A Biomimetic Method to Replicate the Natural Fluid Movements of Swimming Snakes to Design Aquatic Robots
Abstract
1. Introduction
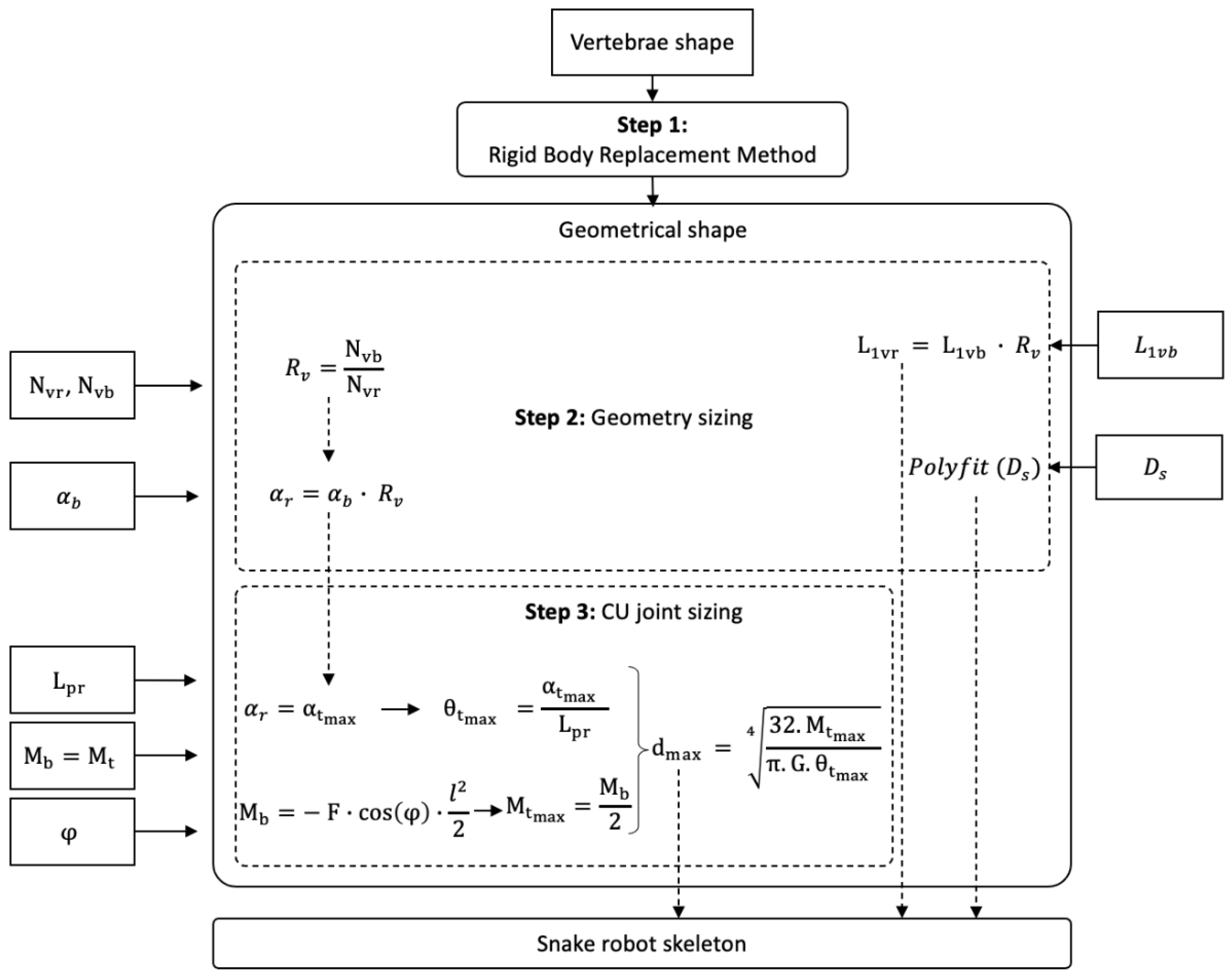
2. BIM: Bio-Inspired Method
2.1. Introduction to BIM
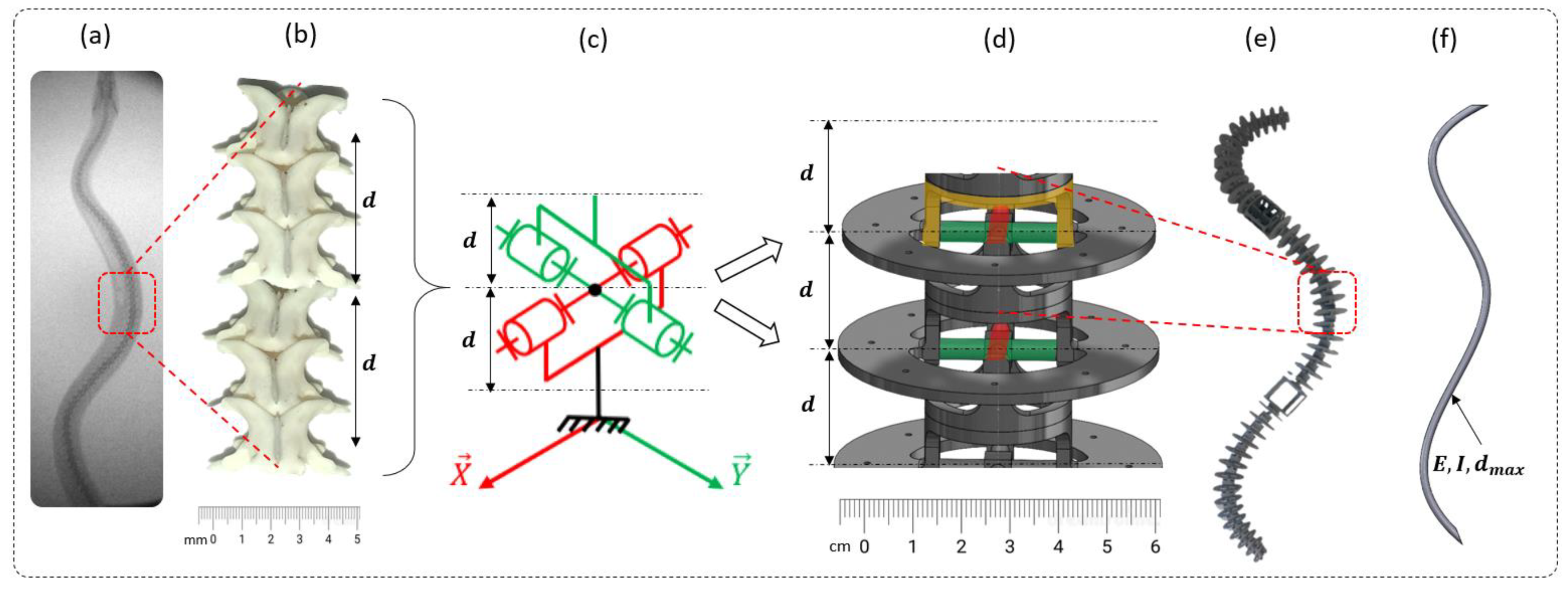
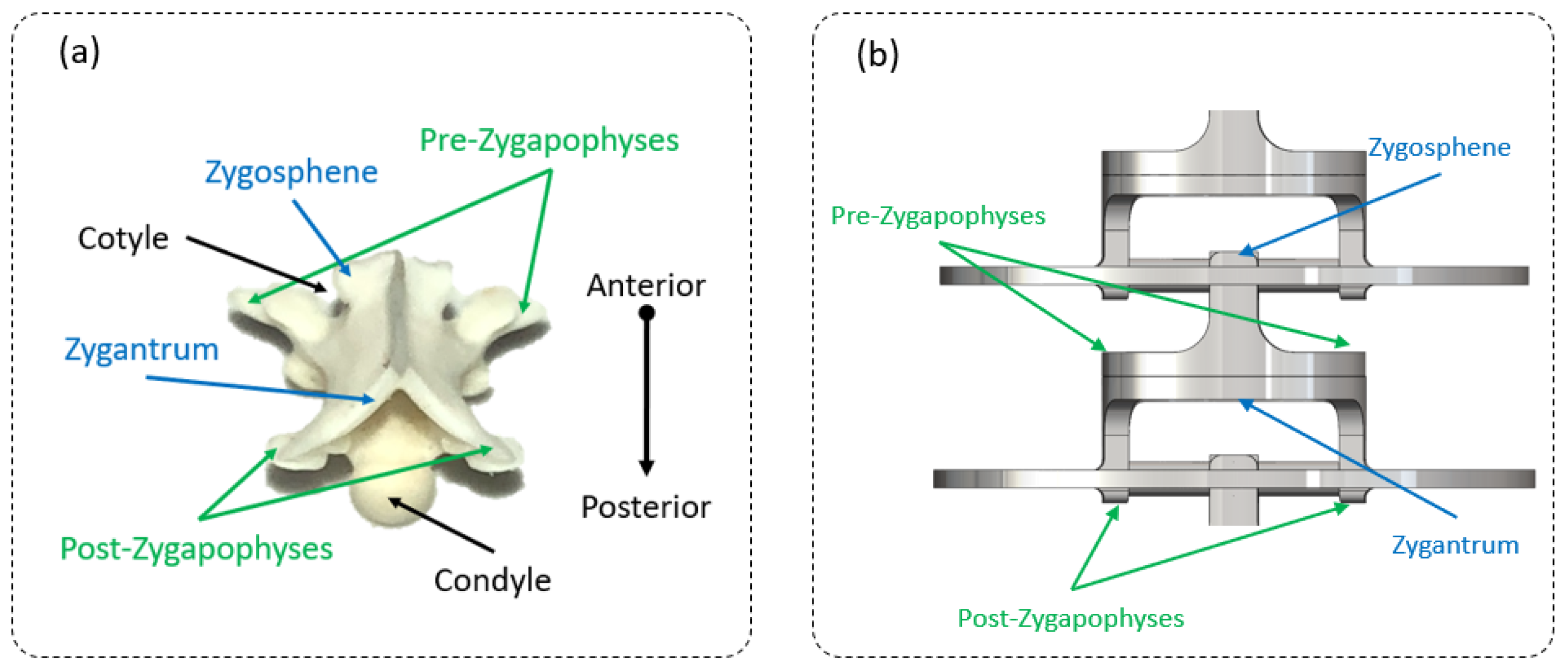
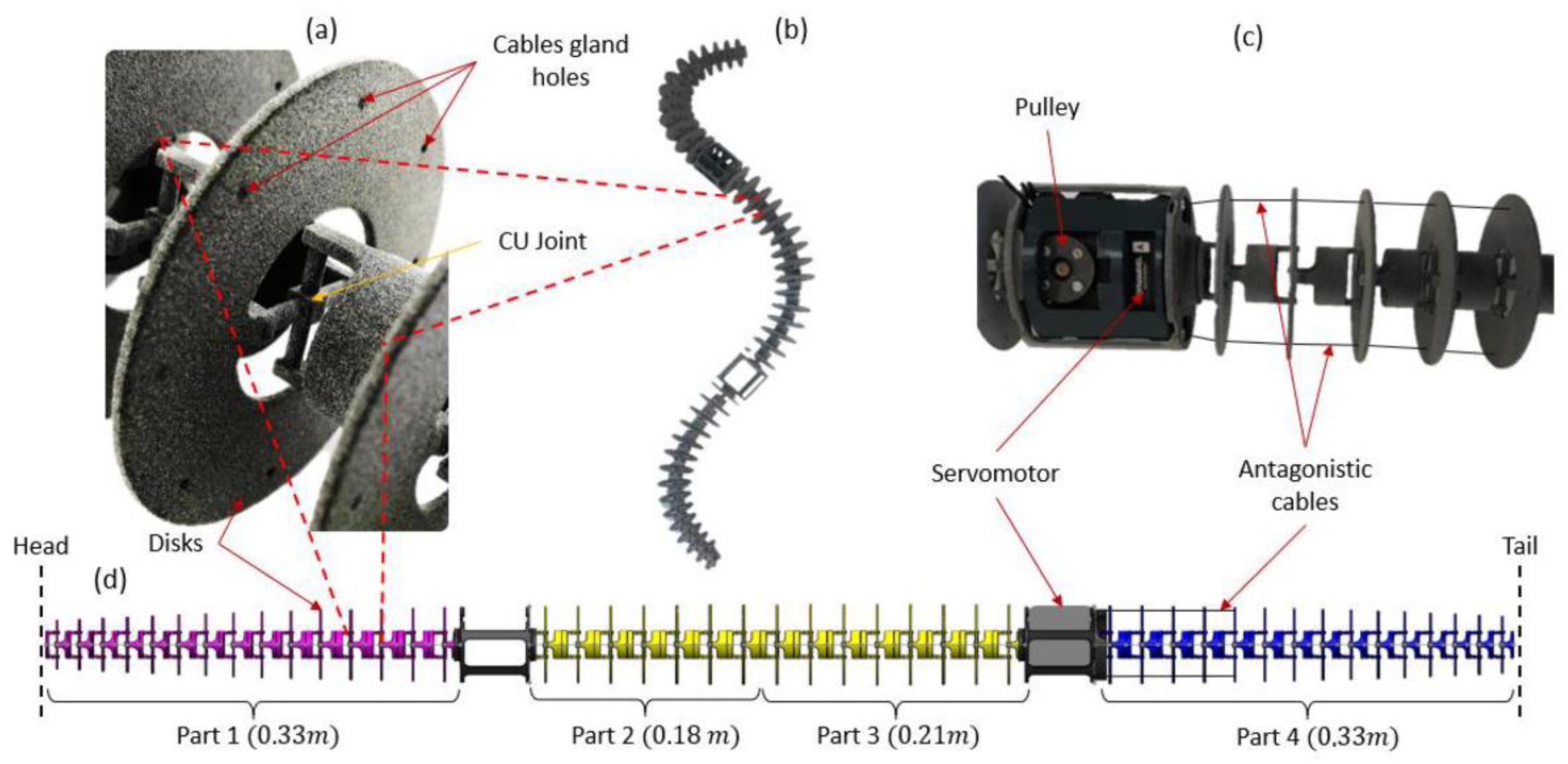
2.2. Step 1: Geometric Shape—The Rigid Body Replacement Method
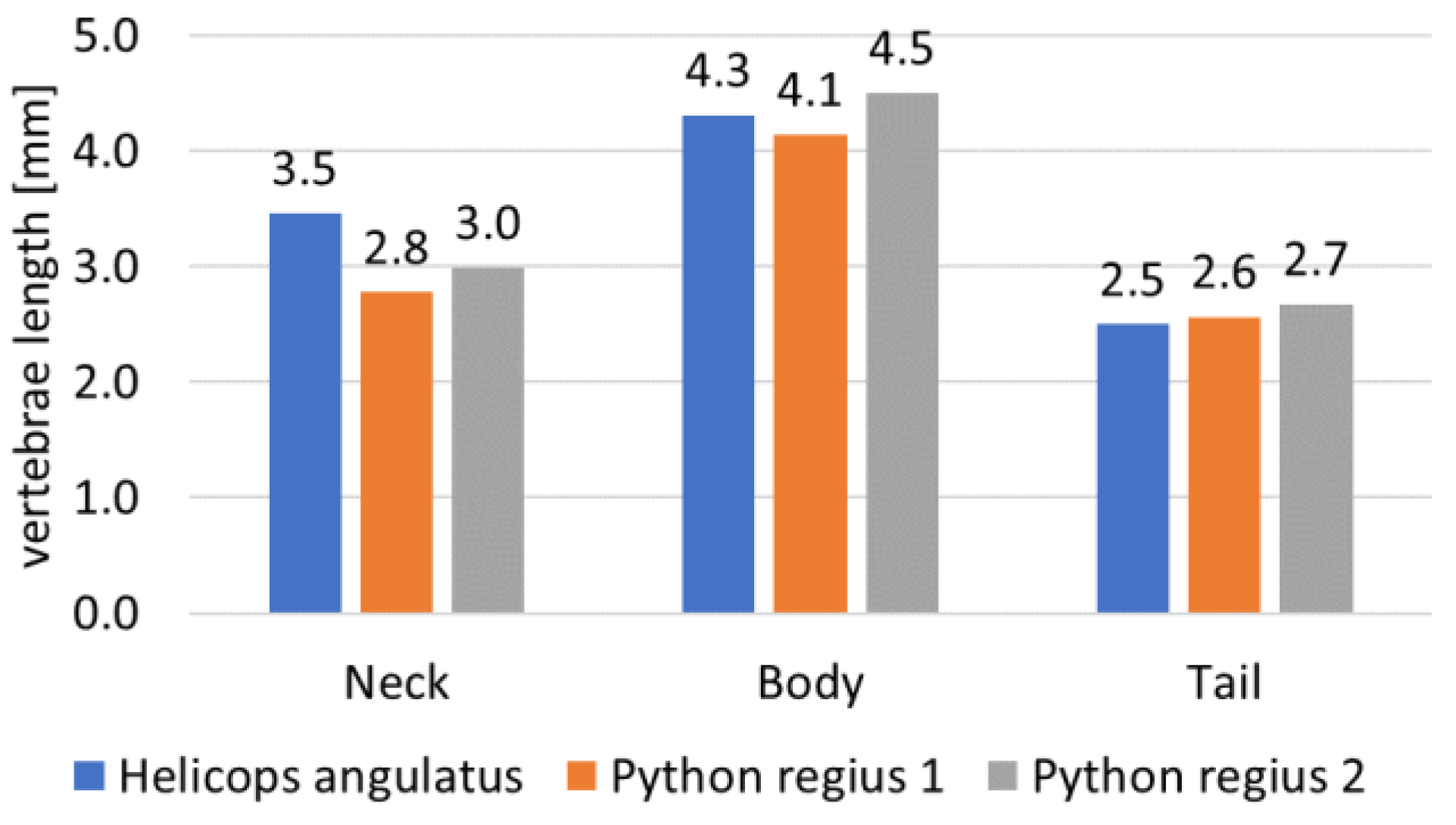
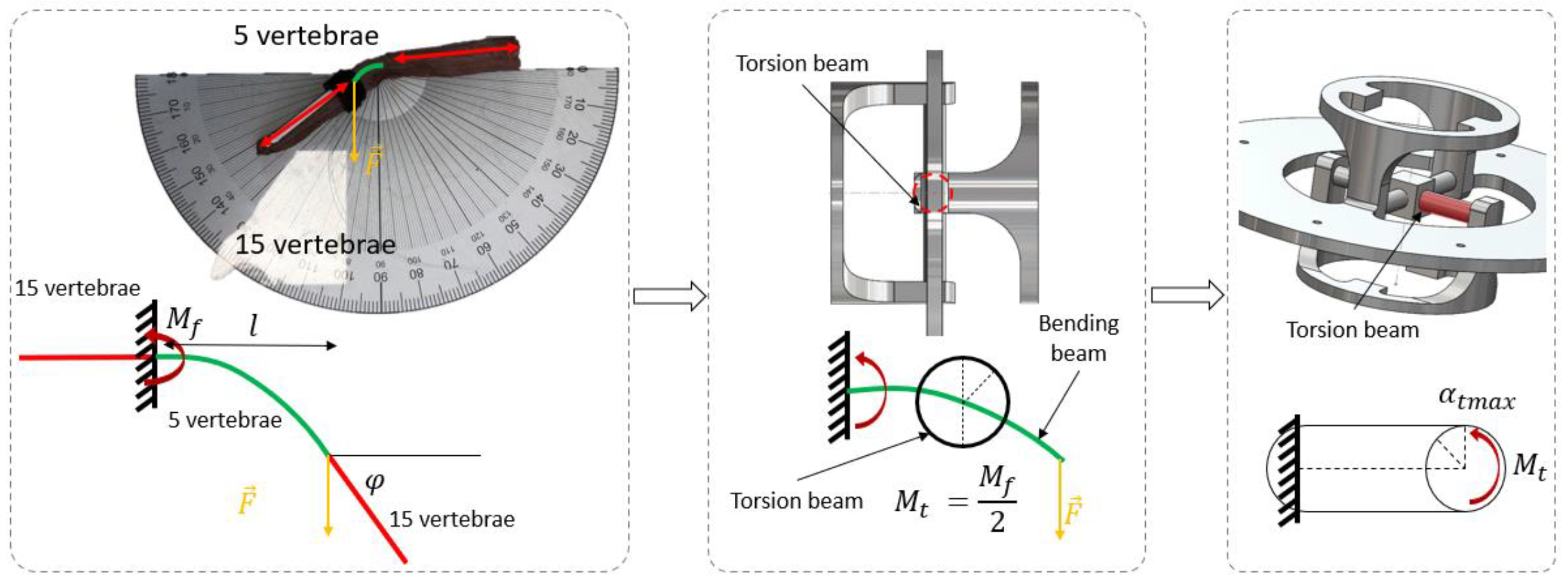
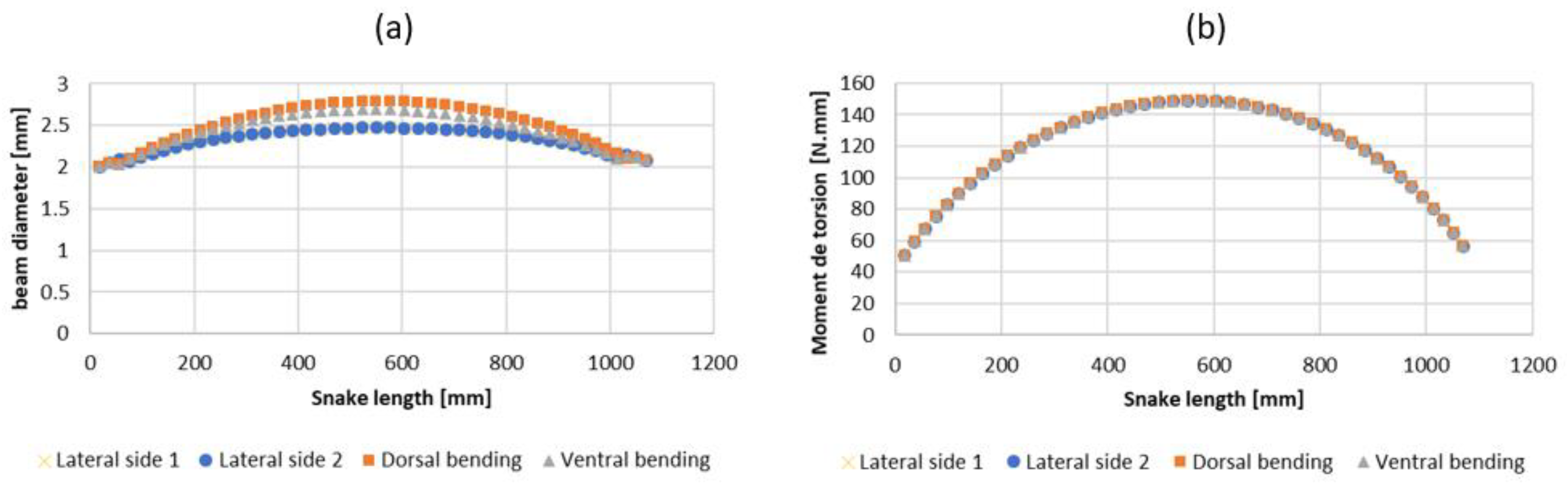
2.3. Step 2: Sizing the Geometric Shape
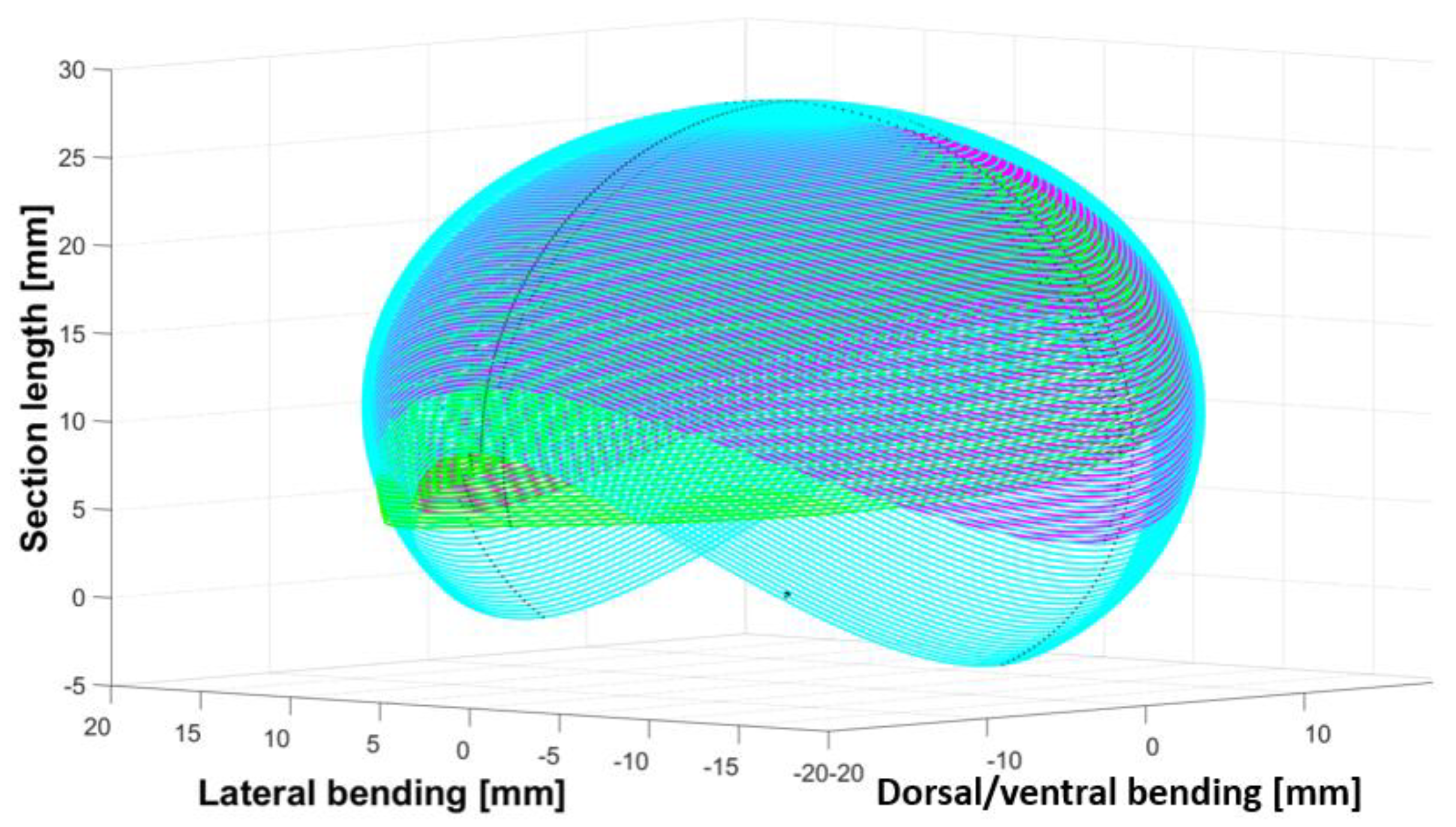
2.4. Step 3: Sizing the CU Joint
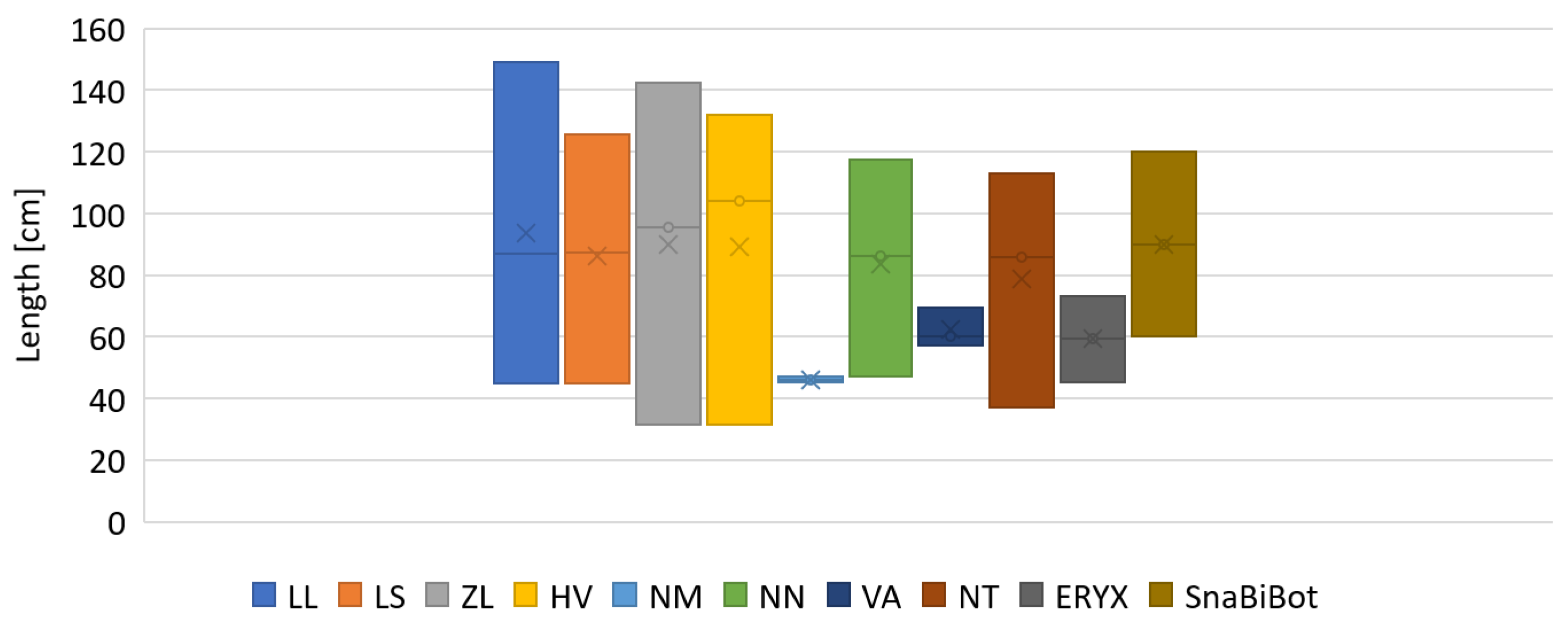
2.5. Bio-Inspired Snake Robot Skeletons
3. Motion Analysis and Design Validation
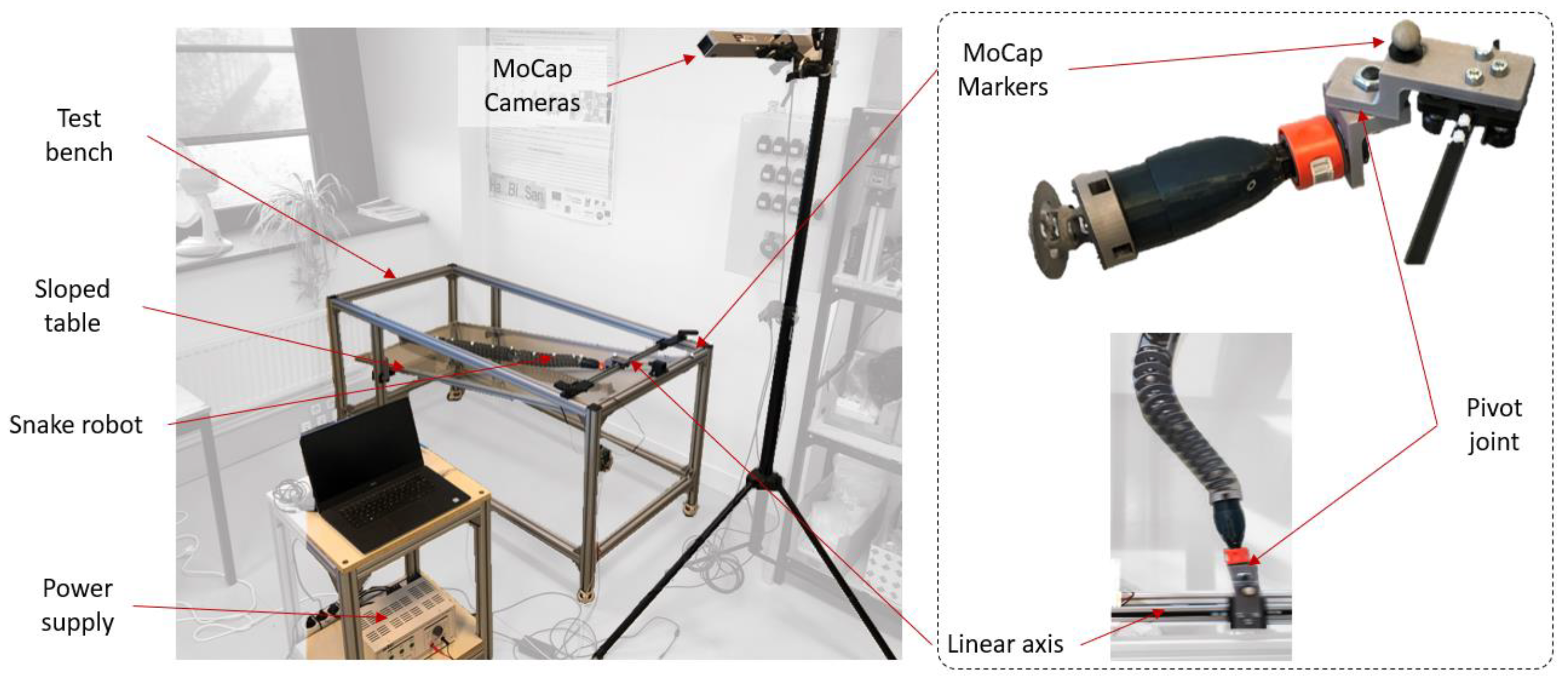
3.1. Materials
3.2. Protocol
- (1)
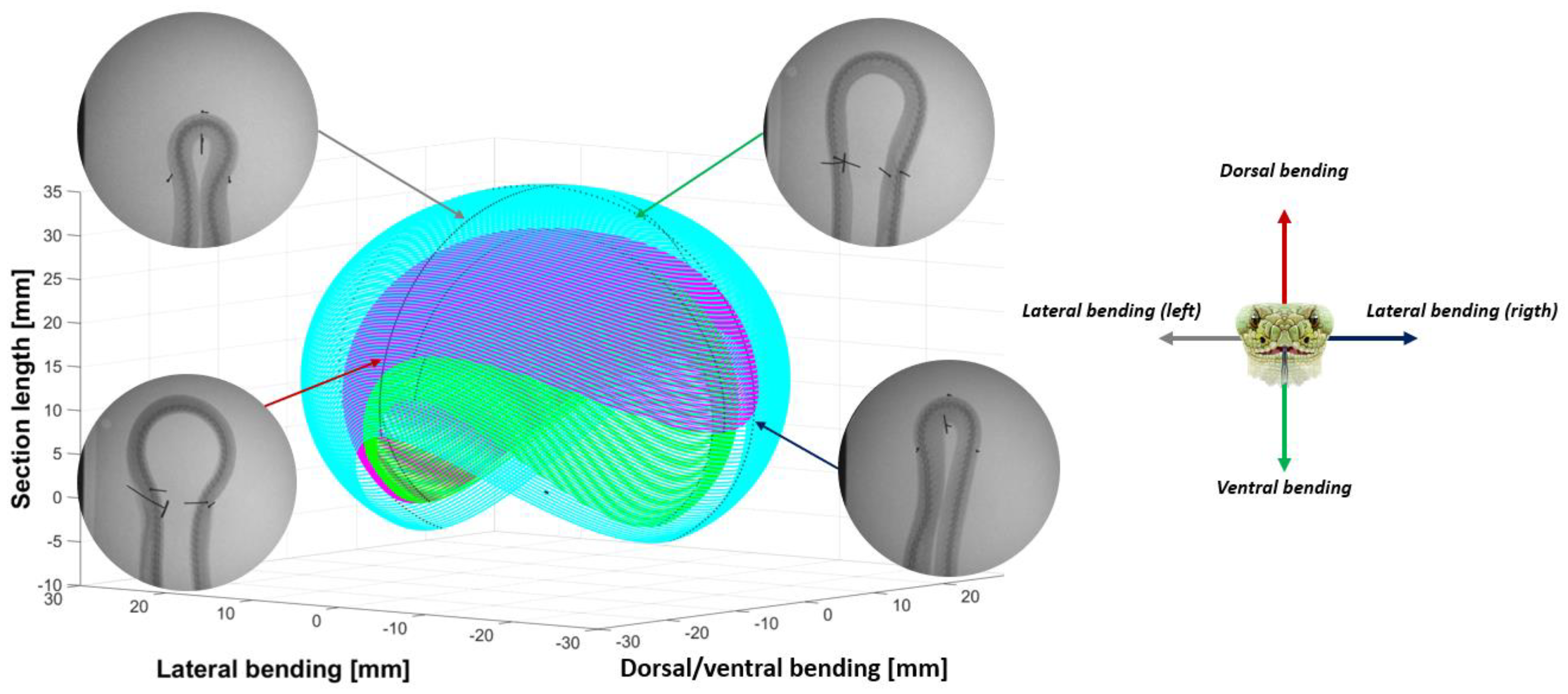
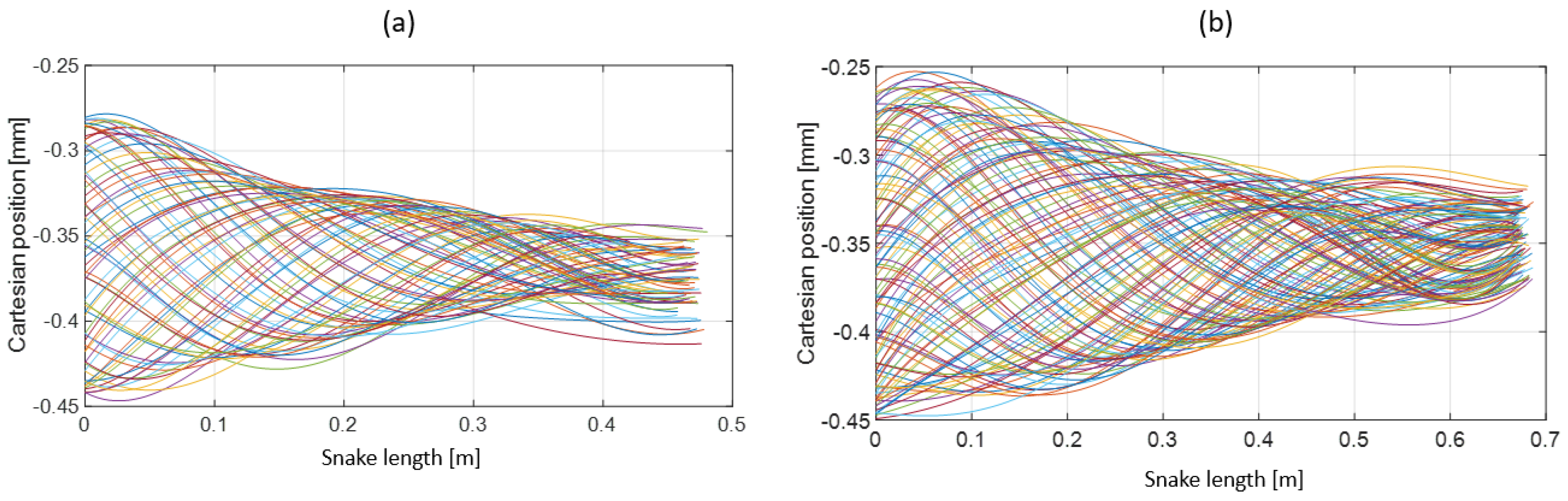
- Extracting the undulation cone required monitoring of the entire body while moving. Thus, 11 reflective markers (see Figure 16) were taped to every fifth vertebra from the head to the tail of the inert robot. On the snakes, seven markers were taped and homogeneously distributed along the snake bodies (each ~40 vertebrae). Undulations were recorded using motion capture.
- (2)
- Each robot and snake (Hierophis viridiflavus, Zamenis longissimus) (see Table 1) head was actuated for nine seconds at a constant speed of 0.9 m/s and with a constant head amplitude of 0.17 m.
- (3)
- The applied head amplitude of the robotic snake varied while maintaining a constant head speed of 0.9 m/s. In the first experiment, an amplitude of 0.2 m was applied to the head; the second time, an amplitude of 0.17 m was applied.
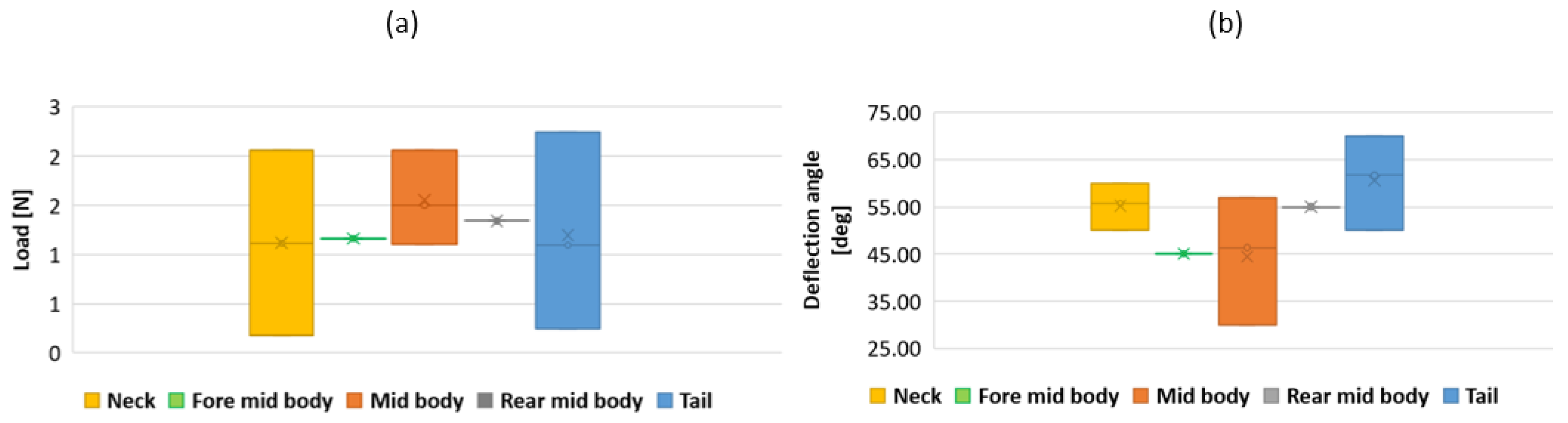
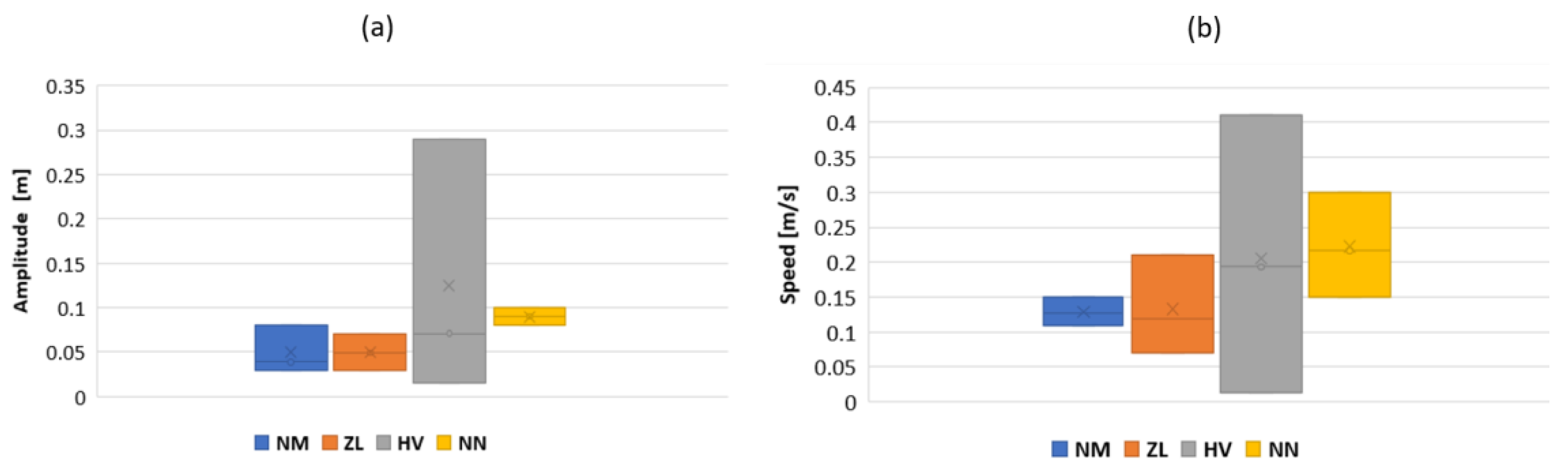
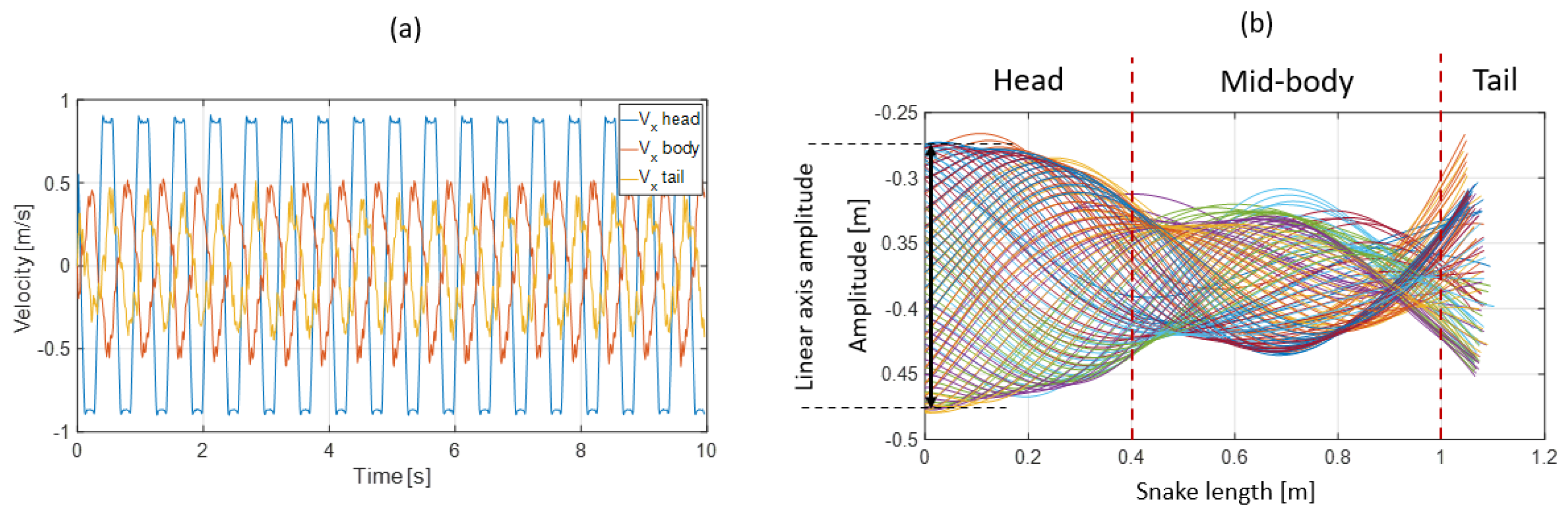
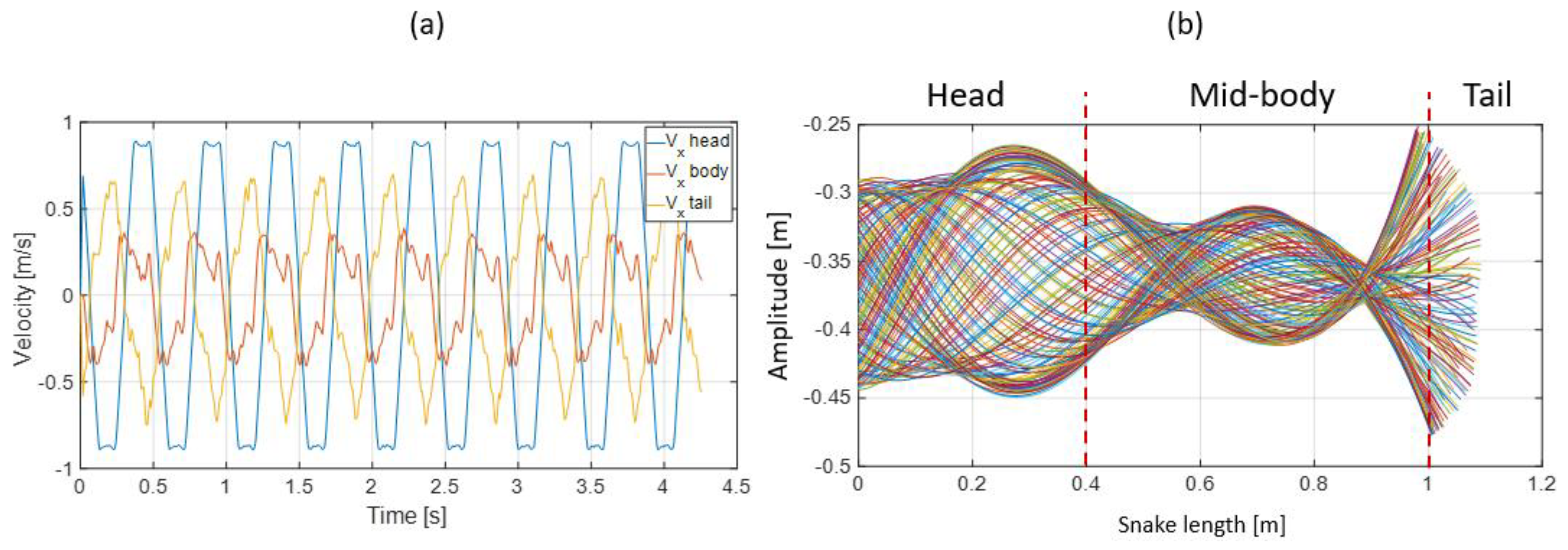
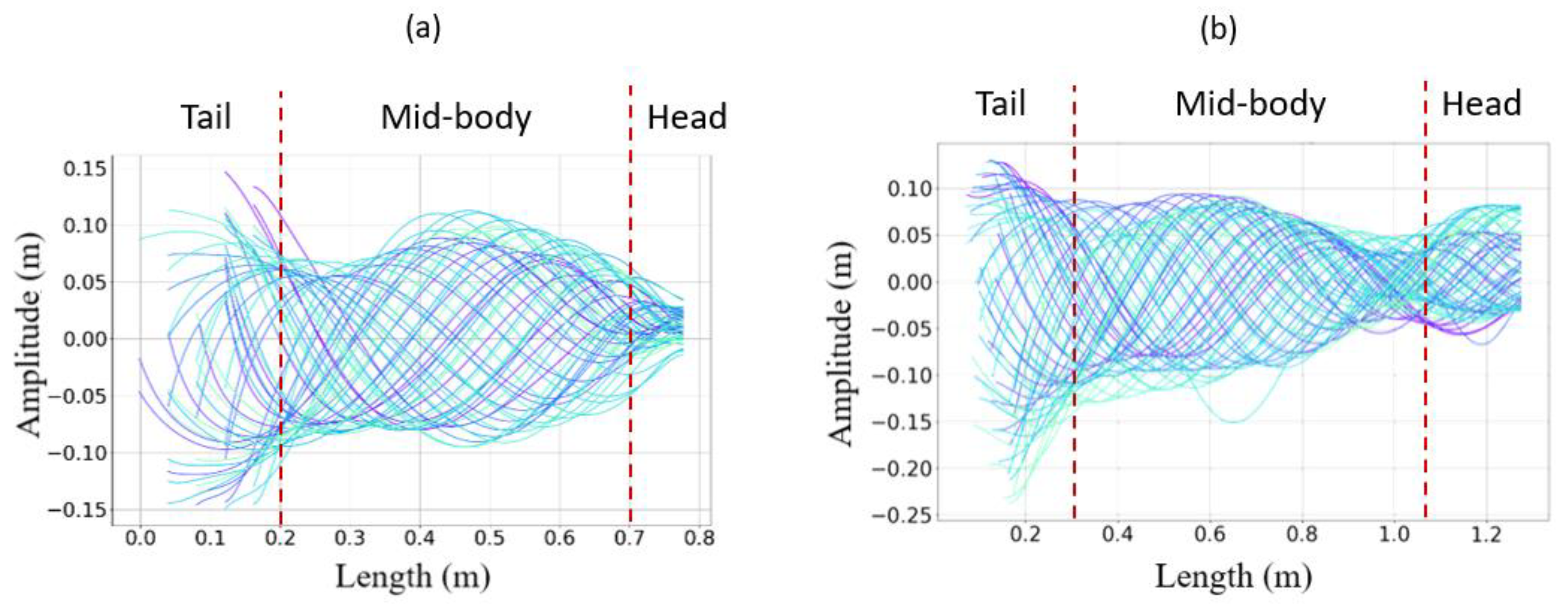
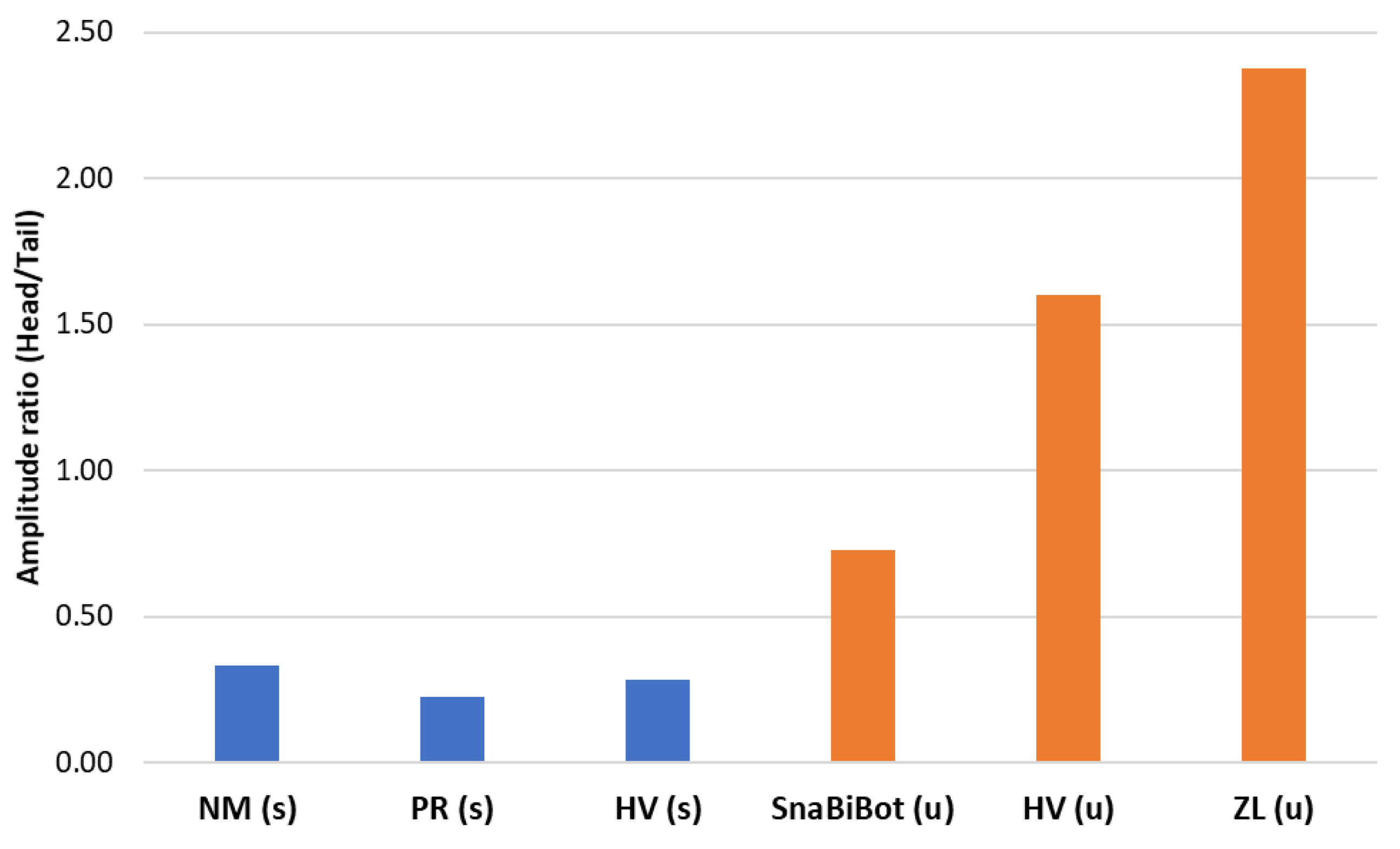
3.3. Results
4. Discussion and Future Work
4.1. Discussion
4.2. Future Works
5. Conclusions
- (1)
- A general comprehensive bio-inspired method for synthesizing specific locomotion was introduced. A BIM was applied to the development of a snake robot to perform undulations. Snake robot mechanical behavior was implemented directly from biological snakes, unlike traditional robots.
- (2)
- A new design to synthesize a snake vertebra based on a compliant universal joint was introduced. The CU joint reproduces the motions in a volume, ensuring stiffness according to the two respective plans. Each joint in a respective plan can be modeled as a beam with a constant Young’s modulus and a variable diameter. The global deformation of the snake robot body realizes fluid undulation, which differs from a traditional snake robot endowed with rigid modules.
- (3)
- The collaboration between biology and robotics led to a comparison of the snake’s behavior using an original testing bench. A comparison of the undulation cones demonstrated that internal actuation (muscles) combined with musculoskeletal system stiffness mostly occurred in the mid-body, where the amplitudes were the greatest. The direct biomimetic investigation between the flexible skeleton of the robot and biological snakes provides answers to biologists and a feedback loop to better understand how snakes move, for instance, which part of the snake should be targeted in future investigations. The snake robot fulfills this central role.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crespi, A.; Ijspeert, A.J. AmphiBot II: An Amphibious Snake Robot that Crawls and Swims using a Central Pattern Generator. In Proceedings of the 9th International Conference on Climbing and Walking Robots CLAWAR 2006; SAGE: Thousand Oaks, CA, USA, 2006. [Google Scholar]
- Manfredi, L.; Assaf, T.; Mintchev, S.; Marrazza, S.; Capantini, L.; Orofino, S.; Ascari, L.; Grillner, S.; Wallén, P.; Ekeberg, Ö.; et al. A bioinspired autonomous swimming robot as a tool for studying goal-directed locomotion. Biol. Cybern. 2013, 107, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Ijspeert, A.J. Central pattern generators for locomotion control in animals and robots: A review. Neural Netw. 2008, 21, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Nyakatura, J.A.; Melo, K.; Horvat, T.; Karakasiliotis, K.; Allen, V.R.; Andikfar, A.; Andrada, E.; Arnold, P.; Lauströer, J.; Hutchinson, J.R.; et al. Reverse-engineering the locomotion of a stem amniote. Nature 2019, 565, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-H.; Kim, K.-S.; Lee, S.-H.; Cho, C.; Ryuh, Y. Design and Control of 3-DOF Robotic Fish ‘ICHTHUS V5.5’. In Intelligent Robotics and Applications; Springer: Berlin/Heidelberg, Germany, 2013; pp. 310–319. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Dukes, I.; Francis, G. Design of 3D Swim Patterns for Autonomous Robotic Fish. In Proceedings of the 2006 IEEE/RSJ International Conference on Intelligent Robots and Systems, Beijing, China, 9–15 October 2006; pp. 2406–2411. [Google Scholar] [CrossRef]
- Martinez-Garcia, E.A.; Lavrenov, R.; Magid, E. Robot Fish Caudal Propulsive Mechanisms: A Mini-Review. AI Comput. Sci. Robot. Technol. 2022, 1–17. [Google Scholar] [CrossRef]
- Sfakiotakis, M.; Lane, D.M.; Davies, J.B.C. Review of fish swimming modes for aquatic locomotion. IEEE J. Ocean. Eng. 1999, 24, 237–252. [Google Scholar] [CrossRef]
- Kopman, V.; Porfiri, M. Design, Modeling, and Characterization of a Miniature Robotic Fish for Research and Education in Biomimetics and Bioinspiration. IEEEASME Trans. Mechatron. 2013, 18, 471–483. [Google Scholar] [CrossRef]
- Katzschmann, R.K.; DelPreto, J.; MacCurdy, R.; Rus, D. Exploration of underwater life with an acoustically controlled soft robotic fish. Sci. Robot. 2018, 3, eaar3449. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Z.; Du, R. A Novel Robot Fish With Wire-Driven Active Body and Compliant Tail. IEEEASME Trans. Mechatron. 2017, 22, 1633–1643. [Google Scholar] [CrossRef]
- Romano, D.; Wahi, A.; Miraglia, M.; Stefanini, C. Development of a Novel Underactuated Robotic Fish with Magnetic Transmission System. Machines 2022, 10, 755. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Z.; Du, R. Robot fish with two-DOF pectoral fins and a wire-driven caudal fin. Adv. Robot. 2018, 32, 25–36. [Google Scholar] [CrossRef]
- Wang, M.; Dong, H.; Li, X.; Zhang, Y.; Yu, J. Control and Optimization of a Bionic Robotic Fish Through a Combination of CPG model and PSO. Neurocomputing 2019, 337, 144–152. [Google Scholar] [CrossRef]
- Salazar, R.; Fuentes, V.; Abdelkefi, A. Classification of biological and bioinspired aquatic systems: A review. Ocean Eng. 2018, 148, 75–114. [Google Scholar] [CrossRef]
- Liljeback, P.; Stavdahl, O.; Pettersen, K.Y.; Gravdahl, J.T. Mamba-A waterproof snake robot with tactile sensing. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; pp. 294–301. [Google Scholar] [CrossRef]
- Boyer, F.; Chablat, D.; Lemoine, P.; Wenger, P. The Eel-Like Robot. In Proceedings of the 33rd Mechanisms and Robotics Conference, Parts A and B, San Diego, CA, USA, 30 August–2 September 2009; Volume 7, pp. 655–662. [Google Scholar] [CrossRef]
- Ijspeert, A.J.; Crespi, A.; Ryczko, D.; Cabelguen, J.-M. From swimming to walking with a salamander robot driven by a spinal cord model. Science 2007, 315, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Mori, M. Biologically Inspired Snake-like Robots. In Proceedings of the 2004 IEEE International Conference on Robotics and Biomimetics, Shenyang, China, 22–26 August 2004; pp. 1–7. [Google Scholar] [CrossRef]
- Orekhov, A.L.; Abah, C.; Simaan, N. Snake-Like Robots for Minimally Invasive, Single Port, and Intraluminal Surgeries. In The Enciclopedia of Medical Robotics; World Scientific: Singapore, 2018; pp. 203–243. [Google Scholar] [CrossRef]
- Crespi, A.; Badertscher, A.; Guignard, A.; Ijspeert, A.J. AmphiBot I: An amphibious snake-like robot. Robot. Auton. Syst. 2005, 50, 163–175. [Google Scholar] [CrossRef]
- Porez, M.; Boyer, F.; Ijspeert, A.J. Improved Lighthill fish swimming model for bio-inspired robots: Modeling, computational aspects and experimental comparisons. Int. J. Robot. Res. 2014, 33, 1322–1341. [Google Scholar] [CrossRef]
- Yamakita, M.; Kamamichi, N.; Kozuki, T.; Asaka, K.; Luo, Z.-W. A snake-like swimming robot using IPMC actuator and verification of doping effect. In Proceedings of the 2005 IEEE/RSJ International Conference on Intelligent Robots and Systems, Edmonton, Alta, 2–6 August 2005; pp. 2035–2040. [Google Scholar] [CrossRef]
- Nguyen, D.Q.; Ho, V.A. Anguilliform Swimming Performance of an Eel-Inspired Soft Robot. Soft Robot. 2022, 9, 425–439. [Google Scholar] [CrossRef]
- Pattishall, A.; Cundall, D. Dynamic changes in body form during swimming in the water snake Nerodia sipedon. Zoology 2008, 111, 48–61. [Google Scholar] [CrossRef]
- Rollinson, D.; Bilgen, Y.; Brown, B.; Enner, F.; Ford, S.; Layton, C.; Rembisz, J.; Schwerin, M.; Willig, A.; Velagapudi, P.; et al. Design and architecture of a series elastic snake robot. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; pp. 4630–4636. [Google Scholar] [CrossRef]
- Gautreau, E.; Sandoval, J.; Bonnet, X.; Arsicault, M.; Zeghloul, S.; Laribi, M.A. A new bio-inspired Hybrid Cable-Driven Robot (HCDR) to design more realistic snakebots. In Proceedings of the 2022 International Conference on Robotics and Automation (ICRA), Philadelphia, PA, USA, 23–27 May 2022; pp. 2134–2140. [Google Scholar] [CrossRef]
- Qi, P.; Qiu, C.; Liu, H.; Dai, J.S.; Seneviratne, L.D.; Althoefer, K. A Novel Continuum Manipulator Design Using Serially Connected Double-Layer Planar Springs. IEEEASME Trans. Mechatron. 2016, 21, 1281–1292. [Google Scholar] [CrossRef]
- Gautreau, E.; Bonnet, X.; Fox, T.; Fosseries, G.; Valle, V.; Herrel, A.; Laribi, M.A. Complementary methods to acquire the kinematics of swimming snakes: A ba-3 sis to design bio-inspired robots. J. Bionic Eng. 2022, 1–15. [Google Scholar] [CrossRef]
- Gallego, J.A.; Herder, J. Synthesis Methods in Compliant Mechanisms: An Overview. In Proceedings of the ASME 2009 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference, San Diego, CA, USA, 30 August–2 September 2009; pp. 193–214. [Google Scholar] [CrossRef]
- Galbusera, F.; Bassani, T. The Spine: A Strong, Stable, and Flexible Structure with Biomimetics Potential. Biomimetics 2019, 4, 60. [Google Scholar] [CrossRef]
- Gautreau, E.; Sandoval, J.; Arsicault, M.; Bonnet, X.; Zeghloul, S.; Laribi, M.A. Kinematic Modelling of a Bioinspired Two Sections Serial Continuum Robot (SCR). In Advances in Service and Industrial Robotics; Müller, A., Brandstötter, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 247–255. [Google Scholar] [CrossRef]
- Penning, D.A. Quantitative axial myology in two constricting snakes: Lampropeltis holbrooki and Pantherophis obsoletus. J. Anat. 2018, 232, 1016–1024. [Google Scholar] [CrossRef]
- Mathou, A.; Bonnet, X.; Daoues, K.; Ksas, R.; Herrel, A. Evolutionary convergence of muscle architecture in relation to locomotor ecology in snakes. J. Anat. 2022. [Google Scholar]
- Vázquez, T.; Neipp, C.; Beléndez, A. Numerical and Experimental Analysis of a Cantilever Beam: A Laboratory Project to Introduce Geometric Nonlinearity in Mechanics of Materials. Int. J. Eng. Educ. 2003, 19, 885–892. [Google Scholar]
- Hall, A.R. The Pseudo-Rigid-Body Model for Fast, Accurate, Non-Linear Elasticity. Master’s Thesis, Brigham Young University, Provo, Utah, 2013. Available online: https://scholarsarchive.byu.edu/etd/3869 (accessed on 28 November 2022).
- Lee, K. Large deflections of cantilever beams of non-linear elastic material under a combined loading. Int. J. Non-Linear Mech. 2002, 37, 439–443. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, B.K. Elastica of Non-Prismatic and Nonlinear Elastic Cantilever Beams under Combined Loading. Appl. Sci. 2019, 9, 877. [Google Scholar] [CrossRef]
- Webster, R.J., III; Jones, B.A. Design and Kinematic Modeling of Constant Curvature Continuum Robots: A Review. J Robot. Res 2010, 29, 1661–1683. [Google Scholar] [CrossRef]
- Boyer, F.; Lebastard, V.; Candelier, F.; Renda, F. Dynamics of continuum and soft robots: A strain parametrization based approach. IEEE Trans. Robot. 2020, 37, 847–863. [Google Scholar] [CrossRef]
- Renda, F.; Cacucciolo, V.; Dias, J.; Seneviratne, L. Discrete Cosserat approach for soft robot dynamics: A new piece-wise constant strain model with torsion and shears. In Proceedings of the 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Daejeon, Korea, 9–14 October 2016; pp. 5495–5502. [Google Scholar] [CrossRef]
- Janabi-Sharifi, F.; Jalali, A.; Walker, I.D. Cosserat Rod-Based Dynamic Modeling of Tendon-Driven Continuum Robots: A Tutorial. IEEE Access 2021, 9, 68703–68719. [Google Scholar] [CrossRef]
- Simaan, N.; Xu, K.; Wei, W.; Kapoor, A.; Kazanzides, P.; Taylor, R.; Flint, P. Design and Integration of a Telerobotic System for Minimally Invasive Surgery of the Throat. Int. J. Robot. Res. 2009, 28, 1134–1153. [Google Scholar] [CrossRef]
- Ouyang, B.; Liu, Y.; Sun, D. Design of a three-segment continuum robot for minimally invasive surgery. Robot. Biomim. 2016, 3, 2. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Chen, W.; Wang, X.; Pfeifer, R. A cable-driven soft robot surgical system for cardiothoracic endoscopic surgery: Preclinical tests in animals. Surg. Endosc. 2017, 31, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
- Althoefer, K. Antagonistic actuation and stiffness control in soft inflatable robots. Nat. Rev. Mater. 2018, 3, 6. [Google Scholar] [CrossRef]
- Koleoso, M.; Feng, X.; Xue, Y.; Li, Q.; Munshi, T.; Chen, X. Micro/nanoscale magnetic robots for biomedical applications. Mater. Today Bio 2020, 8, 100085. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Jiao, N.; Wang, Z.; Liu, L. A Magnetic Continuum Robot With Multi-Mode Control Using Opposite-Magnetized Magnets. IEEE Robot. Autom. Lett. 2021, 6, 2485–2492. [Google Scholar] [CrossRef]
- Edelmann, J.; Petruska, A.J.; Nelson, B.J. Magnetic control of continuum devices. Int. J. Robot. Res. 2017, 36, 68–85. [Google Scholar] [CrossRef]
- Russo, M.; Sriratanasak, N.; Ba, W.; Dong, X.; Mohammad, A.; Axinte, D. Cooperative Continuum Robots: Enhancing Individual Continuum Arms by Reconfiguring Into a Parallel Manipulator. IEEE Robot. Autom. Lett. 2022, 7, 1558–1565. [Google Scholar] [CrossRef]
- Hertel, H. Structure, Form, Movement; Reinhold: New York, NY, USA, 1966. [Google Scholar]





| Snake | Snout–Vent Length (m) | Body Mass (g) |
|---|---|---|
| SnaBiBot | 1.2 | 220 |
| Hierophis viridiflavus | 0.9 | 202 |
| Zamenis longissimus | 0.8 | 196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautreau, E.; Bonnet, X.; Sandoval, J.; Fosseries, G.; Herrel, A.; Arsicault, M.; Zeghloul, S.; Laribi, M.A. A Biomimetic Method to Replicate the Natural Fluid Movements of Swimming Snakes to Design Aquatic Robots. Biomimetics 2022, 7, 223. https://doi.org/10.3390/biomimetics7040223
Gautreau E, Bonnet X, Sandoval J, Fosseries G, Herrel A, Arsicault M, Zeghloul S, Laribi MA. A Biomimetic Method to Replicate the Natural Fluid Movements of Swimming Snakes to Design Aquatic Robots. Biomimetics. 2022; 7(4):223. https://doi.org/10.3390/biomimetics7040223
Chicago/Turabian StyleGautreau, Elie, Xavier Bonnet, Juan Sandoval, Guillaume Fosseries, Anthony Herrel, Marc Arsicault, Saïd Zeghloul, and Med Amine Laribi. 2022. "A Biomimetic Method to Replicate the Natural Fluid Movements of Swimming Snakes to Design Aquatic Robots" Biomimetics 7, no. 4: 223. https://doi.org/10.3390/biomimetics7040223
APA StyleGautreau, E., Bonnet, X., Sandoval, J., Fosseries, G., Herrel, A., Arsicault, M., Zeghloul, S., & Laribi, M. A. (2022). A Biomimetic Method to Replicate the Natural Fluid Movements of Swimming Snakes to Design Aquatic Robots. Biomimetics, 7(4), 223. https://doi.org/10.3390/biomimetics7040223











