Silk-Based Biomaterials for Designing Bioinspired Microarchitecture for Various Biomedical Applications
Abstract
1. Introduction
2. Biomaterials
2.1. Definition
2.2. Biobased Biomaterials
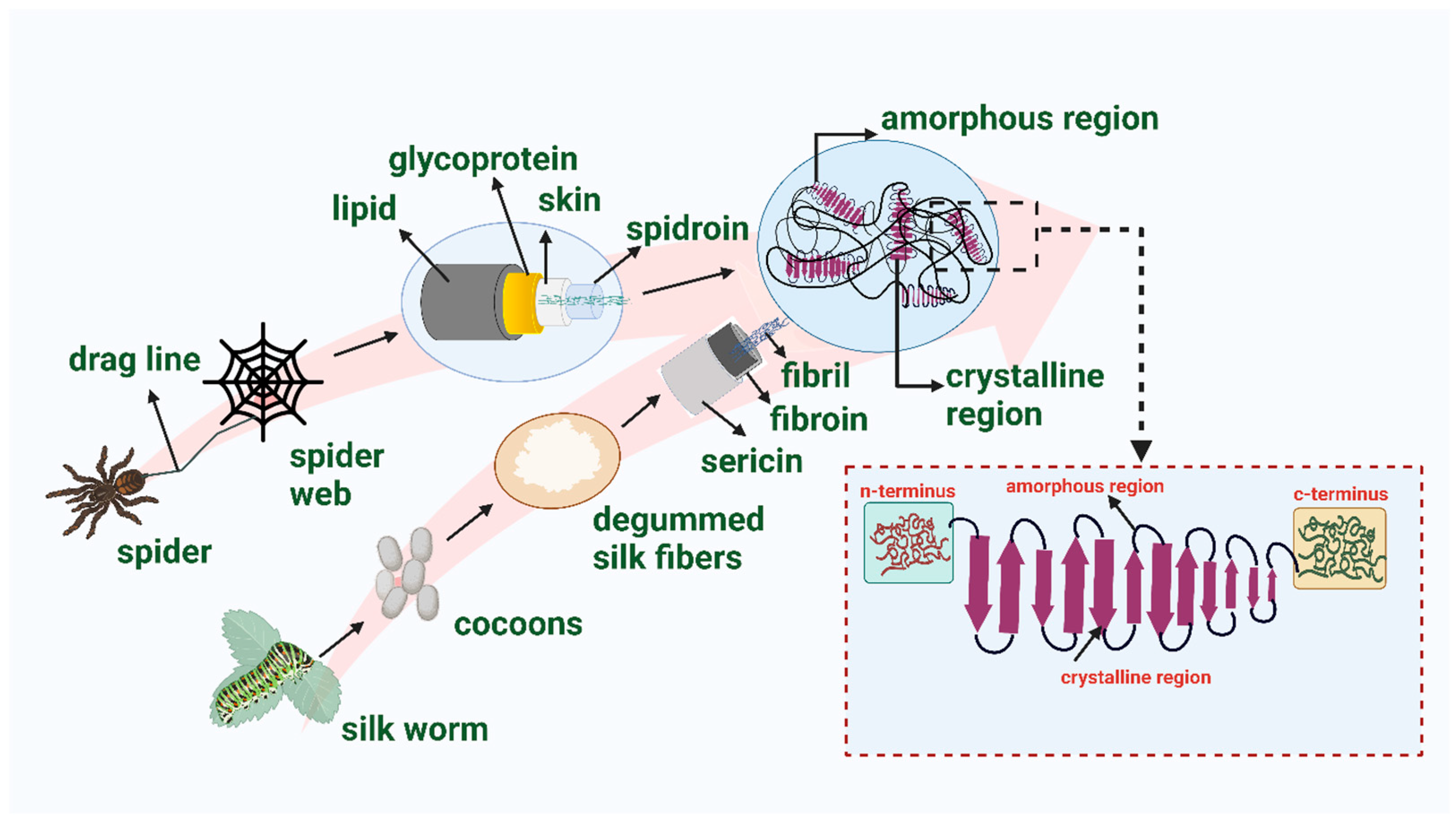
3. Silk
3.1. Global Potential of Silk as a Biopolymer
3.2. Silk Characteristics
3.3. Extraction of Silk Fibroin
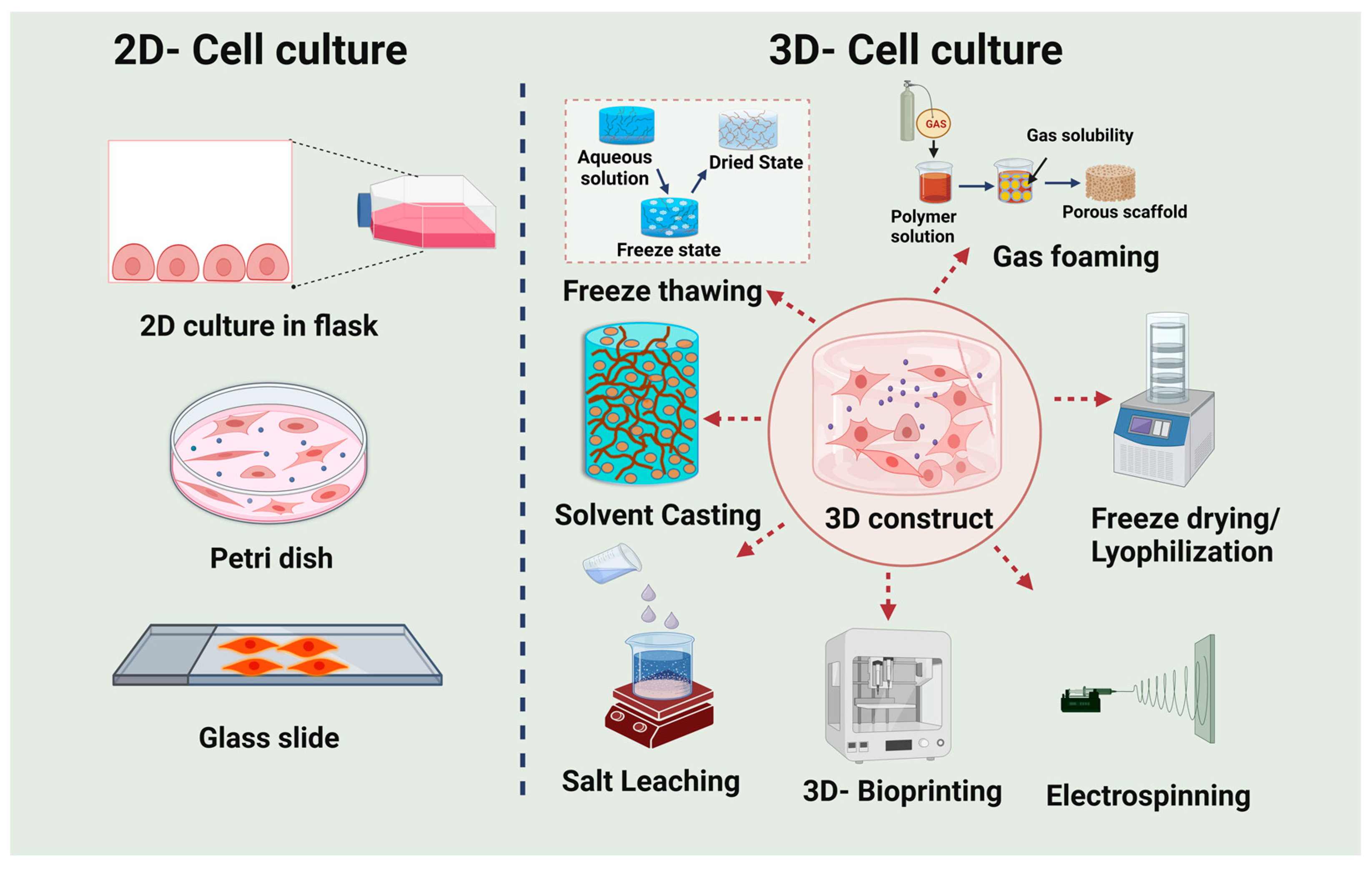
4. Development of 3D Microarchitecture Biomaterials
5. Various Approaches for Fabricating 3D Microarchitecture Scaffolds
5.1. Hydrogels
5.2. Electrospinning
5.3. Salt Leaching
5.4. 3D Bioprinting
5.5. Gas Foaming
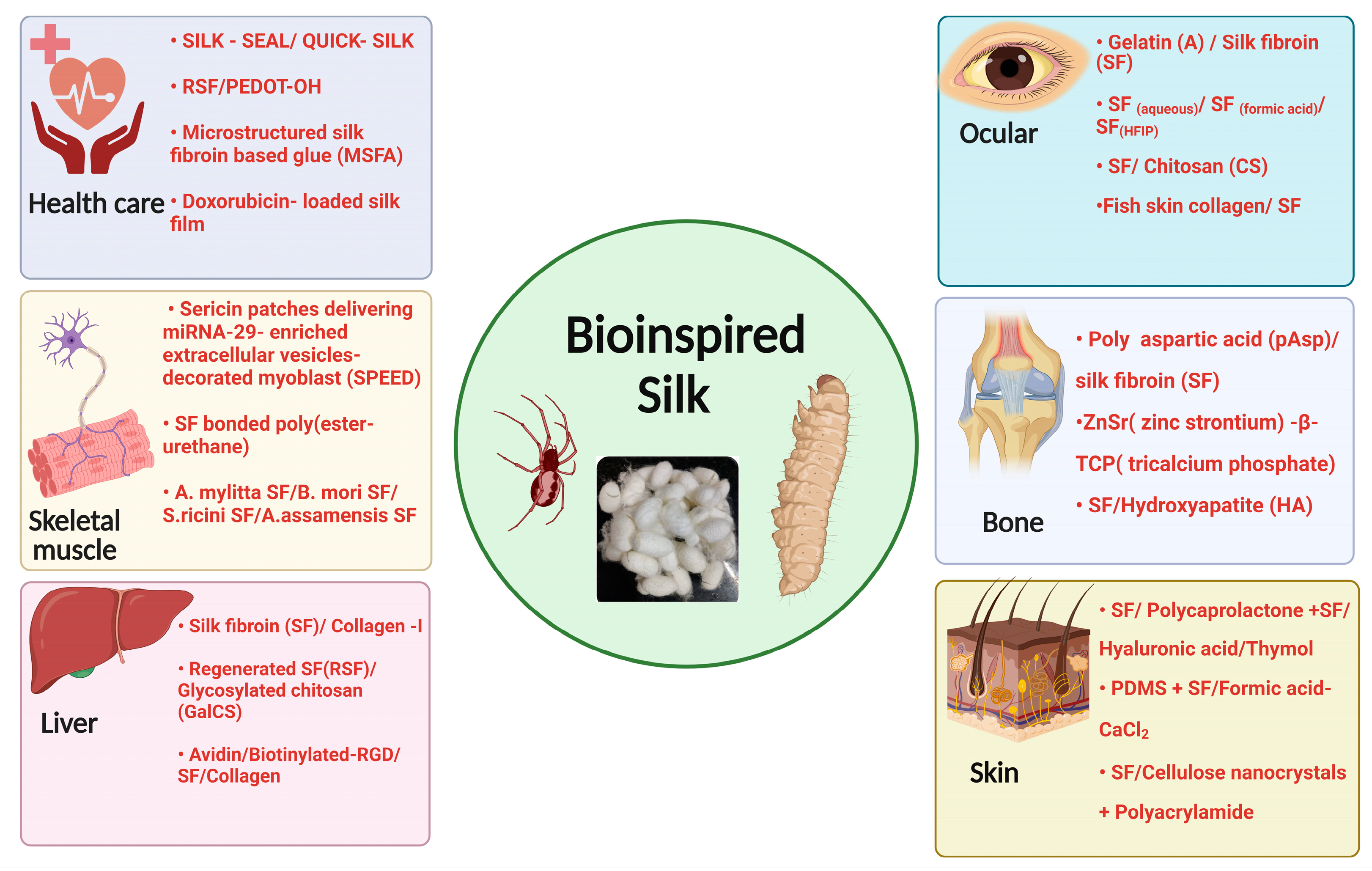
6. Bioinspiration of Silk for Biomedical and Tissue Engineering Applications
6.1. Bioinspiration of Bone Tissue Engineering
6.2. Bioinspiration of Liver Tissue Engineering
6.3. Bioinspiration of Ocular Tissue Engineering
6.4. Bioinspiration of Skeletal Muscle Engineering
6.5. Bioinspiration of Skin Tissue Engineering
6.6. Bioinspiration of Silk in the Area of Healthcare Applications
7. Conclusions and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.; Chang, J.; Zhu, Y.; Wu, C. 3D Printing of Bioinspired Biomaterials for Tissue Regeneration. Adv. Healthc. Mater. 2020, 9, 2000208. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, X.; Li, X.; Chen, Z.; Zhou, C.; Zhou, Q.; Chen, Y. Recent Progress in Biomimetic Additive Manufacturing Technology: From Materials to Functional Structures. Adv. Mater. 2018, 30, 1706539. [Google Scholar] [CrossRef]
- Wu, H.Y.; Zhang, F.; Yue, X.X.; Ming, J.F.; Zuo, B.Q. Wet-Spun Silk Fibroin Scaffold with Hierarchical Structure for Ligament Tissue Engineering. Mater. Lett. 2014, 135, 63–66. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, C.S.; Rath, S.N.; Ramakrishna, S. Electrospun Nanofibres to Mimic Natural Hierarchical Structure of Tissues: Application in Musculoskeletal Regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e604–e619. [Google Scholar] [CrossRef]
- Sadtler, K.; Wolf, M.T.; Ganguly, S.; Moad, C.A.; Chung, L.; Majumdar, S.; Housseau, F.; Pardoll, D.M.; Elisseeff, J.H. Divergent Immune Responses to Synthetic and Biological Scaffolds. Biomaterials 2019, 192, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Roy, K.; Keselowsky, B.G. Materials That Harness and Modulate the Immune System. MRS Bull. 2014, 39, 25–34. [Google Scholar] [CrossRef]
- Irvine, D.J.; Swartz, M.A.; Szeto, G.L. Engineering Synthetic Vaccines Using Cues from Natural Immunity. Nat. Mater. 2013, 12, 978–990. [Google Scholar] [CrossRef]
- Gervaso, F.; Sannino, A.; Peretti, G.M. The Biomaterialist’s Task: Scaffold Biomaterials and Fabrication Technologies. Joints 2013, 1, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Chowdhury, S.K.; Dey, S.; Moses, J.C.; Mandal, B.B. Silk: A Promising Biomaterial Opening New Vistas Towards Affordable Healthcare Solutions. J. Indian Inst. Sci. 2019, 99, 445–487. [Google Scholar] [CrossRef]
- Jin, Y.; Kundu, B.; Cai, Y.; Kundu, S.; Yao, J. Bio-Inspired Mineralization of Hydroxyapatite in 3D Silk Fibroin Hydrogel for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2015, 134, 339–345. [Google Scholar] [CrossRef]
- Yao, Q.; Lan, Q.-H.; Jiang, X.; Du, C.-C.; Zhai, Y.-Y.; Shen, X.; Xu, H.-L.; Xiao, J.; Kou, L.; Zhao, Y.-Z. Bioinspired Biliverdin/Silk Fibroin Hydrogel for Antiglioma Photothermal Therapy and Wound Healing. Theranostics 2020, 10, 11719–11736. [Google Scholar] [CrossRef] [PubMed]
- Dundar Arisoy, F.; Kolewe, K.W.; Homyak, B.; Kurtz, I.S.; Schiffman, J.D.; Watkins, J.J. Bioinspired Photocatalytic Shark-Skin Surfaces with Antibacterial and Antifouling Activity via Nanoimprint Lithography. ACS Appl. Mater. Interfaces 2018, 10, 20055–20063. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, K.; Gao, Q.; Zhang, W.; Zhou, W.; Shi, S.Q.; Xia, C.; Li, J. Bioinspired Design by Gecko Structure and Mussel Chemistry for Bio-Based Adhesive System through Incorporating Natural Fibers. J. Clean. Prod. 2019, 236, 117591. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Zhang, L.; Zhu, C.; Wang, J.; Li, Y.; Wang, Y.; Wang, C.; Zhang, Y.; Yuan, Q. Bioinspired Extracellular Vesicles Embedded with Black Phosphorus for Molecular Recognition-Guided Biomineralization. Nat. Commun. 2019, 10, 2829. [Google Scholar] [CrossRef]
- Liu, X.; Steiger, C.; Lin, S.; Parada, G.A.; Liu, J.; Chan, H.F.; Yuk, H.; Phan, N.V.; Collins, J.; Tamang, S.; et al. Ingestible Hydrogel Device. Nat. Commun. 2019, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for Tissue Engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef]
- Lanza, R.P.; Langer, R.S.; Vacanti, J. Principles of Tissue Engineering, 2nd ed.; Academic Press: San Diego, CA, USA, 2000; ISBN 978-0-12-436630-5. [Google Scholar]
- Komeri, R.; Kasoju, N.; Anil Kumar, P.R. 13—In Vitro Cytotoxicity and Cytocompatibility Assays for Biomaterial Testing under Regulatory Platform. In Biomedical Product and Materials Evaluation; Mohanan, P.V., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2022; pp. 329–353. ISBN 978-0-12-823966-7. [Google Scholar]
- Keane, T.J.; Badylak, S.F. Biomaterials for Tissue Engineering Applications. Semin. Pediatr. Surg. 2014, 23, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered Biomaterials for in Situ Tissue Regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Maksoud, F.J.; de la Paz, M.F.V.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous Biomaterials for Tissue Engineering: A Review. J. Mater. Chem. B 2022, 10, 8111–8165. [Google Scholar] [CrossRef]
- Chaikof, E.L.; Matthew, H.; Kohn, J.; Mikos, A.G.; Prestwich, G.D.; Yip, C.M. Biomaterials and Scaffolds in Reparative Medicine. Ann. N. Y. Acad. Sci. 2002, 961, 96–105. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, C.; Thouas, G.A. Progress and Challenges in Biomaterials Used for Bone Tissue Engineering: Bioactive Glasses and Elastomeric Composites. Prog. Biomater. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic Biomaterials as Instructive Extracellular Microenvironments for Morphogenesis in Tissue Engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hillas, P.J.; Báez, J.A.; Nokelainen, M.; Balan, J.; Tang, J.; Spiro, R.; Polarek, J.W. The Application of Recombinant Human Collagen in Tissue Engineering. BioDrugs 2004, 18, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Gundu, S.; Sahi, A.K.; Varshney, N.; Varghese, J.; Vishwakarma, N.K.; Mahto, S.K. Fabrication and in Vitro Characterization of Luffa-Based Composite Scaffolds Incorporated with Gelatin, Hydroxyapatite and Psyllium Husk for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2022, 33, 2220–2248. [Google Scholar] [CrossRef]
- Agarwal, P.; Agarwal, P.; Sahi, A.; Varshney, N.; Poddar, S.; Mahto, S.K. Revisiting Efforts and Advancements in Tracheal Tissue Engineering. Authorea 2020. [Google Scholar] [CrossRef]
- Sahi, A.K.; Varshney, N.; Poddar, S.; Mahto, S.K. Comparative Behaviour of Electrospun Nanofibers Fabricated from Acid and Alkaline Hydrolysed Gelatin: Towards Corneal Tissue Engineering. J. Polym. Res. 2020, 27, 344. [Google Scholar] [CrossRef]
- Sahi, A.K.; Anjali; Varshney, N.; Poddar, S.; Vajanthri, K.Y.; Mahto, S.K. Optimizing a Detection Method for Estimating Polyunsaturated Fatty Acid in Human Milk Based on Colorimetric Sensors. Mater. Sci. Energy Technol. 2019, 2, 624–628. [Google Scholar] [CrossRef]
- Ha, T.L.B.; Quan, T.M.; Vu, D.N.; Si, D.M.; Ha, T.L.B.; Quan, T.M.; Vu, D.N.; Si, D.M. Naturally Derived Biomaterials: Preparation and Application; IntechOpen: London, UK, 2013; ISBN 978-953-51-1108-5. [Google Scholar]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Fukunishi, T.; Shoji, T.; Shinoka, T. 18—Nanofiber Composites in Vascular Tissue Engineering. In Nanofiber Composites for Biomedical Applications; Ramalingam, M., Ramakrishna, S., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 455–481. ISBN 978-0-08-100173-8. [Google Scholar]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Sobajo, C.; Behzad, F.; Yuan, X.-F.; Bayat, A. Silk: A Potential Medium for Tissue Engineering. Eplasty 2008, 8, e47. [Google Scholar] [PubMed]
- Bargel, H.; Trossmann, V.T.; Sommer, C.; Scheibel, T. Bioselectivity of Silk Protein-Based Materials and Their Bio-Inspired Applications. Beilstein J. Nanotechnol. 2022, 13, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Wang, Y.; Chang, H.-I.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds; IntechOpen: Sawston, UK, 2011; ISBN 978-953-307-663-8. [Google Scholar]
- Garcia-Fuentes, M.; Meinel, A.J.; Hilbe, M.; Meinel, L.; Merkle, H.P. Silk Fibroin/Hyaluronan Scaffolds for Human Mesenchymal Stem Cell Culture in Tissue Engineering. Biomaterials 2009, 30, 5068–5076. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Ravikumar, A. Indian Silk Industry in the Global Scenario. EXCEL Int. J. Multidiscip. Manag. Stud. 2011, 1, 100–110. [Google Scholar]
- Bukhari, R.; Kour, H. Background, Current Scenario and Future Challenges of the Indian Silk Industry. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2448–2463. [Google Scholar] [CrossRef]
- Naskar, D.; Barua, R.R.; Ghosh, A.K.; Kundu, S.C. 1—Introduction to Silk Biomaterials. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine; Kundu, S.C., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 3–40. ISBN 978-0-85709-699-9. [Google Scholar]
- Padaki, N.V.; Das, B.; Basu, A. 1—Advances in Understanding the Properties of Silk. In Advances in Silk Science and Technology; Basu, A., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2015; pp. 3–16. ISBN 978-1-78242-311-9. [Google Scholar]
- Craig, C.L. Evolution of Arthropod Silks. Annu. Rev. Entomol. 1997, 42, 231–267. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.-Q.; Liu, X.; Zhang, K.-Q. Silk Fiber—Molecular Formation Mechanism, Structure-Property Relationship and Advanced Applications; IntechOpen: London, UK, 2014; ISBN 978-953-51-1617-2. [Google Scholar]
- Kiseleva, A.P.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Recent Advances in Development of Functional Spider Silk-Based Hybrid Materials. Front. Chem. 2020, 8, 554. [Google Scholar] [CrossRef]
- Shao, Z.; Vollrath, F. Surprising Strength of Silkworm Silk. Nature 2002, 418, 741. [Google Scholar] [CrossRef]
- Du, N.; Liu, X.Y.; Narayanan, J.; Li, L.; Lim, M.L.M.; Li, D. Design of Superior Spider Silk: From Nanostructure to Mechanical Properties. Biophys. J. 2006, 91, 4528. [Google Scholar] [CrossRef] [PubMed]
- Cranford, S.W. Increasing Silk Fibre Strength through Heterogeneity of Bundled Fibrils. J. R. Soc. Interface 2013, 10, 20130148. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Römer, L.; Scheibel, T. Polymeric Materials Based on Silk Proteins. Polymer 2008, 49, 4309–4327. [Google Scholar] [CrossRef]
- Chen, S.; Liu, M.; Huang, H.; Cheng, L.; Zhao, H.-P. Mechanical Properties of Bombyx mori Silkworm Silk Fibre and Its Corresponding Silk Fibroin Filament: A Comparative Study. Mater. Des. 2019, 181, 108077. [Google Scholar] [CrossRef]
- Hazra, S.; Nandi, S.; Naskar, D.; Guha, R.; Chowdhury, S.; Pradhan, N.; Kundu, S.C.; Konar, A. Non-Mulberry Silk Fibroin Biomaterial for Corneal Regeneration. Sci. Rep. 2016, 6, 21840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Shao, H.; Hu, X. Hybrid Silk Fibers Dry-Spun from Regenerated Silk Fibroin/Graphene Oxide Aqueous Solutions. ACS Appl. Mater. Interfaces 2016, 8, 3349–3358. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk Fibroin as Biomaterial for Bone Tissue Engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef]
- Konwarh, R.; Bhunia, B.K.; Mandal, B. Opportunities and Challenges in Exploring Indian Non-Mulberry Silk for Biomedical Applications. Proc. Indian Natl. Sci. Acad. 2017, 83, 85–101. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, Mechanical Properties and Applications of Silk Fibroin Materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Biswal, B.; Dan, A.K.; Sengupta, A.; Das, M.; Bindhani, B.K.; Das, D.; Parhi, P.K. Extraction of Silk Fibroin with Several Sericin Removal Processes and Its Importance in Tissue Engineering: A Review. J. Polym. Environ. 2022, 30, 2222–2253. [Google Scholar] [CrossRef]
- Wöltje, M.; Kölbel, A.; Aibibu, D.; Cherif, C. A Fast and Reliable Process to Fabricate Regenerated Silk Fibroin Solution from Degummed Silk in 4 Hours. Int. J. Mol. Sci. 2021, 22, 10565. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Nakao, H.; Takasu, Y.; Tsubouchi, K. Preparation of Undegraded Native Molecular Fibroin Solution from Silkworm Cocoons. Mater. Sci. Eng. C 2001, 14, 41–46. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, H.; Wang, C.; Wu, L.; Huang, J.; Guo, C. Tensile Behavior and Morphology of Differently Degummed Silkworm (Bombyx mori) Cocoon Silk Fibres. Mater. Lett. 2006, 60, 919–925. [Google Scholar] [CrossRef]
- Nultsch, K.; Bast, L.K.; Näf, M.; Yakhlifi, S.E.; Bruns, N.; Germershaus, O. Effects of Silk Degumming Process on Physicochemical, Tensile, and Optical Properties of Regenerated Silk Fibroin. Macromol. Mater. Eng. 2018, 303, 1800408. [Google Scholar] [CrossRef]
- Allardyce, B.J.; Rajkhowa, R.; Dilley, R.J.; Atlas, M.D.; Kaur, J.; Wang, X. The Impact of Degumming Conditions on the Properties of Silk Films for Biomedical Applications. Text. Res. J. 2016, 86, 275–287. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zhang, Y.-Q. Effect of Regeneration of Liquid Silk Fibroin on Its Structure and Characterization. Soft Matter 2013, 9, 138–145. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for Tissue Engineering and Regenerative Medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel Scaffolds for Tissue Engineering: Progress and Challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M.; Ahmad, S.R. Fabrication of Polymeric Biomaterials: A Strategy for Tissue Engineering and Medical Devices. J. Mater. Chem. B 2015, 3, 8224–8249. [Google Scholar] [CrossRef] [PubMed]
- Griffanti, G.; Jiang, W.; Nazhat, S.N. Bioinspired Mineralization of a Functionalized Injectable Dense Collagen Hydrogel through Silk Sericin Incorporation. Biomater. Sci. 2019, 7, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Zhu, K.; Duan, J.; Guo, J.; Wu, S.; Lu, A.; Zhang, L. High-Strength Films Consisted of Oriented Chitosan Nanofibers for Guiding Cell Growth. Biomacromolecules 2017, 18, 3904–3912. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Khoerunnisa, F.; Nurhayati, M.; Hiqmah, R.N.; Hendrawan, H.; Dara, F.; Aziz, H.A.; Sonjaya, Y.; Nasir, M. Effect of pH, Temperature, and Electrolytes on Swelling and Release Behaviors of PVA/AAm/GO Based Hydrogel Composites. AIP Conf. Proc. 2021, 2349, 020025. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Mi, S. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials 2016, 9, 614. [Google Scholar] [CrossRef]
- Mulholland, E.J. Electrospun Biomaterials in the Treatment and Prevention of Scars in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 481. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Park, J.-S. Electrospinning and Its Applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 1, 043002. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Hosseini, S.M.; Yousefi, M. Application of Electrospinning Technique in Development of Intelligent Food Packaging: A Short Review of Recent Trends. Food Sci. Nutr. 2020, 8, 4656–4665. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun Nanofibers: New Generation Materials for Advanced Applications. Mater. Sci. Eng. B 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Min, J.; Min, W.; Shiyong, W.; Zhibao, C.; Shichun, M. Aligned Nanofibers Based on Electrospinning Technology. Prog. Chem. 2016, 28, 711. [Google Scholar] [CrossRef]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-Based Systems for Fabrication of Electrospun Nanofibers: Food, Pharmaceutical and Biomedical Applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef]
- Sahi, A.; Varshney, N.; Poddar, S.; Gundu, S.; Mahto, S. Fabrication and Characterization of Silk Fibroin-Based Nanofibrous Scaffolds Supplemented with Gelatin for Corneal Tissue Engineering. Cells Tissues Organs 2021, 210, 173–194. [Google Scholar] [CrossRef]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Mahto, S.K. Soy Protein Isolate Supplemented Silk Fibroin Nanofibers for Skin Tissue Regeneration: Fabrication and Characterization. Int. J. Biol. Macromol. 2020, 160, 112–127. [Google Scholar] [CrossRef]
- Mohammadzadehmoghadam, S.; Dong, Y. Fabrication and Characterization of Electrospun Silk Fibroin/Gelatin Scaffolds Crosslinked With Glutaraldehyde Vapor. Front. Mater. 2019, 6, 91. [Google Scholar] [CrossRef]
- Xue, Y.; Kim, H.-J.; Lee, J.; Liu, Y.; Hoffman, T.; Chen, Y.; Zhou, X.; Sun, W.; Zhang, S.; Cho, H.-J.; et al. Co-Electrospun Silk Fibroin and Gelatin Methacryloyl Sheet Seeded with Mesenchymal Stem Cells for Tendon Regeneration. Small 2022, 18, 2107714. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Tse, J.W.; Nori, A.; Leong, K.W.; Yim, E.K.F. Chapter 27—Cell–Substrate Interactions. In Principles of Regenerative Medicine, 3rd ed.; Atala, A., Lanza, R., Mikos, A.G., Nerem, R., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 437–468. ISBN 978-0-12-809880-6. [Google Scholar]
- Varshney, N.; Sahi, A.K.; Vajanthri, K.Y.; Poddar, S.; Balavigneswaran, C.K.; Prabhakar, A.; Rao, V.; Mahto, S.K. Culturing Melanocytes and Fibroblasts within Three-Dimensional Macroporous PDMS Scaffolds: Towards Skin Dressing Material. Cytotechnology 2019, 71, 287–303. [Google Scholar] [CrossRef]
- Iis, S.; Toibah, A.R.; Toibah, A. Recent Progress on the Development of Porous Bioactive Calcium Phosphate for Biomedical Applications. Recent Pat. Biomed. Eng. 2008, 1, 213–229. [Google Scholar]
- Yao, D.; Dong, S.; Lu, Q.; Hu, X.; Kaplan, D.L.; Zhang, B.; Zhu, H. Salt-Leached Silk Scaffolds with Tunable Mechanical Properties. Biomacromolecules 2012, 13, 3723–3729. [Google Scholar] [CrossRef]
- Lin, S.; Lu, G.; Liu, S.; Bai, S.; Liu, X.; Lu, Q.; Zuo, B.; Kaplan, D.L.; Zhu, H. Nanoscale Control of Silks for Nanofibrous Scaffold Formation with Improved Porous Structure. J. Mater. Chem. B Mater. Biol. Med. 2014, 2, 2622–2633. [Google Scholar] [CrossRef]
- Somers, N.; Lasgorceix, M. Overview of Substitutes for Bone Replacement: Natural and Synthetic Products. In Encyclopedia of Materials: Technical Ceramics and Glasses; Pomeroy, M., Ed.; Elsevier: Oxford, UK, 2021; pp. 473–492. ISBN 978-0-12-822233-1. [Google Scholar]
- Martins, P.M.; Nunes-Pereira, J.; Lanceros-Méndez, S.; Costa, C.M. Chapter 17—Synthetic Polymer-Based Membranes for Lithium-Ion Batteries. In Synthetic Polymeric Membranes for Advanced Water Treatment, Gas Separation, and Energy Sustainability; Ismail, A.F., Salleh, W.N.W., Yusof, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 383–415. ISBN 978-0-12-818485-1. [Google Scholar]
- Shivalkar, S.; Singh, S. Solid Freeform Techniques Application in Bone Tissue Engineering for Scaffold Fabrication. Tissue Eng. Regen. Med. 2017, 14, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Sittinger, M.; Risbud, M.V. Scaffold-Based Tissue Engineering: Rationale for Computer-Aided Design and Solid Free-Form Fabrication Systems. Trends Biotechnol. 2004, 22, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A Review on Stereolithography and Its Applications in Biomedical Engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Roche, C.D.; Brereton, R.J.L.; Ashton, A.W.; Jackson, C.; Gentile, C. Current Challenges in Three-Dimensional Bioprinting Heart Tissues for Cardiac Surgery. Eur. J. Cardio-Thorac. Surg. 2020, 58, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Gan, S.; Wang, X.; Liu, W.; Li, X. Applications of 3D Bioprinting in Tissue Engineering: Advantages, Deficiencies, Improvements, and Future Perspectives. J. Mater. Chem. B 2021, 9, 5385–5413. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-J.; Nam, H.; Jang, J.; Lee, S.-J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering 2020, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Zandrini, T.; Florczak, S.; Levato, R.; Ovsianikov, A. Breaking the Resolution Limits of 3D Bioprinting: Future Opportunities and Present Challenges. Trends Biotechnol. 2022. [Google Scholar] [CrossRef]
- Placone, J.K.; Mahadik, B.; Fisher, J.P. Addressing Present Pitfalls in 3D Printing for Tissue Engineering to Enhance Future Potential. APL Bioeng. 2020, 4, 010901. [Google Scholar] [CrossRef]
- Persaud, A.; Maus, A.; Strait, L.; Zhu, D. 3D Bioprinting with Live Cells. Eng. Regen. 2022, 3, 292–309. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Gorth, D.; J Webster, T. 10—Matrices for Tissue Engineering and Regenerative Medicine. In Biomaterials for Artificial Organs; Lysaght, M., Webster, T.J., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2011; pp. 270–286. ISBN 978-1-84569-653-5. [Google Scholar]
- Giannitelli, S.M.; Basoli, F.; Mozetic, P.; Piva, P.; Bartuli, F.N.; Luciani, F.; Arcuri, C.; Trombetta, M.; Rainer, A.; Licoccia, S. Graded Porous Polyurethane Foam: A Potential Scaffold for Oro-Maxillary Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 51, 329–335. [Google Scholar] [CrossRef]
- Abdelaal, O.A.M.; Darwish, S.M.H. Review of Rapid Prototyping Techniques for Tissue Engineering Scaffolds Fabrication. In Characterization and Development of Biosystems and Biomaterials; Öchsner, A., da Silva, L.F.M., Altenbach, H., Eds.; Advanced Structured Materials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 33–54. ISBN 978-3-642-31470-4. [Google Scholar]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A Novel Carrier for Cell and Drug Delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Harris, L.D.; Kim, B.S.; Mooney, D.J. Open Pore Biodegradable Matrices Formed with Gas Foaming. J. Biomed. Mater. Res. 1998, 42, 396–402. [Google Scholar] [CrossRef]
- Costantini, M.; Barbetta, A. 6—Gas Foaming Technologies for 3D Scaffold Engineering. In Functional 3D Tissue Engineering Scaffolds; Deng, Y., Kuiper, J., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 127–149. ISBN 978-0-08-100979-6. [Google Scholar]
- Santos-Rosales, V.; Iglesias-Mejuto, A.; García-González, C.A. Solvent-Free Approaches for the Processing of Scaffolds in Regenerative Medicine. Polymers 2020, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Gao, W.; Bai, H. Silk-Based Bioinspired Structural and Functional Materials. iScience 2022, 25, 103940. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic Delivery of Signals for Bone Tissue Engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef]
- Gugjoo, M.B.; Amarpal; Sharma, G.T.; Aithal, H.P.; Kinjavdekar, P. Cartilage Tissue Engineering: Role of Mesenchymal Stem Cells along with Growth Factors & Scaffolds. Indian J. Med. Res. 2016, 144, 339–347. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y. A Mussel-Inspired Osteogenesis Microenvironment with Bioactive Peptides for the Dual-Functionalization of Biomedical Substrates. New J. Chem. 2020, 44, 14256–14265. [Google Scholar] [CrossRef]
- Song, X.; Li, X.; Wang, F.; Wang, L.; Lv, L.; Xie, Q.; Zhang, X.; Shao, X. Bioinspired Protein/Peptide Loaded 3D Printed PLGA Scaffold Promotes Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 832727. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qi, Z.; Zheng, C.; Xue, P.; Fu, C.; Pan, S.; Yang, X. Enhanced Cell Proliferation and Osteogenesis Differentiation through a Combined Treatment of Poly-L-Lysine-Coated PLGA/Graphene Oxide Hybrid Fiber Matrices and Electrical Stimulation. J. Nanomater. 2020, 2020, 5892506. [Google Scholar] [CrossRef]
- Panseri, S.; Montesi, M.; Hautcoeur, D.; Dozio, S.M.; Chamary, S.; De Barra, E.; Tampieri, A.; Leriche, A. Bone-like Ceramic Scaffolds Designed with Bioinspired Porosity Induce a Different Stem Cell Response. J. Mater. Sci. Mater. Med. 2021, 32, 3. [Google Scholar] [CrossRef]
- Collins, A.M.; Skaer, N.J.V.; Gheysens, T.; Knight, D.; Bertram, C.; Roach, H.I.; Oreffo, R.O.C.; Von-Aulock, S.; Baris, T.; Skinner, J.; et al. Bone-like Resorbable Silk-Based Scaffolds for Load-Bearing Osteoregenerative Applications. Adv. Mater. 2009, 21, 75–78. [Google Scholar] [CrossRef]
- de Wildt, B.W.M.; van der Meijden, R.; Bartels, P.A.A.; Sommerdijk, N.A.J.M.; Akiva, A.; Ito, K.; Hofmann, S. Bioinspired Silk Fibroin Mineralization for Advanced In Vitro Bone Remodeling Models. Adv. Funct. Mater. 2022, 32, 2206992. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Costa, J.B.; Carneiro, S.M.; Pina, S.; Veloso, A.C.A.; Reis, R.L.; Oliveira, J.M. Bioinspired Silk Fibroin-Based Composite Grafts as Bone Tunnel Fillers for Anterior Cruciate Ligament Reconstruction. Pharmaceutics 2022, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-Printed Silk-Hydroxyapatite Scaffolds for Enhanced Bone Regeneration with Innervation and Vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D.L. Silk Fibroin/Hydroxyapatite Composites for Bone Tissue Engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Liu, Y.; Zhao, Q.; Wu, W.; Li, D.; Jin, Z. Biomaterial Scaffolds with Biomimetic Fluidic Channels for Hepatocyte Culture. J. Bionic Eng. 2013, 10, 57–64. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, L.S.; Yin, F.; Shi, Y.Q.; Han, Y.; Zhang, L.; Jin, H.F.; Nie, Y.Z.; Wang, J.B.; Hao, X.; et al. In Vitro and in Vivo Characterization of Silk Fibroin/Gelatin Composite Scaffolds for Liver Tissue Engineering. J. Dig. Dis. 2012, 13, 168–178. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, Z.; Gao, C.; Chen, K.; Lu, S.; Yan, H.; Liu, W.; Wang, M.; Ding, Y.; Huang, L.; et al. Development of Biomimetic Hepatic Lobule-Like Constructs on Silk-Collagen Composite Scaffolds for Liver Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 940634. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Kundu, S.C. Bio-Inspired Fabrication of Fibroin Cryogels from the Muga Silkworm Antheraea Assamensis for Liver Tissue Engineering. Biomed. Mater. 2013, 8, 055003. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.; Coburn, J.M. HepaRG Maturation in Silk Fibroin Scaffolds: Toward Developing a 3D In Vitro Liver Model. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk Fibroin Based Biomimetic Artificial Extracellular Matrix for Hepatic Tissue Engineering Applications. Biomed. Mater. 2012, 7, 045004. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.H.; Wilson, C.G.; Seib, F.P. A Review of the Emerging Role of Silk for the Treatment of the Eye. Pharm. Res. 2018, 35, 248. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lawrence, B.D.; Liu, A.; Schwab, I.R.; Oliveira, L.A.; Rosenblatt, M.I. Silk Fibroin as a Biomaterial Substrate for Corneal Epithelial Cell Sheet Generation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4130–4138. [Google Scholar] [CrossRef]
- Doutch, J.; Quantock, A.J.; Smith, V.A.; Meek, K.M. Light Transmission in the Human Cornea as a Function of Position across the Ocular Surface: Theoretical and Experimental Aspects. Biophys. J. 2008, 95, 5092–5099. [Google Scholar] [CrossRef]
- Meek, K.M.; Boote, C. The Organization of Collagen in the Corneal Stroma. Exp. Eye Res. 2004, 78, 503–512. [Google Scholar] [CrossRef]
- Wu, J.; Rnjak-Kovacina, J.; Du, Y.; Funderburgh, M.L.; Kaplan, D.L.; Funderburgh, J.L. Corneal Stromal Bioequivalents Secreted on Patterned Silk Substrates. Biomaterials 2014, 35, 3744–3755. [Google Scholar] [CrossRef]
- Gil, E.S.; Park, S.-H.; Marchant, J.; Omenetto, F.; Kaplan, D.L. Response of Human Corneal Fibroblasts on Silk Film Surface Patterns. Macromol. Biosci. 2010, 10, 664–673. [Google Scholar] [CrossRef]
- Le, Q.; Xu, J.; Deng, S.X. The Diagnosis of Limbal Stem Cell Deficiency. Ocul. Surf. 2018, 16, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Yang, L.; Zeng, Y.; Gao, X.; Xu, H. Poly(Ethylene Glycol)-Modified Silk Fibroin Membrane as a Carrier for Limbal Epithelial Stem Cell Transplantation in a Rabbit LSCD Model. Stem Cell Res. Ther. 2017, 8, 256. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, N.; Rodríguez-Barrientos, C.A.; Aznar-Cervantes, S.D.; Chacón, M.; Cenis, J.L.; Riestra, A.C.; Sánchez-Avila, R.M.; Persinal, M.; Brea-Pastor, A.; Fernández-Vega Cueto, L.; et al. Silk Fibroin Films for Corneal Endothelial Regeneration: Transplant in a Rabbit Descemet Membrane Endothelial Keratoplasty. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3357–3365. [Google Scholar] [CrossRef] [PubMed]
- Wulle, K.G.; Lerche, W. Electron Microscopic Observations of the Early Development of the Human Corneal Endothelium and Descemet’s Membrane. Ophthalmologica 1969, 157, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Konomi, H.; Hirosawa, K. Characterization of the Collagen in the Hexagonal Lattice of Descemet’s Membrane: Its Relation to Type VIII Collagen. J. Cell Biol. 1990, 110, 219–227. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Marchant, J.K.; Pindrus, M.; Omenetto, F.; Kaplan, D.L. Silk Film Biomaterials for Cornea Tissue Engineering. Biomaterials 2009, 30, 1299–1308. [Google Scholar] [CrossRef]
- Gosselin, E.A.; Torregrosa, T.; Ghezzi, C.E.; Mendelsohn, A.C.; Gomes, R.; Funderburgh, J.L.; Kaplan, D.L. Multi-Layered Silk Film Co-Culture System for Human Corneal Epithelial and Stromal Stem Cells. J. Tissue Eng. Regen. Med. 2018, 12, 285–295. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Oyen, M.L. Composite Electrospun Gelatin Fiber-Alginate Gel Scaffolds for Mechanically Robust Tissue Engineered Cornea. J. Mech. Behav. Biomed. Mater. 2013, 21, 185–194. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Kwee, B.J.; Mooney, D.J. Biomaterials for Skeletal Muscle Tissue Engineering. Curr. Opin. Biotechnol. 2017, 47, 16–22. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Naskar, D.; Kinnear, B.F.; Grenik, E.; Dye, D.E.; Grounds, M.D.; Kundu, S.C.; Coombe, D.R. Silk Fibroin Scaffolds with Muscle-like Elasticity Support in Vitro Differentiation of Human Skeletal Muscle Cells. J. Tissue Eng. Regen. Med. 2017, 11, 3178–3192. [Google Scholar] [CrossRef]
- Clegg, M.H.; Harris, T.I.; Zhang, X.; Barney, J.T.; Jones, J.A.; Vargis, E. Silkworm Silk Fiber Bundles as Improved In Vitro Scaffolds for Skeletal Muscle. ACS Biomater. Sci. Eng. 2020, 6, 6853–6863. [Google Scholar] [CrossRef]
- Song, Y.; Li, M.; Lei, S.; Hao, L.; Lv, Q.; Liu, M.; Wang, G.; Wang, Z.; Fu, X.; Wang, L. Silk Sericin Patches Delivering MiRNA-29-Enriched Extracellular Vesicles-Decorated Myoblasts (SPEED) Enhances Regeneration and Functional Repair after Severe Skeletal Muscle Injury. Biomaterials 2022, 287, 121630. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, F.; Zhang, Y.; You, Y.; Wang, Z.; Guan, Z. Bioinspired Design of Nanostructured Elastomers with Cross-Linked Soft Matrix Grafting on the Oriented Rigid Nanofibers to Mimic Mechanical Properties of Human Skin. ACS Nano 2015, 9, 271–278. [Google Scholar] [CrossRef]
- Tran, L.-G.; Nguyen, T.-Q.; Park, W.-T. Bio-Inspired Barbed Microneedle for Skin Adhesion with Interlocking Mechanics. In Proceedings of the 2019 IEEE 32nd International Conference on Micro Electro Mechanical Systems (MEMS), Seoul, Republic of Korea, 27–31 January 2019; pp. 547–550. [Google Scholar]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y.; Jiang, D.; Zhang, J.; Sheng, Z.; Xie, C.; Peng, Z.; et al. Rapid Printing of Bio-Inspired 3D Tissue Constructs for Skin Regeneration. Biomaterials 2020, 258, 120287. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P.; Kaplan, D.L. Doxorubicin-Loaded Silk Films: Drug-Silk Interactions and in Vivo Performance in Human Orthotopic Breast Cancer. Biomaterials 2012, 33, 8442–8450. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Liu, Q.; Li, R.; Wang, J.; Zhen, X.; Yue, G.; Wang, H.; Cui, F.; Wu, F.; Yang, M.; et al. Facile Preparation of Paclitaxel Loaded Silk Fibroin Nanoparticles for Enhanced Antitumor Efficacy by Locoregional Drug Delivery. ACS Appl. Mater. Interfaces 2013, 5, 12638–12645. [Google Scholar] [CrossRef]
- Patil, A.C.; Bandla, A.; Liu, Y.-H.; Luo, B.; Thakor, N.V. Nontransient Silk Sandwich for Soft, Conformal Bionic Links. Mater. Today 2020, 32, 68–83. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Wang, J.; Wang, T.; Jiang, Y.; Hu, J.; Liu, Z.; Chen, X.; Yu, J. Bioinspired, Microstructured Silk Fibroin Adhesives for Flexible Skin Sensors. ACS Appl. Mater. Interfaces 2020, 12, 5601–5609. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar Sahi, A.; Gundu, S.; Kumari, P.; Klepka, T.; Sionkowska, A. Silk-Based Biomaterials for Designing Bioinspired Microarchitecture for Various Biomedical Applications. Biomimetics 2023, 8, 55. https://doi.org/10.3390/biomimetics8010055
Kumar Sahi A, Gundu S, Kumari P, Klepka T, Sionkowska A. Silk-Based Biomaterials for Designing Bioinspired Microarchitecture for Various Biomedical Applications. Biomimetics. 2023; 8(1):55. https://doi.org/10.3390/biomimetics8010055
Chicago/Turabian StyleKumar Sahi, Ajay, Shravanya Gundu, Pooja Kumari, Tomasz Klepka, and Alina Sionkowska. 2023. "Silk-Based Biomaterials for Designing Bioinspired Microarchitecture for Various Biomedical Applications" Biomimetics 8, no. 1: 55. https://doi.org/10.3390/biomimetics8010055
APA StyleKumar Sahi, A., Gundu, S., Kumari, P., Klepka, T., & Sionkowska, A. (2023). Silk-Based Biomaterials for Designing Bioinspired Microarchitecture for Various Biomedical Applications. Biomimetics, 8(1), 55. https://doi.org/10.3390/biomimetics8010055






