Enhanced Adhesion of Synthetic Discs with Micro-Patterned Margins
Abstract
:1. Introduction
2. Materials and Methods
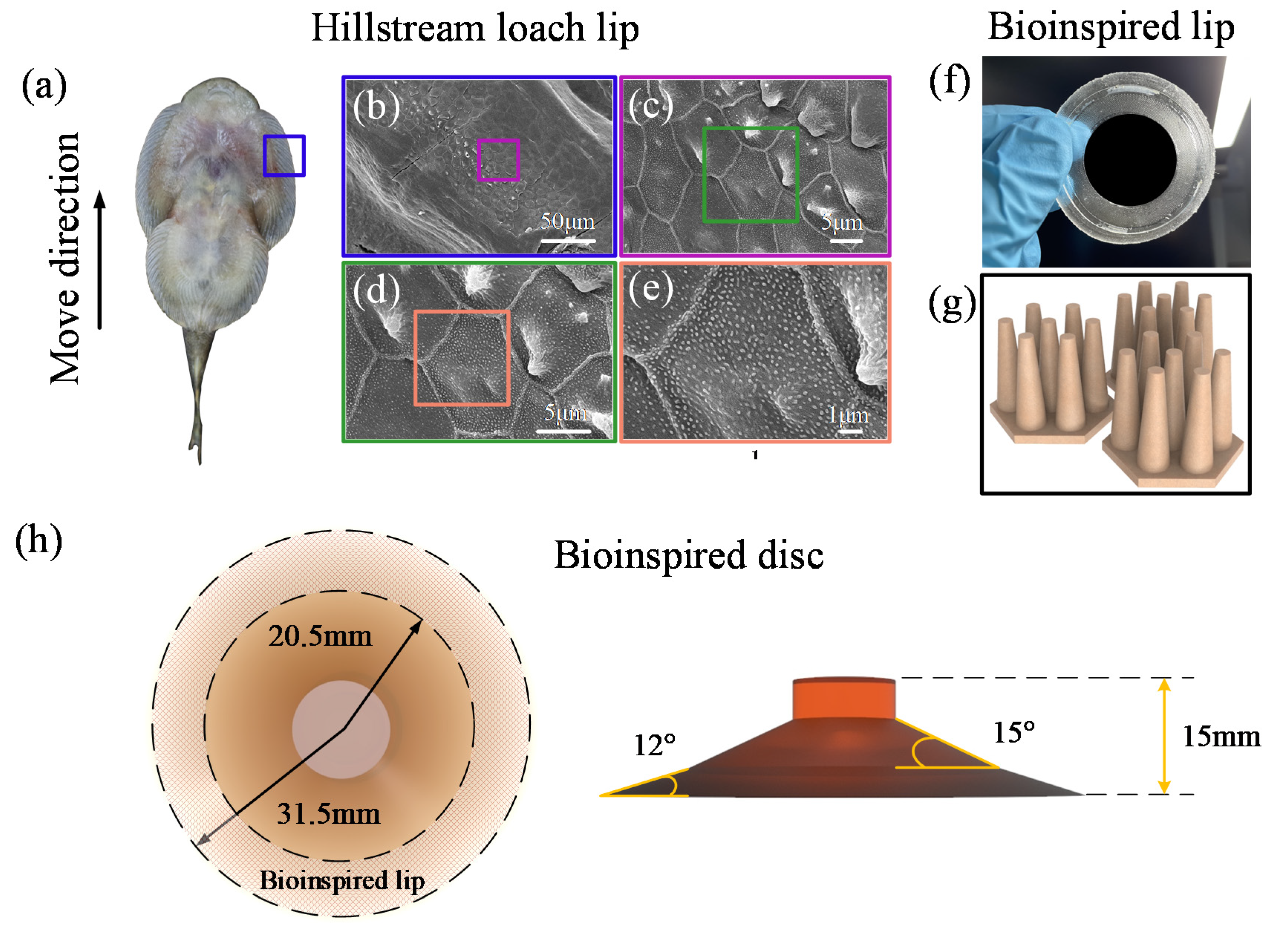
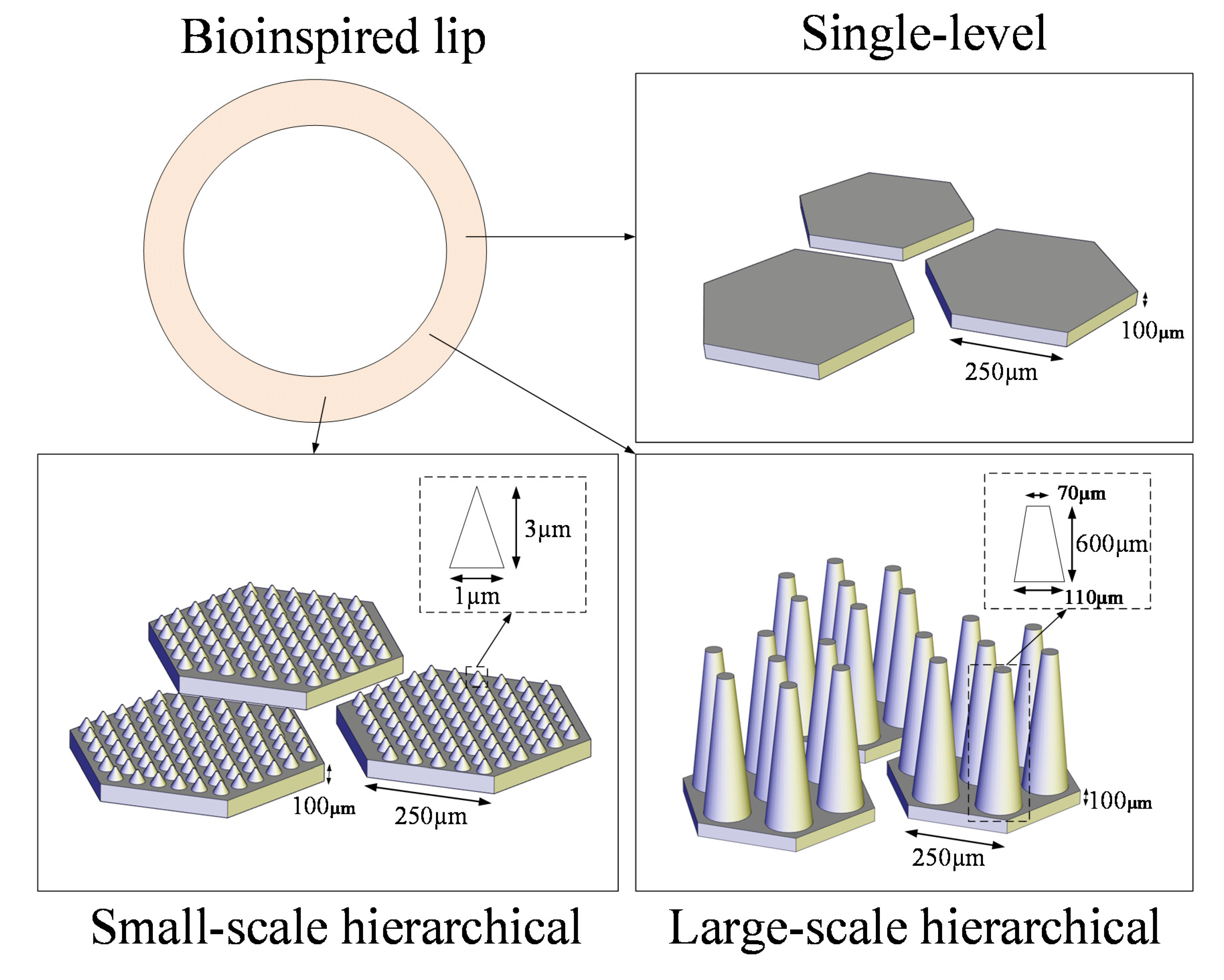
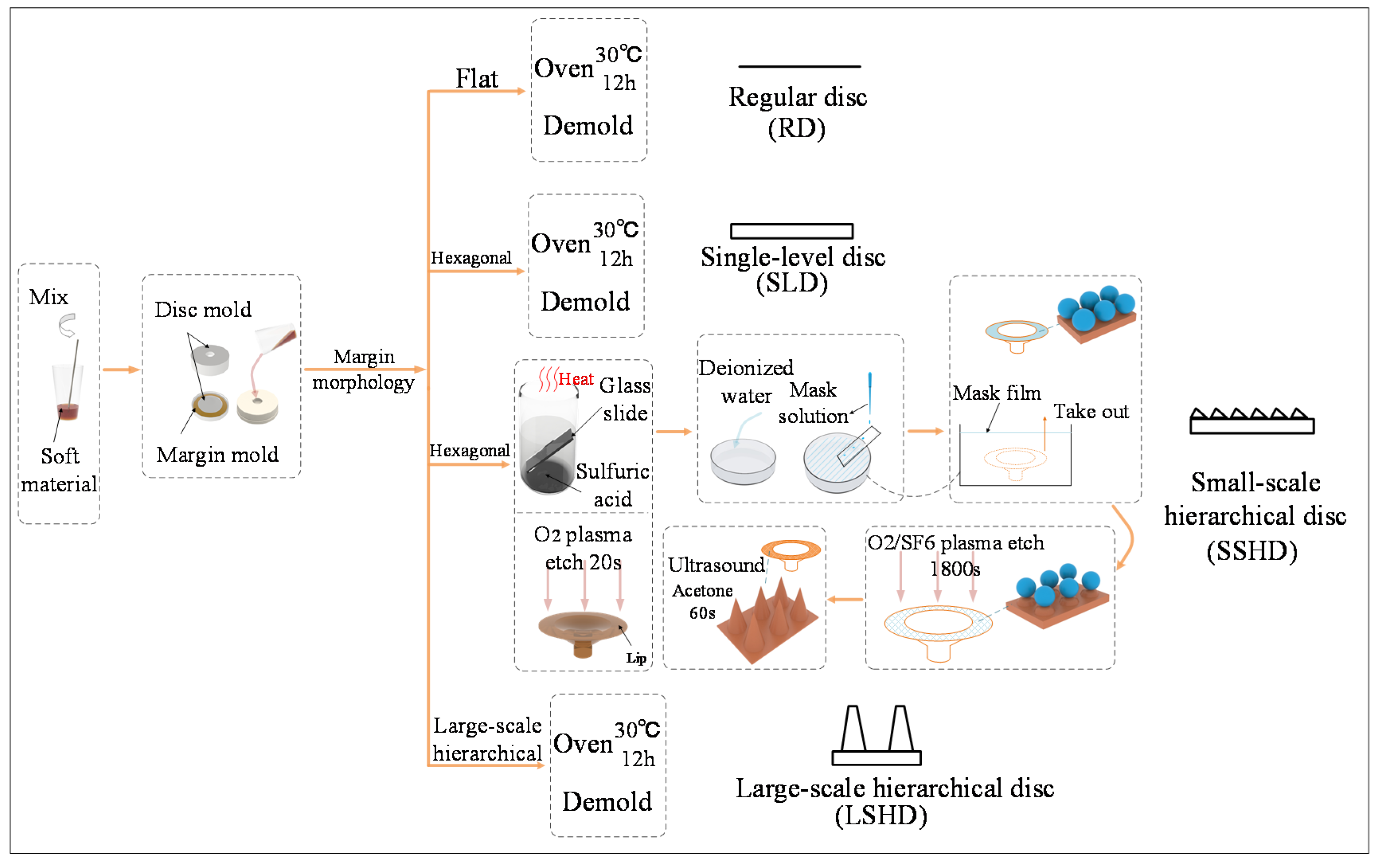
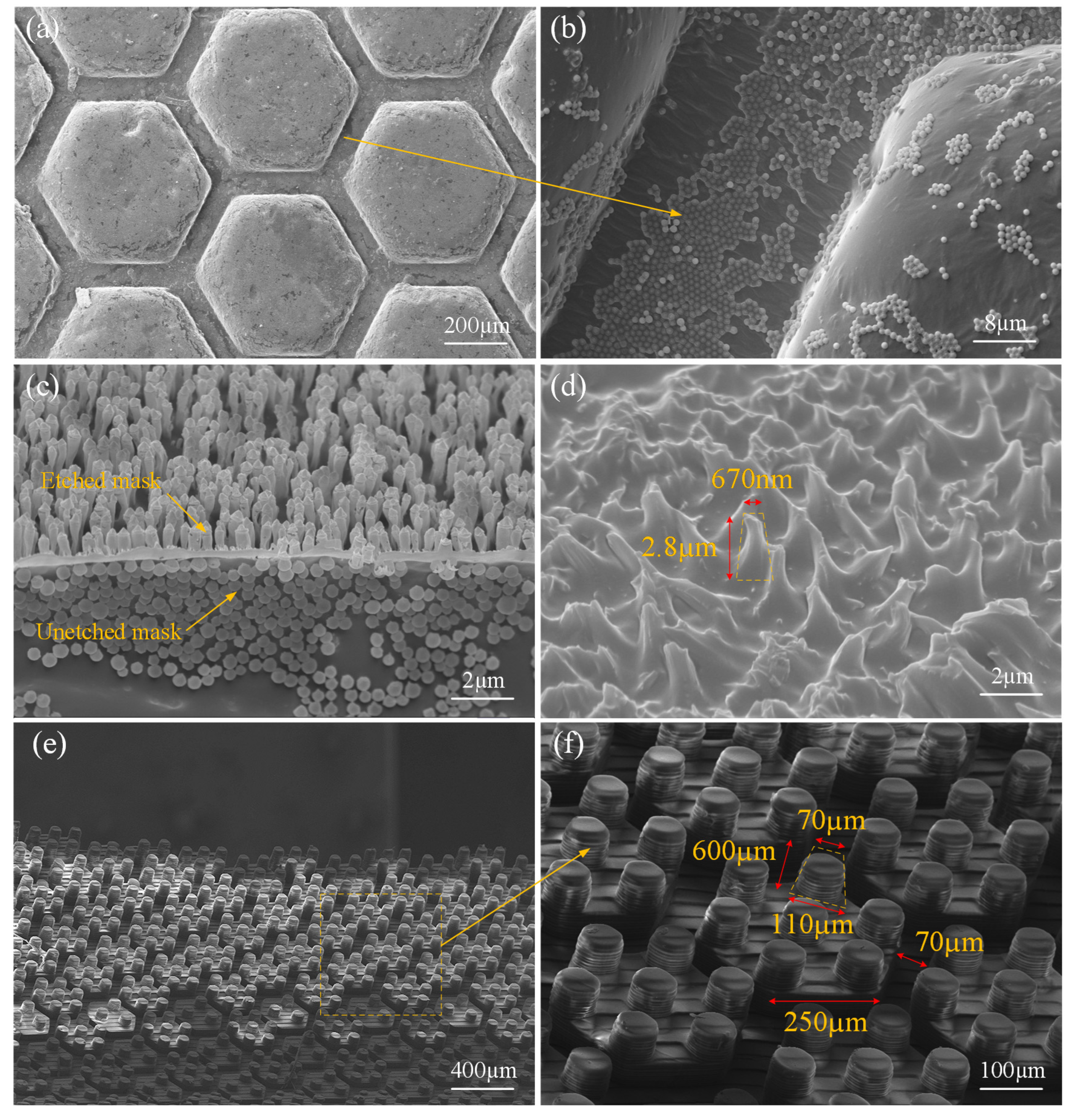
2.1. Bioinspiration and Design
2.2. Test Platforms
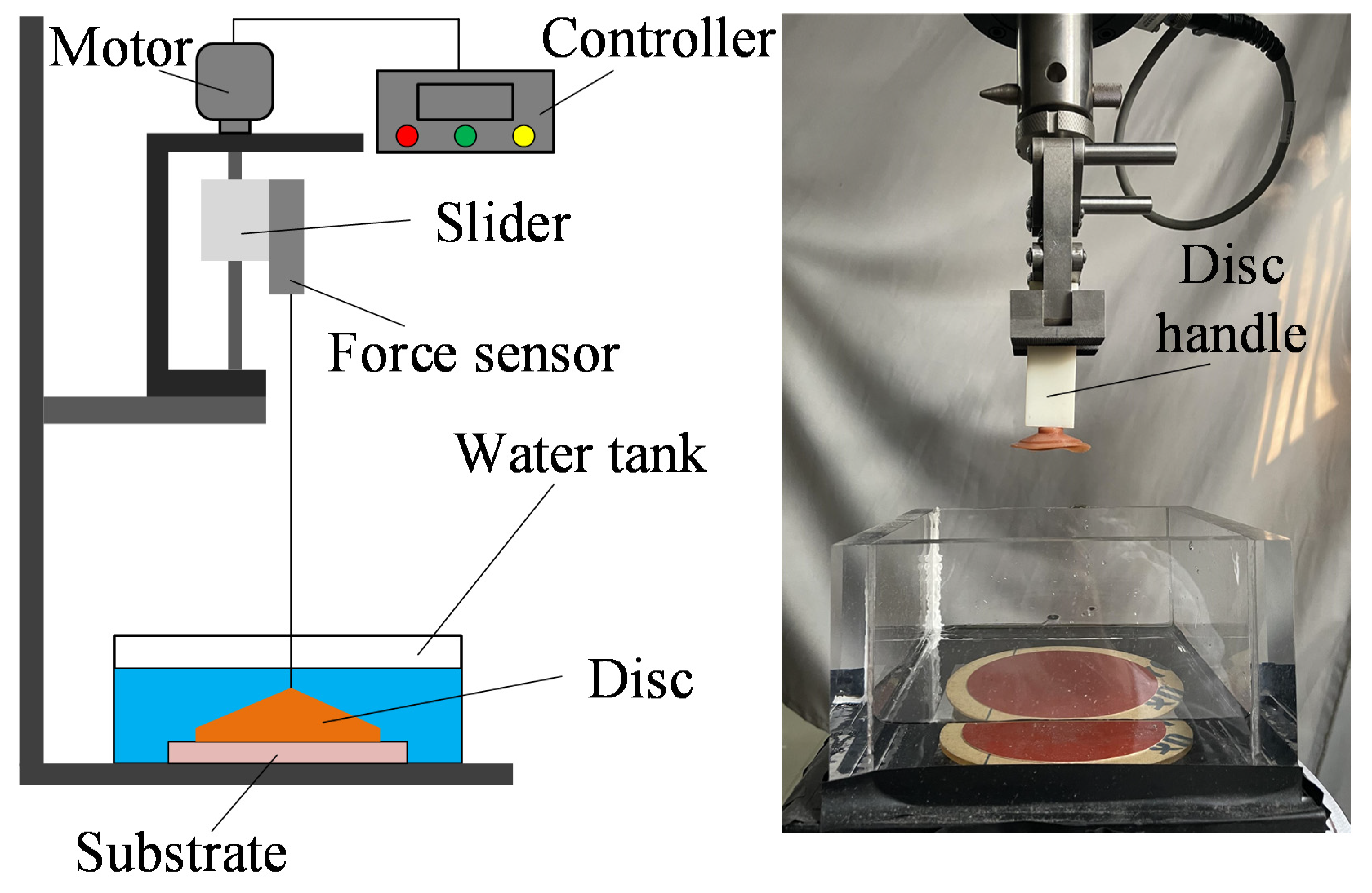
2.2.1. Mechanical Testing Setup
2.2.2. Interfacial Observing Setup
2.3. Testing Procedure
2.3.1. Pull-Off Force Testing
2.3.2. Interfacial Observation
3. Results
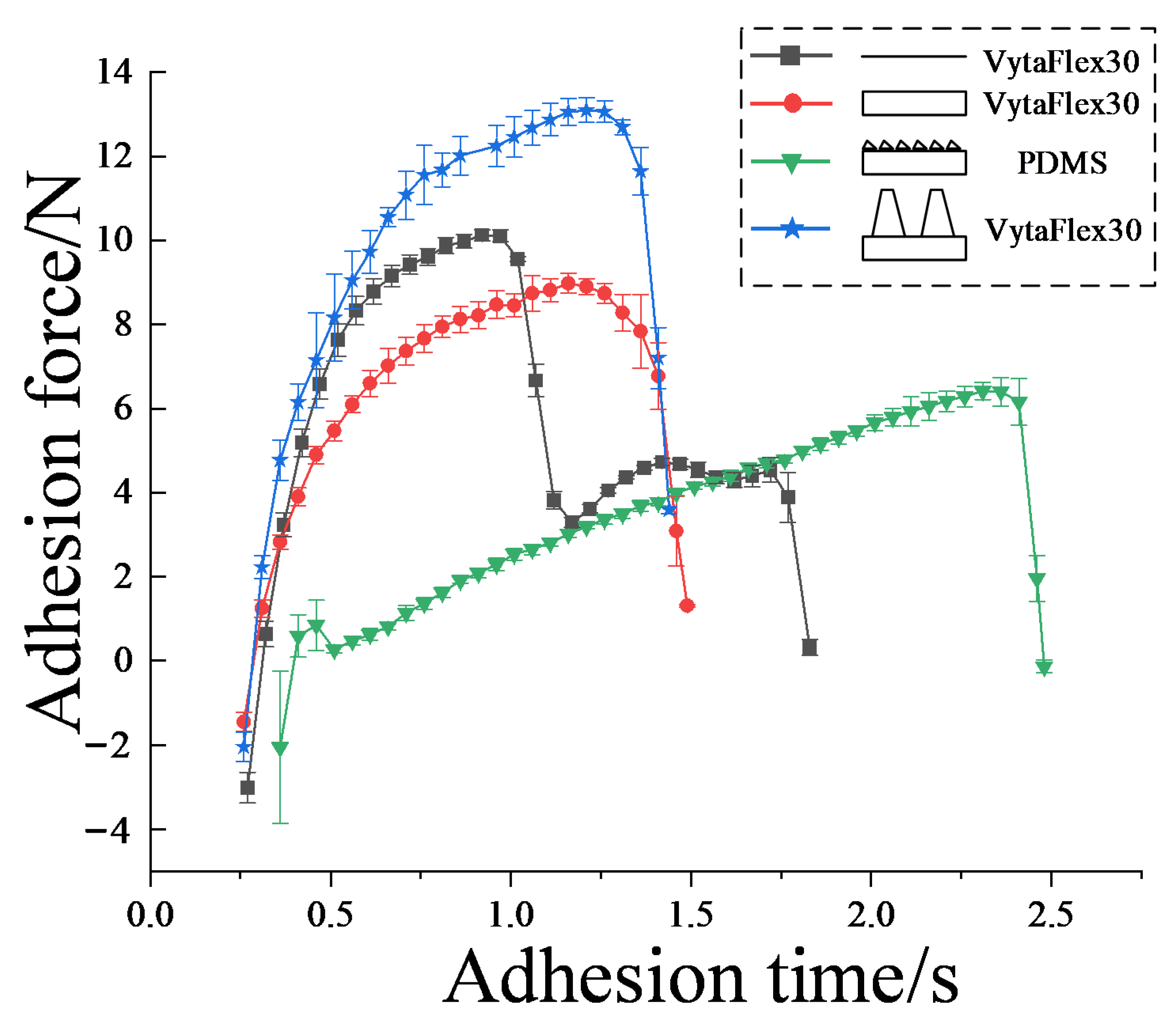
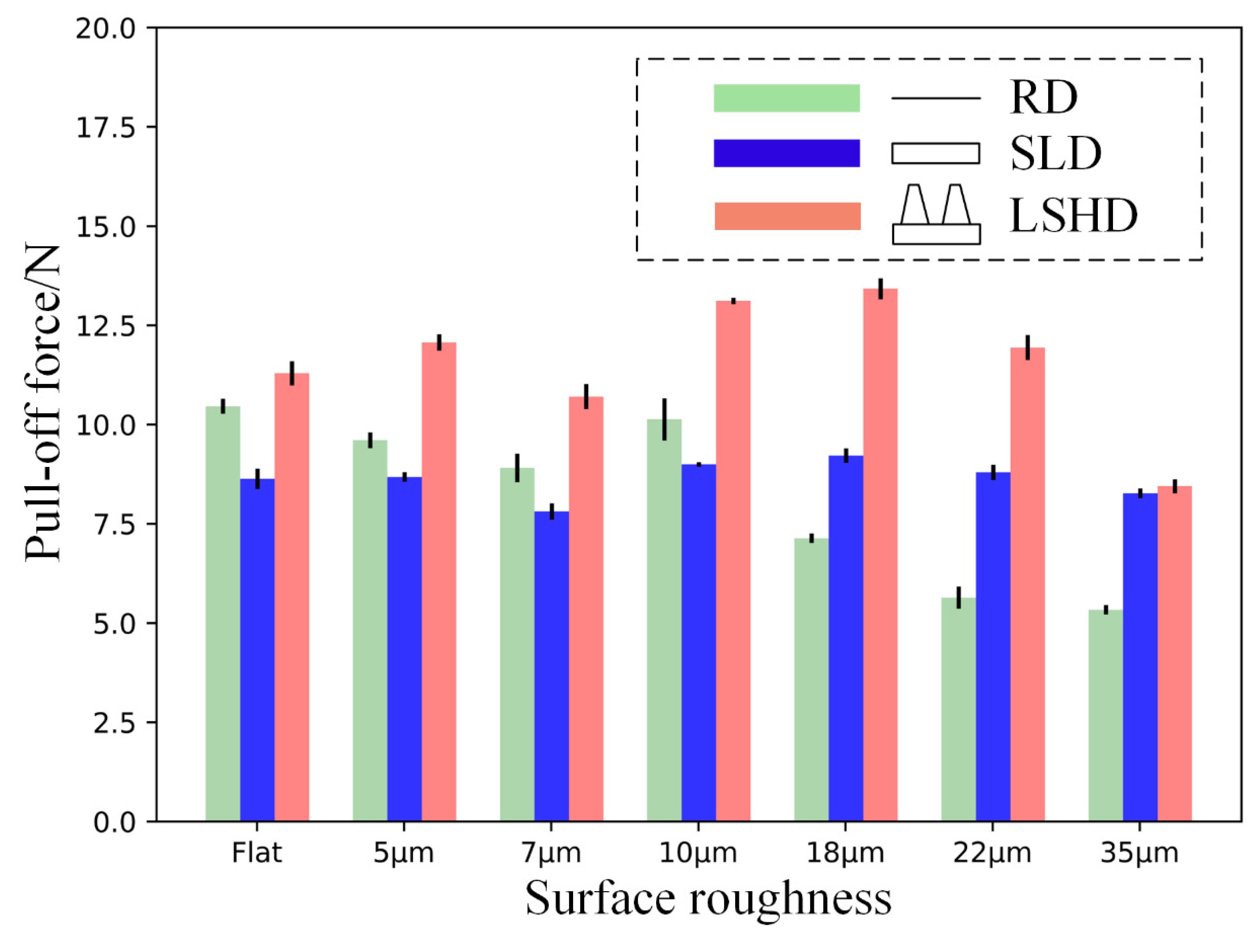
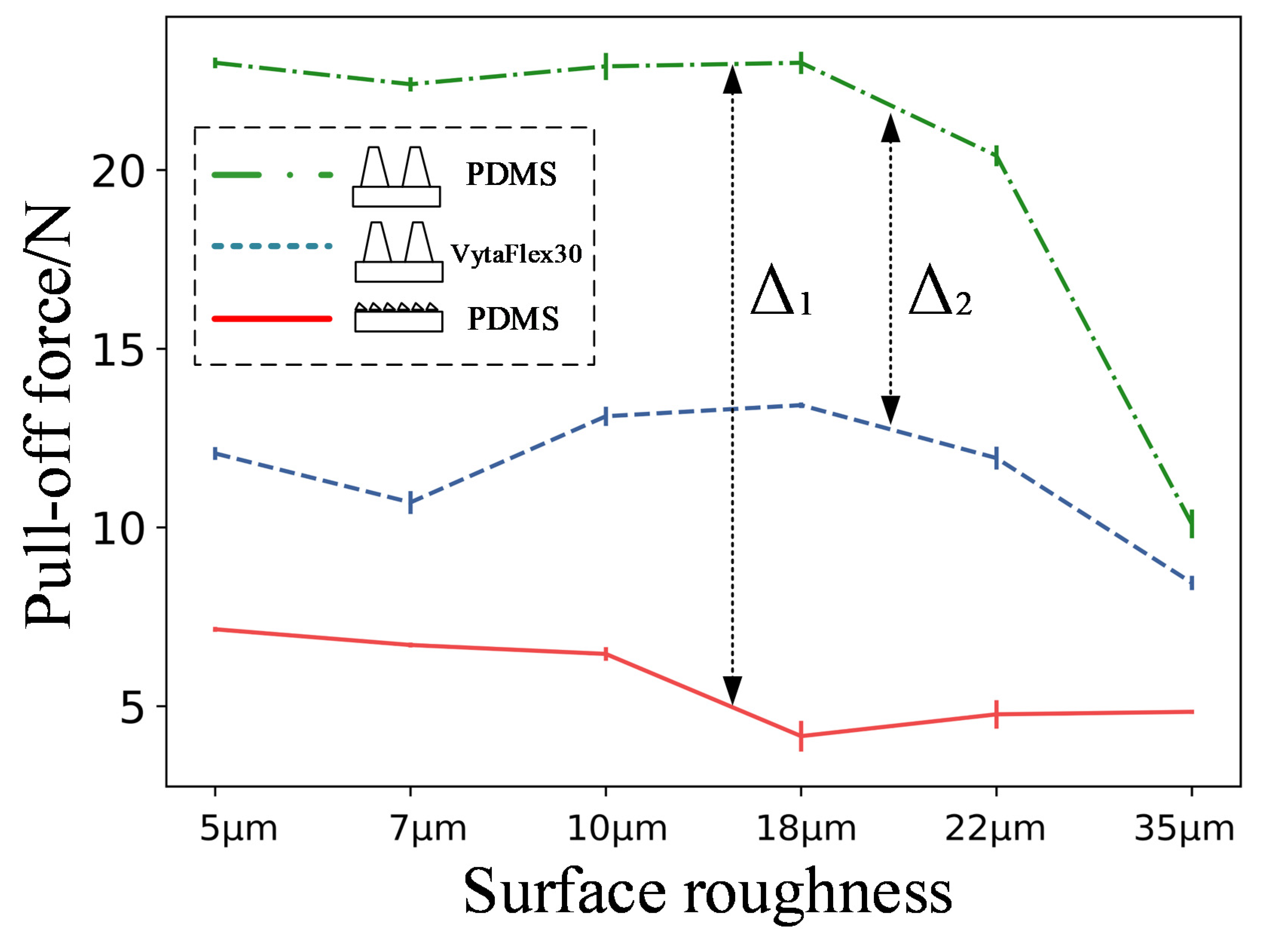
3.1. Pull-Off Force Results
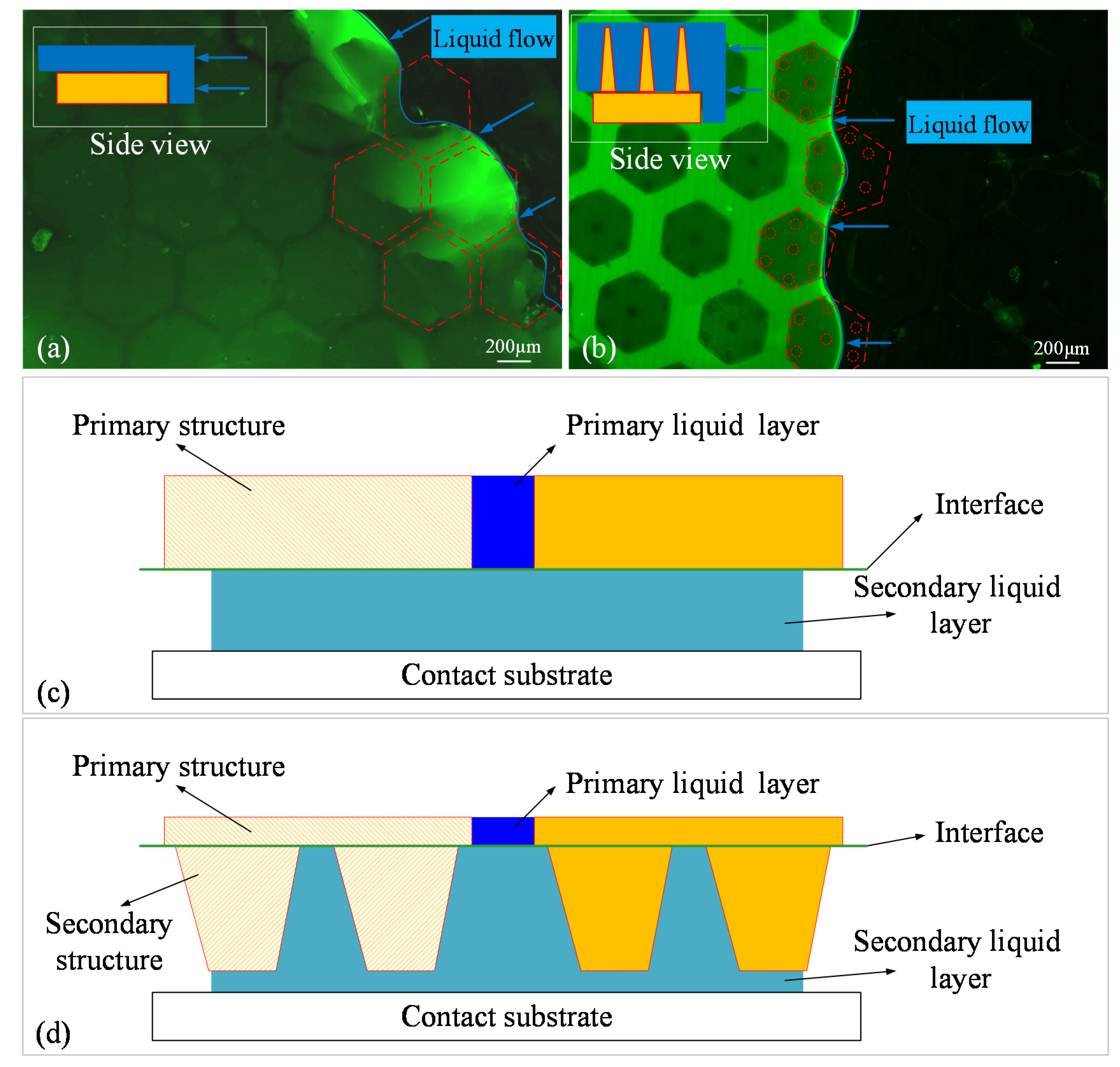
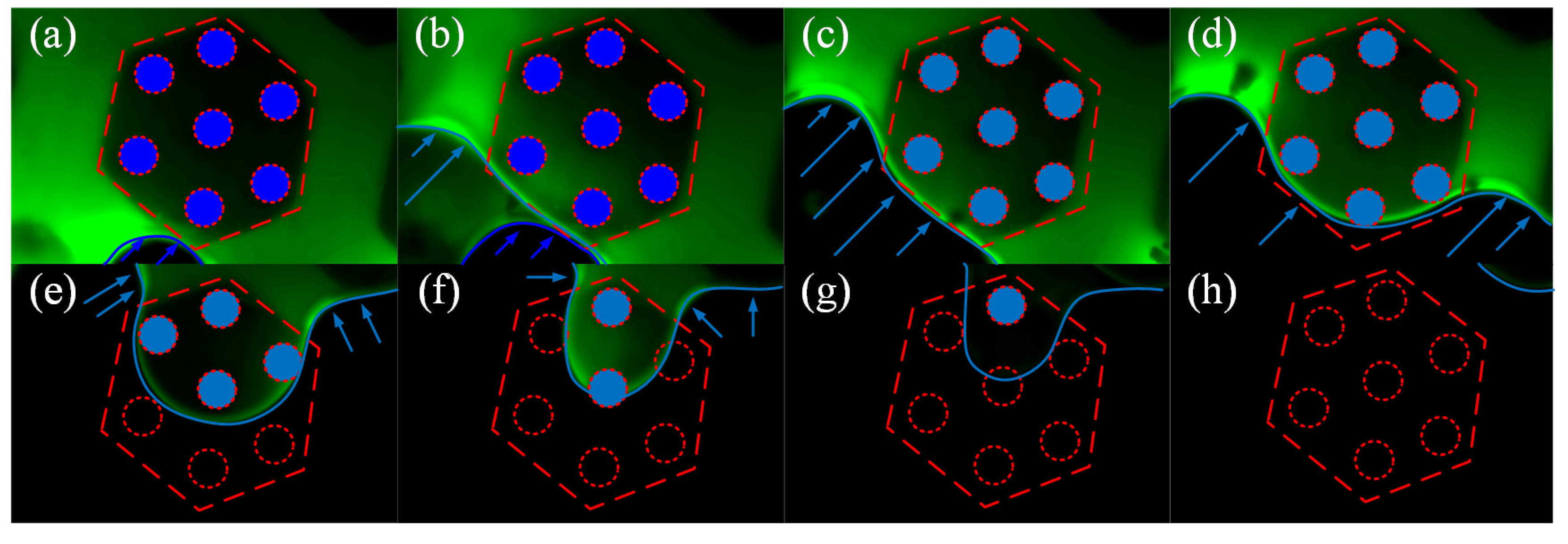
3.2. Interfacial Observation Results
3.2.1. Static Observation
3.2.2. Dynamic Observation
4. Discussion
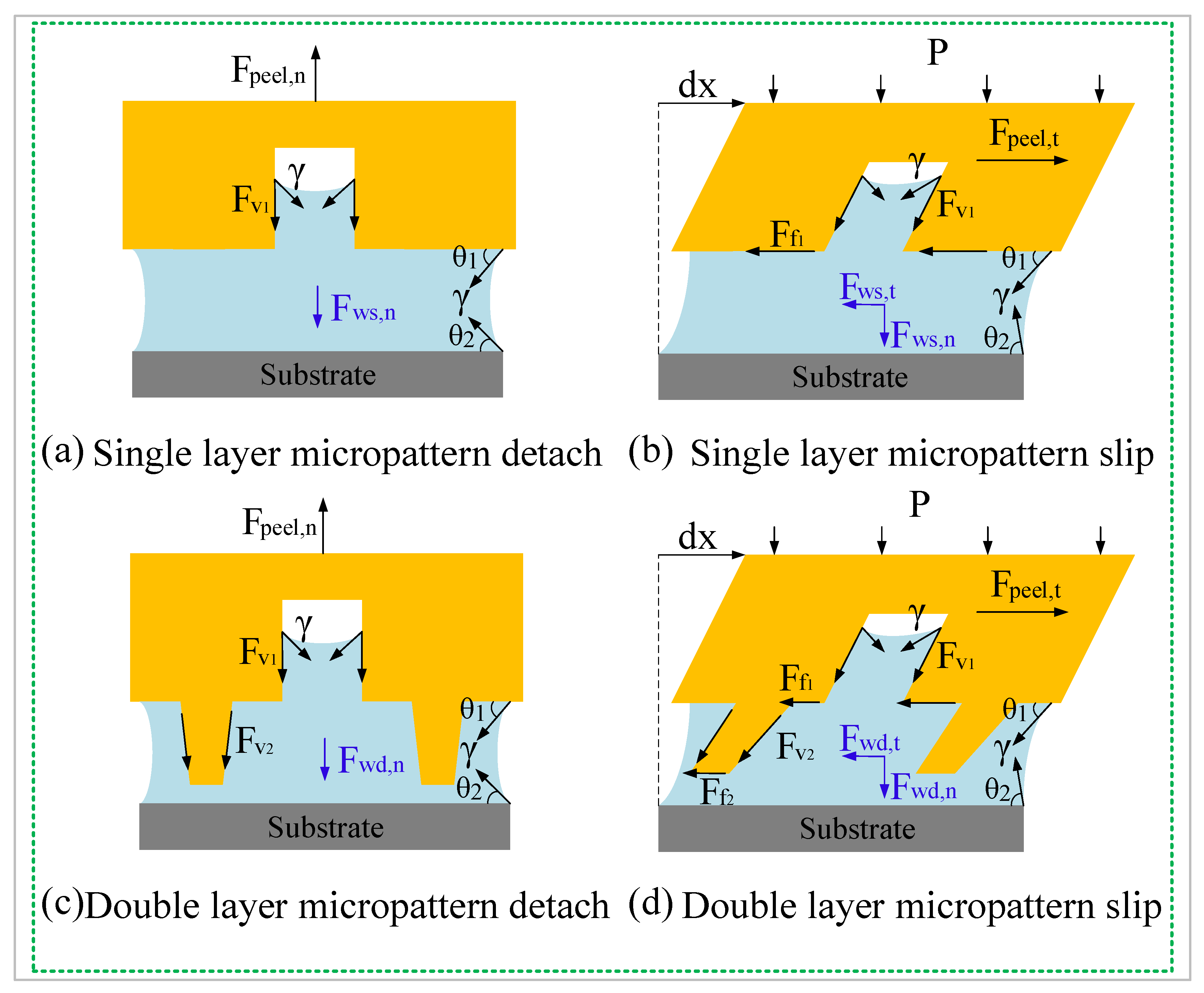
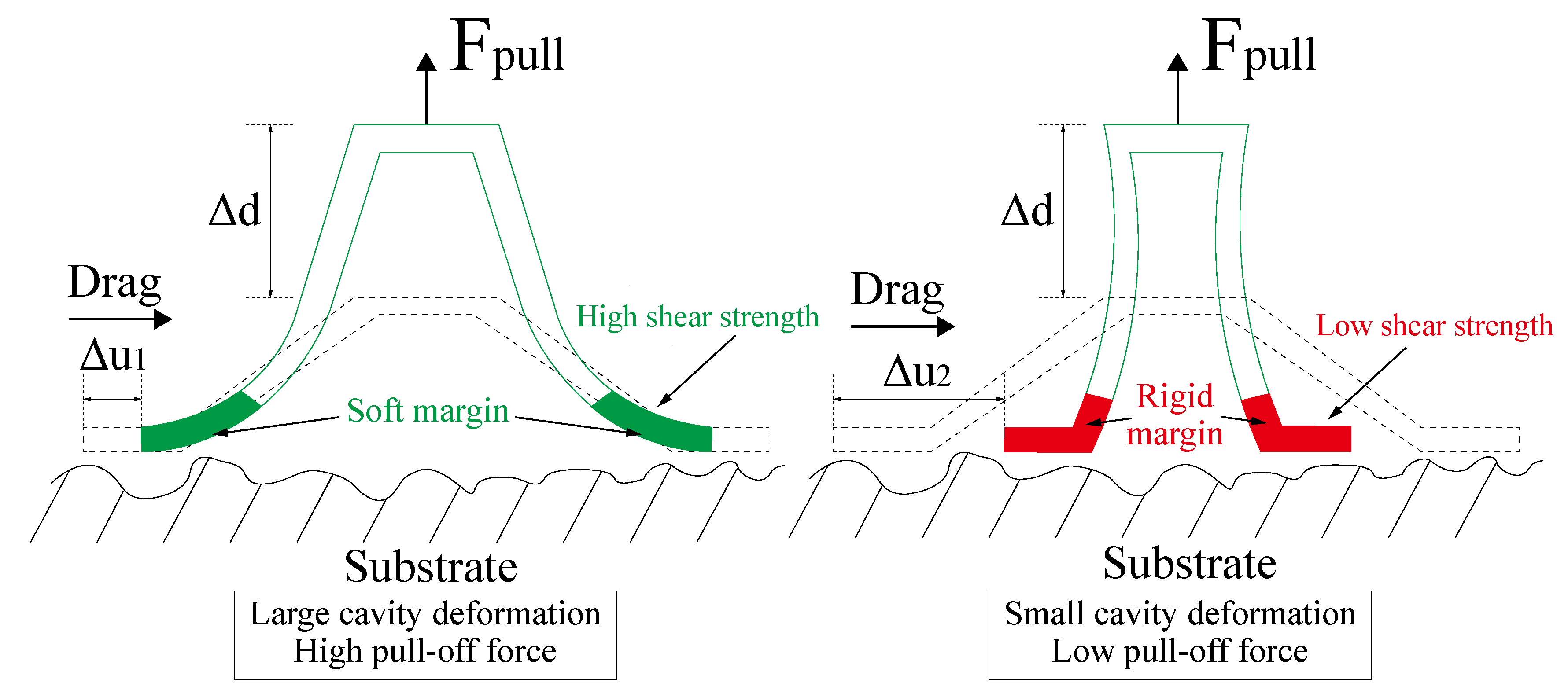
4.1. Enhanced Adhesion Force Due to Higher Capillary Force
4.2. Enhanced Adhesion Force Due to the Synergy Effect
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kier, W.M.; Smith, A.M. The Structure and Adhesive Mechanism of Octopus Suckers. Integr. Comp. Biol. 2002, 42, 1146–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadler, J.H.; Mercer, A.J.; Culler, M.; Ledford, K.A.; Bloomquist, R.; Lin, A. Structures and Function of Remora Adhesion. MRS Proc. 2013, 1498, 159–168. [Google Scholar] [CrossRef]
- Wainwright, D.K.; Kleinteich, T.; Kleinteich, A.; Gorb, S.N.; Summers, A.P. Stick tight: Suction adhesion on irregular surfaces in the northern clingfish. Biol. Lett. 2013, 9, 20130234. [Google Scholar] [CrossRef] [Green Version]
- De Meyer, J.; Geerinckx, T. Using the whole body as a sucker: Combining respiration and feeding with an attached lifestyle in hill stream loaches (Balitoridae, Cypriniformes). J. Morphol. 2014, 275, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wang, J.; Ji, C. The Adhesive System and Anisotropic Shear Force of Guizhou Gastromyzontidae. Sci. Rep. 2016, 6, 37221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, X.; Chen, Y.; Wainwright, D.K.; Kenaley, C.P.; Gong, Z.; Liu, Z.; Liu, H.; Guan, J.; Wang, T.; et al. A biorobotic adhesive disc for underwater hitchhiking inspired by the remora suckerfish. Sci. Robot. 2017, 2, eaan8072. [Google Scholar] [CrossRef] [Green Version]
- Chuang, Y.-C.; Chang, H.-K.; Liu, G.-L.; Chen, P.-Y. Climbing upstream: Multi-scale structural characterization and underwater adhesion of the Pulin river loach (Sinogastromyzon puliensis). J. Mech. Behav. Biomed. Mater. 2017, 73, 76–85. [Google Scholar] [CrossRef]
- Tramacere, F.; Appel, E.; Mazzolai, B.; Gorb, S.N. Hairy suckers: The surface microstructure and its possible functional significance in the Octopus vulgaris sucker. Beilstein J. Nanotechnol. 2014, 5, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Ditsche, P.; Wainwright, D.K.; Summers, A.P. Attachment to challenging substrates–fouling, roughness and limits of adhesion in the northern clingfish (Gobiesox maeandricus). J. Exp. Biol. 2014, 217, 2548–2554. [Google Scholar] [CrossRef] [Green Version]
- Beckert, M.; Flammang, B.E.; Nadler, J.H. Remora fish suction pad attachment is enhanced by spinule friction. J. Exp. Biol. 2015, 218, 3551–3558. [Google Scholar]
- Tramacere, F.; Beccai, L.; Kuba, M.; Gozzi, A.; Bifone, A.; Mazzolai, B. The Morphology and Adhesion Mechanism of Octopus vulgaris Suckers. PLoS ONE 2013, 8, e65074. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, S.; Zhang, Y.; Song, S.; Wang, C.; Tan, S.; Zhao, W.; Wang, G.; Sun, W.; Yang, F.; et al. Aerial-aquatic robots capable of crossing the air-water boundary and hitchhiking on surfaces. Sci. Robot. 2022, 7, eabm6695. [Google Scholar] [CrossRef] [PubMed]
- Ditsche, P.; Summers, A. Learning from Northern clingfish (Gobiesox maeandricus): Bioinspired suction cups attach to rough surfaces. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.R. Unculi (Horny Projections Arising from Single Cells), an Adaptive Feature of the Epidermis of Ostariophysan Fishes. Zool. Scr. 1982, 11, 55–76. [Google Scholar] [CrossRef]
- Das, D.; Nag, T.C. Adhesion by paired pectoral and pelvic fins in a mountain-stream catfish, Pseudocheneis sulcatus (Sisoridae). Environ. Biol. Fishes 2004, 71, 1–5. [Google Scholar] [CrossRef]
- Sandoval, J.A.; Jadhav, S.; Quan, H.; Deheyn, D.D.; Tolley, M.T. Reversible adhesion to rough surfaces both in and out of water, inspired by the clingfish suction disc. Bioinspir. Biomim. 2019, 14, 06601. [Google Scholar] [CrossRef]
- Tramacere, F.; Beccai, L.; Mattioli, F.; Sinibaldi, E.; Mazzolai, B. Artificial Adhesion Mechanisms Inspired by Octopus Suckers. In Proceedings of the 2012 IEEE International Conference on Robotics and Automation, Guangzhou, China, 11–14 December 2012; pp. 3846–3851. [Google Scholar]
- Zhang, Y.; Wu, X.; Gong, L.; Chen, G.; Wang, X. A Bionic Adhesive Disc for Torrent Immune Locomotion Inspired by the Guizhou Gastromyzontidae. In Proceedings of the 2019 IEEE International Conference on Robotics and Biomimetics (ROBIO), Dali, China, 6–8 December 2019; pp. 132–135. [Google Scholar]
- Tramacere, F.; Pugno, N.M.; Kuba, M.J.; Mazzolai, B. Unveiling the morphology of the acetabulum in octopus suckers and its role in attachment. Interface Focus 2015, 5, 20140050. [Google Scholar] [CrossRef] [Green Version]
- Barnes, W.J.P. Functional Morphology and Design Constraints of Smooth Adhesive Pads. MRS Bull. 2007, 32, 479–4857. [Google Scholar] [CrossRef]
- Persson, B. Wet adhesion with application to tree frog adhesive toe pads and tires. J. Phys. Condens. Matter 2007, 19, 376110. [Google Scholar] [CrossRef]
- Ditsche-Kuru, P.; Koop, J.H.E.; Gorb, S.N. Underwater attachment in current: The role of setose attachment structures on the gills of the mayfly larvaeEpeorus assimilis(Ephemeroptera, Heptageniidae). J. Exp. Biol. 2010, 213, 1950–1959. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhao, A.; Li, L.; He, Q.; Guo, H.; Sun, H.; Gao, Q. Bioinspired ribbed hair arrays with robust superhydrophobicity fabricated by micro/nanosphere lithography and plasma etching. RSC Adv. 2015, 5, 96404–96411. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, A.; Sun, H.; Chen, P.; He, Q. Bio-inspired hierarchical hair arrays with tunable adhesive superhydrophobicity. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 262–269. [Google Scholar] [CrossRef]
- Tsougeni, K.; Vourdas, N.; Tserepi, A.; Gogolides, E.; Cardinaud, C. Mechanisms of Oxygen Plasma Nanotexturing of Organic Polymer Surfaces: From Stable Super Hydrophilic to Super Hydrophobic Surfaces. Langmuir 2009, 25, 11748–11759. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Persson, B.N.J. Physics of suction cups. Soft Matter 2019, 15, 9482–9499. [Google Scholar] [CrossRef]
- Ge, D.; Matsuno, T.; Sun, Y.; Ren, C.; Tang, Y.; Ma, S. Quantitative study on the attachment and detachment of a passive suction cup. Vacuum 2015, 116, 13–20. [Google Scholar] [CrossRef]
- Pesika, N.S.; Tian, Y.; Zhao, B.; Rosenberg, K.; Zeng, H.; McGuiggan, P.; Autumn, K.; Israelachvili, J.N. Peel-Zone Model of Tape Peeling Based on the Gecko Adhesive System. J. Adhes. 2007, 83, 383–401. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Wu, X. Enhanced Adhesion of Synthetic Discs with Micro-Patterned Margins. Biomimetics 2022, 7, 202. https://doi.org/10.3390/biomimetics7040202
Zhou W, Wu X. Enhanced Adhesion of Synthetic Discs with Micro-Patterned Margins. Biomimetics. 2022; 7(4):202. https://doi.org/10.3390/biomimetics7040202
Chicago/Turabian StyleZhou, Weimian, and Xuan Wu. 2022. "Enhanced Adhesion of Synthetic Discs with Micro-Patterned Margins" Biomimetics 7, no. 4: 202. https://doi.org/10.3390/biomimetics7040202
APA StyleZhou, W., & Wu, X. (2022). Enhanced Adhesion of Synthetic Discs with Micro-Patterned Margins. Biomimetics, 7(4), 202. https://doi.org/10.3390/biomimetics7040202





