amidoamine) Dendrimer Nanoconjugates for Enhanced Photodynamic Therapy. Nanomaterials 2018, 8, 445. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Gupta, L.; Sahu, H.; Qayum, A.; Singh, S.K.; Nakhate, K.T.; Gupta, U. Chitosan Engineered PAMAM Dendrimers as Nanoconstructs for the Enhanced Anti-Cancer Potential and Improved in Vivo Brain Pharmacokinetics of Temozolomide. Pharm. Res. 2018, 35, 9. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Huang-Fu, M.-Y.; Guo, W.-W.; Guo, N.-N.; Chen, J.; Liu, H.-N.; Xie, Z.-Q.; Lin, M.-T.; Wei, Q.-C.; Gao, J.-Q. MMP-2-Sensitive HA End-Conjugated Poly(Amidoamine) Dendrimers via Click Reaction to Enhance Drug Penetration into Solid Tumor. ACS Appl. Mater. Interfaces 2017, 9, 42459–42470. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Motoyama, K.; Higashi, T. Sugar-Appended Polyamidoamine Dendrimer Conjugates with Cyclodextrins as Cell-Specific Non-Viral Vectors. Adv. Drug Deliv. Rev. 2013, 65, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched Polymers: Advances from Synthesis to Applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Li, T.; Smet, M.; Dehaen, W.; Xu, H. Selenium–Platinum Coordination Dendrimers with Controlled Anti-Cancer Activity. ACS Appl. Mater. Interfaces 2015, 8, 3609–3614. [Google Scholar] [CrossRef]
- Zhao, L.; Wan, J. Synthesis and Applications of Dendrimer-Modified Mesoporous Nanoparticles. Mater. Lab 2022, 1, 220018. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Yu, Q.; Chen, S.; Cui, Y.; Sun, H.; Gao, D.; Lan, X.; Yang, Q.; Xiao, H. Enhanced Glucose Detection Using Dendrimer Encapsulated Gold Nanoparticles Benefiting from Their Zwitterionic Surface. J. Biomater. Sci. Polym. Ed. 2018, 29, 2267–2280. [Google Scholar] [CrossRef]
- Fang, R.; Liu, Y.; Wang, Z.; Zhang, X. Water-Soluble Supramolecular Hyperbranched Polymers Based on Host-Enhanced π–π Interaction. Polym. Chem. 2013, 4, 900. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, T.; Qiu, Y.; Lin, Y.; Yao, Y.; Lian, W.; Lin, L.; Song, J.; Yang, H. An Inorganic Prodrug, Tellurium Nanowires with Enhanced ROS Generation and GSH Depletion for Selective Cancer Therapy. Chem. Sci. 2019, 10, 7068–7075. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.-W.; Sies, H. ChemInform Abstract: Chemistry of Biologically Important Synthetic Organoselenium Compounds. ChemInform 2010, 32, 2125–2180. [Google Scholar] [CrossRef]
- Mugesh, G.; Singh, H.B. ChemInform Abstract: Synthetic Organoselenium Compounds as Antioxidants: Glutathione Peroxidase Activity. ChemInform 2000, 31, 347–357. [Google Scholar] [CrossRef]
- Kheirabadi, R.; Izadyar, M. Computational Modeling of the Kinetics and Mechanism of the New Generation of Glutathione Peroxidase Nanomimic: Selenosubtilisin and Tellurosubtilisin. J. Iran. Chem. Soc. 2020, 17, 2119–2131. [Google Scholar] [CrossRef]
- Newton, T.D.; Pluth, M.D. Development of a Hydrolysis-Based Small-Molecule Hydrogen Selenide (H2Se) Donor. Chem. Sci. 2019, 10, 10723–10727. [Google Scholar] [CrossRef]
- Blackburn, A.C.; Tzeng, H.-F.; Anders, M.W.; Board, P.G. Discovery of a Functional Polymorphism in Human Glutathione Transferase Zeta by Expressed Sequence Tag Database Analysis. Pharmacogenetics 2000, 10, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Board, P.G.; Fei, X.; Sun, Y.; Lv, S.; Yan, G.; Liu, J.; Shen, J.; Luo, G. A Novel Selenium-Containing Glutathione Transferase Zeta1-1, the Activity of Which Surpasses the Level of Some Native Glutathione Peroxidases. Int. J. Biochem. Cell Biol. 2008, 40, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, R.; Rozovsky, S. Synthesis and Semisynthesis of Selenopeptides and Selenoproteins. Curr. Opin. Chem. Biol. 2018, 46, 41–47. [Google Scholar] [CrossRef]
- Szoniec, G.; Ogorzalek, M.J. Entropy of Never Born Protein Sequences. SpringerPlus 2013, 2, 200. [Google Scholar] [CrossRef]
- Kaushik, R.; Zhang, K.Y.J. A Protein Sequence Fitness Function for Identifying Natural and Nonnatural Proteins. Proteins Struct. Funct. Bioinform. 2020, 88, 1271–1284. [Google Scholar] [CrossRef]
- Gelly, J.-C.; de Brevern, A.G. Protein Peeling 3D: New Tools for Analyzing Protein Structures. Bioinformatics 2010, 27, 132–133. [Google Scholar] [CrossRef]
- Zehfus, M.H. Continuous Compact Protein Domains. Proteins Struct. Funct. Genet. 1987, 2, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Cowie, D.B.; Cohen, G.N. Biosynthesis by Escherichia coli of Active Altered Proteins Containing Selenium instead of Sulfur. Biochim. Biophys. Acta 1957, 26, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Bogicevic, B.; Berthoud, H.; Portmann, R.; Bavan, T.; Meile, L.; Irmler, S. Cysteine Biosynthesis in Lactobacillus casei: Identification and Characterization of a Serine Acetyltransferase. FEMS Microbiol. Lett. 2016, 363, fnw012. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Qi, Z.; Wang, Y.; Liu, X.; Li, J.; Xu, J.; Liu, J.; Shen, J. Engineered Selenium-Containing Glutaredoxin Displays Strong Glutathione Peroxidase Activity Rivaling Natural Enzyme. Int. J. Biochem. Cell Biol. 2009, 41, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, R.; Izadyar, M. Computational Modeling of the Catalytic Cycle of Glutathione Peroxidase Nanomimic. J. Phys. Chem. A 2016, 120, 10108–10115. [Google Scholar] [CrossRef]
- Netto, L.E.S.; de Oliveira, M.A.; Tairum, C.A.; da Silva Neto, J.F. Conferring Specificity in Redox Pathways by Enzymatic Thiol/Disulfide Exchange Reactions. Free Radic. Res. 2016, 50, 206–245. [Google Scholar] [CrossRef]
- Qi, Y.; Grishin, N.V. Structural Classification of Thioredoxin-like Fold Proteins. Proteins Struct. Funct. Bioinform 2004, 58, 376–388. [Google Scholar] [CrossRef]
- Goemans, C.V.; Beaufay, F.; Wahni, K.; Van Molle, I.; Messens, J.; Collet, J.-F. An Essential Thioredoxin Is Involved in the Control of the Cell Cycle in the Bacterium Caulobacter Crescentus. J. Biol. Chem. 2018, 293, 3839–3848. [Google Scholar] [CrossRef]
- Niedźwiedź, A.; Nicpoń, J.; Zawadzki, M.; Służewska-Niedźwiedź, M.; Januszewska, L. The Influence of Road Transport on the Activities of Glutathione Reductase, Glutathione Peroxidase, and Glutathione-S-Transferase in Equine Erythrocytes. Vet. Clin. Pathol. 2012, 41, 123–126. [Google Scholar] [CrossRef]
- Qian, Z.-G.; Pan, F.; Xia, X.-X. Synthetic Biology for Protein-Based Materials. Curr. Opin. Biotechnol. 2020, 65, 197–204. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Chen, H.; Tian, R.; Li, F.; Luo, Q.; Xu, J.; Hou, C.; Liu, J. Hierarchical Protein Self-Assembly into Dynamically Controlled 2D Nanoarrays Via Host–Guest Chemistry. Chem. Commun. 2021, 57, 10620–10623. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Avakyan, N.; Kakkis, A.; Hoffnagle, A.M.; Han, K.; Li, Y.; Zhang, Z.; Choi, T.S.; Na, Y.; Yu, C.-J.; et al. Protein Assembly by Design. Chem. Rev. 2021, 121, 13701–13796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mo, S.; Liu, M.; Liu, L.; Yu, L.; Wang, C. Rationally Designed Protein Building Blocks for Programmable Hierarchical Architectures. Front. Chem. 2020, 8, 587975. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, X.; Si, C.; Gao, Y.; Zhao, L.; Hou, C.; Shoseyov, O.; Luo, Q.; Liu, J. Construction of a Highly Stable Artificial Glutathione Peroxidase on a Protein Nanoring. Org. Biomol. Chem. 2014, 12, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Han, J.; Zhang, H.; Zhao, L.; Si, C.; Zhang, X.; Hou, C.; Luo, Q.; Xu, J.; Liu, J. Quantum-Dot-Induced Self-Assembly of Cricoid Protein for Light Harvesting. ACS Nano 2014, 8, 3743–3751. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Fan, Q.; Zhao, L.; Qiao, Q.; Zhang, X.; Hou, C.; Xu, J.; Luo, Q.; Liu, J. The Construction of Functional Protein Nanotubes by Small Molecule-Induced Self-Assembly of Cricoid Proteins. Chem. Commun. 2016, 52, 4092–4095. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Liu, Y.; Sun, H.; Xu, J.; Liu, J. Reversible Switch of a Selenium-Containing Antioxidant System Regulated by Protein Assembly. ACS Catal. 2020, 10, 9735–9740. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, Q.; Miao, L.; Hou, C.; Bai, Y.; Dong, Z.; Xu, J.; Liu, J. Self-Assembly of Glutathione S-Transferase into Nanowires. Nanoscale 2012, 4, 5847. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Luo, Q.; Zhang, W.; Miao, L.; Xu, J.; Li, H.; Liu, J. Highly Ordered Protein Nanorings Designed by Accurate Control of Glutathione S-Transferase Self-Assembly. J. Am. Chem. Soc. 2013, 135, 10966–10969. [Google Scholar] [CrossRef]
- Shen, T.; Cao, Y.; Zhuang, S.; Li, H. Engineered Bi-Histidine Metal Chelation Sites Map the Structure of the Mechanical Unfolding Transition State of an Elastomeric Protein Domain GB1. Biophys. J. 2012, 103, 807–816. [Google Scholar] [CrossRef]
- Henry, A.P.; Probert, K.; Stewart, C.E.; Thakker, D.; Bhaker, S.; Azimi, S.; Hall, I.P.; Sayers, I. Defining a Role for Lung Function Associated Gene GSTCD in Cell Homeostasis. Respir. Res. 2019, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Li, J.; Zhao, L.; Zhang, W.; Luo, Q.; Dong, Z.; Xu, J.; Liu, J. Construction of Protein Nanowires through Cucurbit [8] Uril-Based Highly Specific Host-Guest Interactions: An Approach to the Assembly of Functional Proteins. Angew. Chem. 2013, 125, 5700–5703. [Google Scholar] [CrossRef]
- Yu, S.; Yin, Y.; Zhu, J.; Huang, X.; Luo, Q.; Xu, J.; Shen, J.; Liu, J. A Modulatory Bifunctional Artificial Enzyme with Both SOD and GPx Activities Based on a Smart Star-Shaped Pseudo-Block Copolymer. Soft Matter 2010, 6, 5342. [Google Scholar] [CrossRef]
- Sun, H.; Miao, L.; Li, J.; Fu, S.; An, G.; Si, C.; Dong, Z.; Luo, Q.; Yu, S.; Xu, J.; et al. Self-Assembly of Cricoid Proteins Induced by “Soft Nanoparticles”: An Approach to Design Multienzyme-Cooperative Antioxidative Systems. ACS Nano 2015, 9, 5461–5469. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Zhang, Y.-H.; Ren, H.; Wang, Y.-L.; Jiang, W.; Fang, B.-S. Strategies and Perspectives of Assembling Multi-Enzyme Systems. Crit. Rev. Biotechnol. 2017, 37, 1024–1037. [Google Scholar] [CrossRef]
- Wang, S.; Tian, R.; Liu, B.; Wang, H.; Liu, J.; Li, C.; Li, M.; Evivie, S.E.; Li, B. Effects of Carbon Concentration, Oxygen, and Controlled PH on the Engineering Strain Lactiplantibacillus Casei E1 in the Production of Bioethanol from Sugarcane Molasses. AMB Express 2021, 11, 95. [Google Scholar] [CrossRef]
- Osman, A.; Oner, E.T.; Eroglu, M.S. Novel Levan and PNIPA Temperature Sensitive Hydrogels for 5-ASA Controlled Release. Carbohydr. Polym. 2017, 165, 61–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Shang, J.; Shao, W.; Jin, L.; Quan, C.; Li, J. Emerging Nanozyme-Based Multimodal Synergistic Therapies in Combating Bacterial Infections. Theranostics 2022, 12, 5995–6020. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Xu, C.; Wang, T.; Liu, J. Biomimetic Construction of Artificial Selenoenzymes. Biomimetics 2023, 8, 54. https://doi.org/10.3390/biomimetics8010054
Zhao H, Xu C, Wang T, Liu J. Biomimetic Construction of Artificial Selenoenzymes. Biomimetics. 2023; 8(1):54. https://doi.org/10.3390/biomimetics8010054
Chicago/Turabian StyleZhao, Hanqing, Chengchen Xu, Tingting Wang, and Junqiu Liu. 2023. "Biomimetic Construction of Artificial Selenoenzymes" Biomimetics 8, no. 1: 54. https://doi.org/10.3390/biomimetics8010054
APA StyleZhao, H., Xu, C., Wang, T., & Liu, J. (2023). Biomimetic Construction of Artificial Selenoenzymes. Biomimetics, 8(1), 54. https://doi.org/10.3390/biomimetics8010054






