Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration
Abstract
:1. Introduction
- Stem cell adhesion on the scaffold, proliferation, and differentiation: These phenomena require hydrophilic scaffold surface and bioactive chemical composition, permitting the exchange of osteogenic signals that promote osteoblastic differentiation [7].
- The complete colonization of the scaffold by bone-forming cells, assisted by substantial vascularization: This requires scaffolds with osteoconductive ability, which is, once again promoted by the chemical composition as well as the existence of a wide open and interconnected porosity.
- The activation of osteoclastic bio-resorption, which is, once again, substantially related to the chemical composition, thus permitting the replacement of the scaffold with the newly formed bone by the natural physiologic process. In this context, nanostructured scaffold materials can offer high specific surface areas and a more chemically active surface, facilitating the bio-resorption process.
- Bone mechano-transduction, consisting of a complex cascade of phenomena that translate mechanical forces into bioelectric signals, is a major source of cell signaling, in which bone tissue is able to continuously renew and remodel over time, and to self-repair following damage of limited entity [8].
2. Bone Tissue: Composition and Structure
2.1. Bone Composition
2.2. Hierarchical Bone Organization
- The macrostructure: Cancellous and cortical bones.
- The microstructure (from 10 to 500 microns): Haversian systems, osteons.
- The sub-microstructure (1–10 microns): Lamellar and woven bones.
- The nanostructure (from a few hundred nanometers to 1 micron): Fibrillar collagen and embedded mineral phase.
- The molecular structure (below a few hundred nanometers): Molecular structure of constituent elements, such as mineral, collagen, and non-collagenous organic proteins [25].
2.2.1. The Macrostructure
- Cortical bone (also called compact bone) is a dense structure organized as regular layers of lamellae tissue, with different thicknesses depending on the location of the bone. The location of cortical tissue is on the outer layer of bone tissue, constituting about 80% of the total mass of the skeleton. Transverse sections of lamellae arrangement in load-bearing bones, such as the femur and other lower extremity bones exhibit a denser and thicker structure compared with other tissues, such as lateral and posterior cortex.
- Cancellous bone (also called trabecular or spongy bone) is less dense tissue in which collagen fibers, and therefore, lamellae are arranged in an irregular way, interconnecting and forming the trabecular tissue network. Cancellous bone is located within metaphysis and epiphysis at the end of long bones, and in short bones, as well. Metabolic activity, such as bone cell production and mineral exchange is higher in cancellous bone compared with the cortical bone as its porous structure is highly vascularized and contains red bone marrow [12,26].
2.2.2. The Microstructure
2.2.3. The Sub-Microstructure
2.2.4. The Nanostructure
2.2.5. The Molecular Structure
3. Bone Tissue: Formation and Metabolism
3.1. Mineralization Process of Bone Tissue
- Chemical factors: Precipitation of ions naturally present in the environment, mediated by complex macromolecular organic structures, which act as sites of heterogeneous nucleation and control specific chemical interactions.
- Spatial factors: Confinement of the nuclei growth, as well as a constraint in their shape and contact with the organic substrate.
- Structural factors: Inducing peculiar crystallographic features driven by the interaction between the mineral phase and the organic template.
- Morphologic factors (morphogenesis): Where the mineral phase takes place in a complex architecture on a macroscopic scale, strictly dependent on the combination of the various phenomena above-mentioned, which hierarchically occur on different dimensional scales in correspondence with the sites of heterogeneous nucleation [36].
3.2. Bone Modeling and Remodeling Processes
- Osteoclasts are highly specialized cells that are known to be capable of resorbing bone tissue, derived from mononuclear monocyte-macrophage precursor cells. In addition, RANKL and macrophage CSF (M-CSF) are two cytokines produced by marrow stromal cells that lead to osteoclast formation. Osteoclasts bind to bone matrix via integrins (transmembrane receptors that facilitate cell–cell and cell–extracellular matrix adhesion [48]) in the osteoclast membrane linked to bone matrix peptides. The β1 family of integrin receptors in osteoclasts binds to collagen, fibronectin, and laminin, but the main integrin receptor facilitating bone resorption is the αvβ3 integrin, which binds to osteopontin and bone sialoprotein [49]. Bone resorption depends on osteoclast secretion of hydrogen ions and cathepsin K enzyme, which define the active resorption process in two distinct phases: Acidification and proteolysis. Hydrogen ions, secreted by the osteoclastic vacuolar adenosine triphosphatase (V-ATPase) channels, acidify the resorption compartment called the “sealing zone” and dissolve the mineral component of the bone matrix, coupled with the transport of chloride ions via an electrochemical gradient [50]. The proteolysis occurs when cathepsin K digests the organic matrix, which is mostly composed of type I collagen [51,52].
- Osteoblasts are the cells responsible for the formation of bone tissue, derived by the differentiation of bMSC recruited from bone marrow to the bone surface by cytokines and growth factors, for example, transforming growth factor-β and insulin-like growth factor 1, which are released from the bone matrix during the resorption phase [53,54]. Osteoblasts express high levels of alkaline phosphatase (ALP) and osteocalcin and secrete large quantities of type I collagen and other specialized matrix proteins, which form osteoid tissue. The organic matrix acts as a template for the deposition of the mineral inorganic phase of HA. Interaction of osteoblasts among themselves, with lining cells, and with bone marrow cells are established by adherent junctions, tight junctions, and gap junctions. Adherent junctions, mainly mediated by cadherins, in addition to tight junctions serve to join cells and facilitate their anchorage to the extracellular matrix (ECM) [55].
- Activation: Osteoclast precursors lay on the bone tissue surface and differentiate to mature and functional osteoclast.
- Resorption: Osteoclast cells stick on the surface of the mineralized matrix to begin the process of bone resorption. The results of bone tissue dissolution are released in body fluids, such as blood and urine, to be useful biomarkers for the next resorption steps.
- Reversal: Osteoclasts interrupt bone tissue resorption and osteoblasts begin bone formation. Although the mechanism is still under investigation, direct cell–cell interaction between osteoclasts and osteoblasts (or their precursors) may trigger the disruption of one process and the initiation of the other [58].
- Formation: Osteoblasts lay down an unmineralized organic matrix (osteoid), which is primarily composed of type I collagen fibers and serves as a template for inorganic HA crystals [59].
3.3. Osteoinductivity and Osteoconductivity
4. Translation of the Biomimetic Concept to 3D Scaffold Development
4.1. Limitations of Current Approaches and Further Challenges in Tissue Engineering
4.2. Guiding Bone Regeneration by Chemistry and Crystal Structure
4.2.1. Synthesis Processes for the Production of Biomimetic Apatites
- The precipitation process is the most common aqueous synthesis method used to produce HA powders and it is performed at atmospheric pressure, low temperature, and inside a reaction vessel. Precipitation typically involves a reaction between orthophosphoric acid and dilute calcium hydroxide, with the former added drop-wise under continuous stirring. Precipitation occurs at a very slow rate and the reaction temperatures can be varied between 25 and 90 °C, which is suitable for tailoring the crystallinity of the final product [114,115].
- Sol-gel materials can be manufactured by gelation of colloidal powders, hypercritical drying or by controlling the hydrolysis and condensation of precursors, then incorporating a drying step at room temperature [113,116]. Sol-gel methods are generally preferable for the achievement of apatitic nanopowders with chemical and morphological uniformity. However, the use of expensive reactants and a general difficulty in hydrolyzing phosphate sources may limit the use of this technique for large scale production [117,118].
- Hydrothermal methods involve the reaction between calcium and phosphate solutions at very high pressures and temperatures (typically in a range between 60 and 250 °C), enabling the development of well-crystallized HA particles [119,120]. Crystallinity and ion content under hydrothermal treatments can be modulated by acting on the temperature and the ionic strength, thus enabling multiple doping [121].
4.2.2. Tailoring the Dissolution Mechanism and Solubility of Apatites
- After the placement of HA in an acidic aqueous solution, adsorption of water molecules and acid ions takes place, with the formation of a solid–liquid interface. The transport of H+ and An− occurs through the Nernst diffusion layer.
- The adsorption of H+ and An− ions on the apatite surface results in the formation of various complexes [124,125] and the protonation of orthophosphoric and hydroxyl groups. As hydroxyl groups have higher basicity and mobility in their crystalline structure than ≡POH surface groups (“≡” stands for the surface), adsorption processes usually occur faster and later diffuse away from the crystal into the bulk solution.
- The hydroxyl ion detachment from the crystal surface leaves the crystal with calcium and orthophosphate groups, that cannot be further protonated due to charge repulsion. As an electrical double layer with a positive charge on apatite cannot be continuous at the atomic (ionic) scale, the detachment of calcium atoms and their diffusion into the bulk solution is favored [123].
- A dissolution nucleus is formed after the detachment of calcium, with the formation of multiple crystal vacancies for Ca2+ and PO43− [126]. In addition, the detachment caused the formation of a charge vacancy, which is immediately compensated by the addition of protons from the acidic solution [127].
- The removal of each calcium results in decreasing attraction forces between the nearest (to calcium) orthophosphate group and the remaining part of the crystals since calcium occupies definite lattice positions, favoring the detachment of the remaining orthophosphates [128].
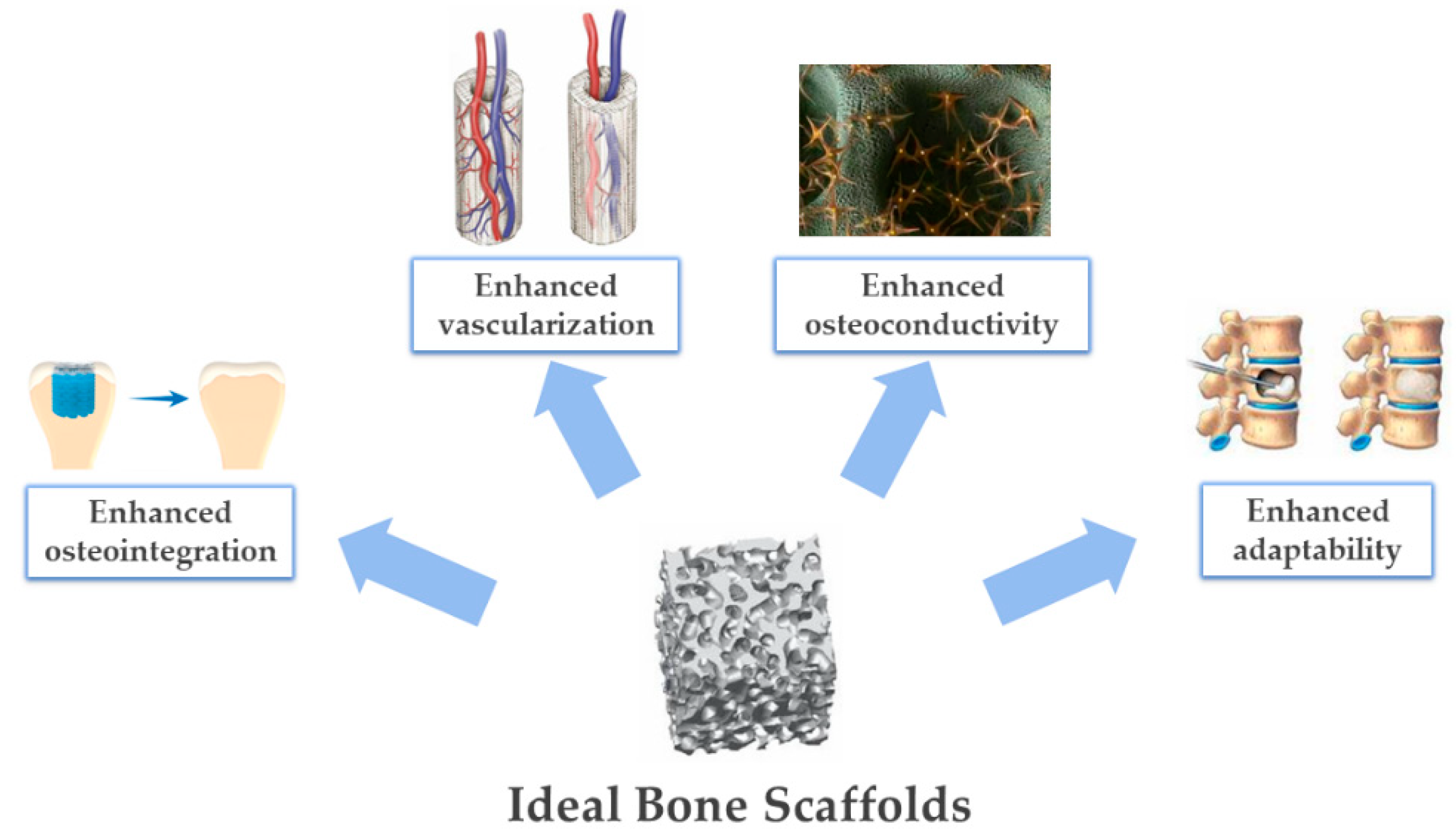
4.3. Guiding Bone Regeneration by 3D Scaffold Architecture and Porosity
5. Recent Approaches Yielding Biomimetic Ceramic-Based Scaffolds
5.1. Organic/Inorganic Scaffolds by 3D Printing
5.2. 3D Hybrid Scaffolds Using Natural Polymers and Bio-Inspired Mineralization Processes
5.3. Bioactive Glass Scaffolds
- Leaching through the exchange of protons from the physiological medium with labile network-modifying ions, such as Na+, K+, Ca2+, Mg2+, etc.:
- 2.
- The previous pH rise facilitates dissolution of the network and formation of additional silanol groups according to the reaction:
- 3.
- Polymerization of the SiO2− rich layer through condensation of neighboring Si–OH groups, which produces a layer rich in amorphous silica.
- 4.
- Migration of Ca2+ ions to the surface of the silica-rich layer to form an amorphous film rich in CaO–P2O5, followed by thickening of the film by incorporation of soluble Ca2+ and PO43− ions from the solution.
- 5.
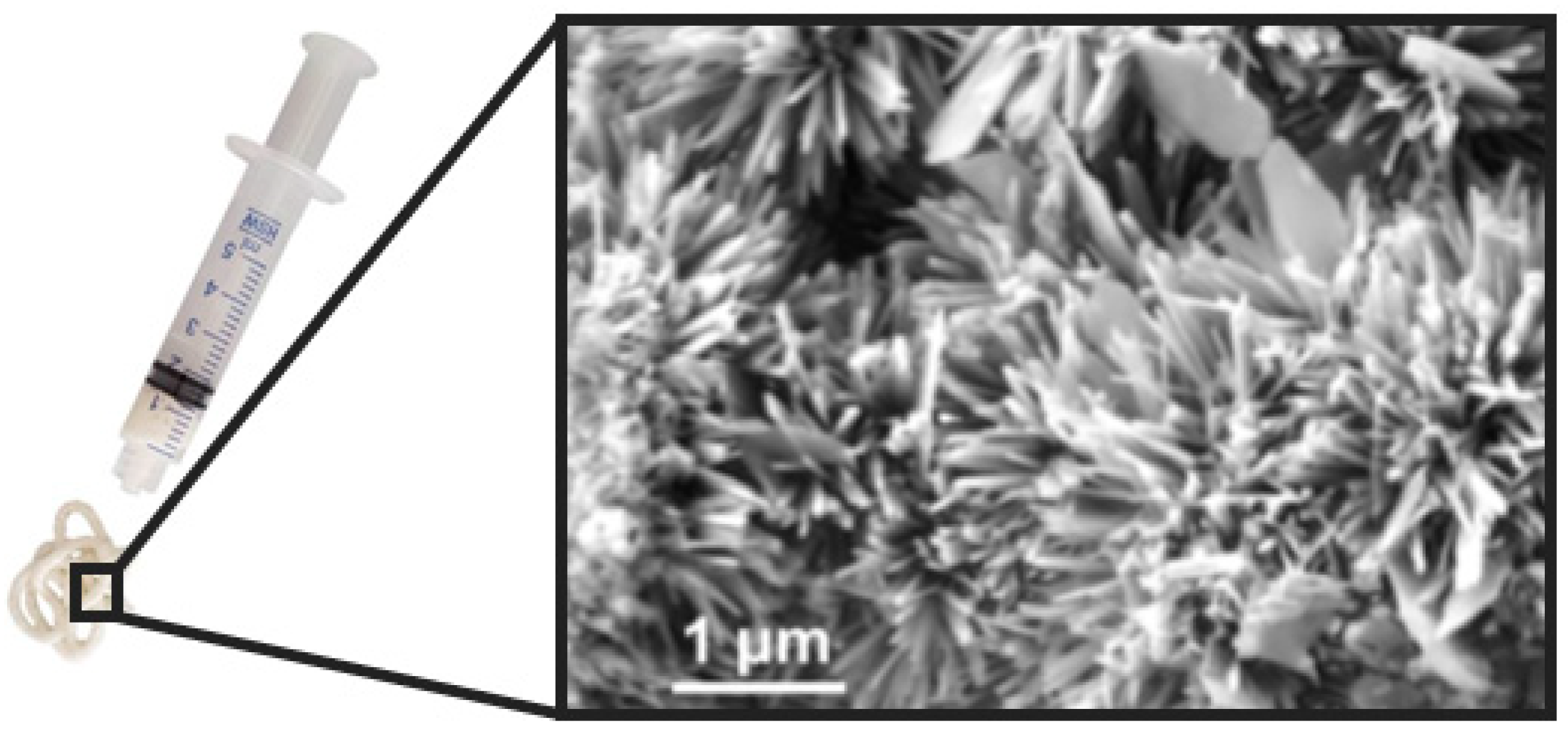
5.4. Self-Hardening Apatitic Scaffolds
5.5. Mechanically Bearing, Biomorphic 3D Scaffolds
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baroli, B. From Natural Bone Grafts to Tissue Engineering Therapeutics: Brainstorming on Pharmaceutical Formulative Requirements and Challenges. J. Pharm. Sci. 2009, 98, 1317–1375. [Google Scholar] [CrossRef]
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone Allografts: What They Can Offer and What They Cannot. J. Bone Jt. Surg. Br. 2007, 89, 574–579. [Google Scholar] [CrossRef]
- St John, T.A.; Vaccaro, A.R.; Sah, A.P.; Schaefer, M.; Berta, S.C.; Albert, T.; Hilibrand, A. Physical and Monetary Costs Associated with Autogenous Bone Graft Harvesting. Am. J. Orthop. 2003, 32, 18–23. [Google Scholar]
- Banwart, J.C.; Asher, M.A.; Hassanein, R.S. Iliac Crest Bone Graft Harvest Donor Site Morbidity. Spine. 1995, 20, 1055–1060. [Google Scholar] [CrossRef]
- Weber, F.E. Reconsidering Osteoconduction in the Era of Additive Manufacturing. In Tissue Engineering—Part B: Reviews; Mary Ann Liebert Inc.: Larchmont, NY, USA, 2019; pp. 375–386. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, Y.; Hu, Y.; Zou, L.; Chen, J. New Perspectives: In-Situ Tissue Engineering for Bone Repair Scaffold. Compos. Part B Eng. 2020, 202, 108445. [Google Scholar] [CrossRef]
- Ingber, D.E. Cellular Mechanotransduction: Putting All the Pieces Together Again. FASEB J. 2006, 20, 811–827. [Google Scholar] [CrossRef]
- Ruffini, A.; Sandri, M.; Dapporto, M.; Campodoni, E.; Tampieri, A.; Sprio, S. Nature-Inspired Unconventional Approaches to Develop 3D Bioceramic Scaffolds with Enhanced Regenerative Ability. Biomedicines 2021, 9, 916. [Google Scholar] [CrossRef]
- Tampieri, A.; Ruffini, A.; Ballardini, A.; Montesi, M.; Panseri, S.; Salamanna, F.; Fini, M.; Sprio, S. Heterogeneous Chemistry in the 3-D State: An Original Approach to Generate Bioactive, Mechanically-Competent Bone Scaffolds. Biomater. Sci. 2019, 7, 307–321. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, J.; Zhang, C.; Barbieri, D.; Yuan, H.; Moroni, L.; Feng, G. The Role of Calcium Phosphate Surface Structure in Osteogenesis and the Mechanisms Involved. Acta Biomaterialia 2020, 106, 22–33. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Manzano García, M.; Colilla, M. Biomedical Applications of Mesoporous Ceramics Drug Delivery, Smart Materials and Bone Tissue Engineering; Taylor & Francis Group, Ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Uskoković, V. The Role of Hydroxyl Channel in Defining Selected Physicochemical Peculiarities Exhibited by Hydroxyapatite. RSC Adv. 2015, 5, 36614–36633. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Sakae, T.; Nakada, H.; Kaneda, T.; Okada, H. Confusion between Carbonate Apatite and Biological Apatite (Carbonated Hydroxyapatite) in Bone and Teeth. Minerals 2022, 12, 170. [Google Scholar] [CrossRef]
- Cazalbou, S.; Combes, C.; Eichert, D.; Rey, C.; Glimcher, M.J. Poorly Crystalline Apatites: Evolution and Maturation in Vitro and in Vivo. J. Bone Miner. Metab. 2004, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Aufray, M.; Rollin-Martinet, S.; Vandecandelaère, N.; Grossin, D.; Rossignol, F.; Champion, E.; Navrotsky, A.; Rey, C. Nanocrystalline Apatites: The Fundamental Role of Water. Am. Mineral. 2018, 103, 550–564. [Google Scholar] [CrossRef]
- Wang, Y.; von Euw, S.; Fernandes, F.M.; Cassaignon, S.; Selmane, M.; Laurent, G.; Pehau-Arnaudet, G.; Coelho, C.; Bonhomme-Coury, L.; Giraud-Guille, M.M.; et al. Water-Mediated Structuring of Bone Apatite. Nat. Mater. 2013, 12, 1144–1153. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Reznikov, N.; Shahar, R.; Weiner, S. Acta Biomaterialia Bone Hierarchical Structure in Three Dimensions q. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef]
- Boskey, A.L. Bone Tissue Engineering; Jeffrey, O.H., Thomas, A.E., Doll, B., Sfeir, C., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Ruff, C.; Holt, B.; Trinkaus, E. Who’s Afraid of the Big Bad Wolff?: “Wolff’s Law” and Bone Functional Adaptation. Am. J. Phys. Anthropol. 2006, 129, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ogawa, R. Mechanotransduction in Bone Repair and Regeneration. FASEB J. 2010, 24, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Landis, W.J. The Strength of a Calcified Tissue Depends in Part on the Molecular Structure and Organization of Its Constituent Mineral Crystals in Their Organic Matrix. Bone 1995, 16, 533–544. [Google Scholar] [CrossRef]
- Weiner, S.; Traub, W. Bone Structure: From Angstroms to Microns. FASEB 1992, 6, 879–885. [Google Scholar] [CrossRef]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical Properties and the Hierarchical Structure of Bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone Tissue Engineering: A Review in Bone Biomimetics and Drug Delivery Strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D. Bones: Structure and Mechanics, 2nd ed.; Oxford Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Choi, K.; Goldstein, S.A. A Comparison of the Fatigue Behavior of Human Trabecular and Cortical Bone Tissue. J. Biomech. 1992, 25, 1371–1381. [Google Scholar] [CrossRef]
- Marotti, G.; Muglia, M.A.; Palumbo, C. Structure and Function of Lamellar Bone. Clin. Rheumatol. 1994, 13, 63–68. [Google Scholar] [PubMed]
- Fratzl, P.; Weinkamer, R. Nature’s Hierarchical Materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Glimcher, M. Mechanisms of calcification: Role of collagen fibrils and collagen–phosphoprotein complexes in vitro and in vivo. Anat. Rec. 1989, 224, 139–153. [Google Scholar] [CrossRef]
- Kuhn-Spearing, L.; Rey, C.; Kim, H.M.; Glimcher, M.J. Carbonated Apatite Nanocrystals of Bone. In Synthesis and Processing of Nanocrystalline Powder; TMS: Pittsburgh, PA, USA, 1996; pp. 57–68. [Google Scholar]
- Rey, C.; Miquel, J.L.; Facchini, L.; Legrand, A.P.; Glimcher, M.J. Hydroxyl Groups in Bone Mineral. Bone 1995, 16, 583–586. [Google Scholar] [CrossRef]
- Roach, H. Why Does Bone Matrix Contain Non-Collagenous Proteins? The Possible Roles of Osteocalcin, Osteonectin, Osteopontin and Bone Sialoprotein in Bone Mineralisation and Resorption. Cell Biol. Int. 1994, 18, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, S.; Giannoni, P.; Bianchini, P.; Sandri, M.; Marotta, R.; Firpo, G.; Valbusa, U.; Tampieri, A.; Diaspro, A.; Bianco, P.; et al. Order versus Disorder: In Vivo Bone Formation within Osteoconductive Scaffolds. Sci. Rep. 2012, 2, 274. [Google Scholar] [CrossRef]
- Sprio, S.; Sandri, M.; Panseri, S.; Cunha, C.; Tampieri, A. Hybrid Scaffolds for Tissue Regeneration: Chemotaxis and Physical Confinement as Sources of Biomimesis. J. Nanomater. 2012, 2012, 418281. [Google Scholar] [CrossRef]
- Yu, L.; Wei, M. Biomineralization of Collagen-Based Materials for Hard Tissue Repair. Int. J. Mol. Sci. 2021, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, N.; Qi, Y.; Dai, L.; Bryan, T.E.; Mao, J.; Pashley, D.H.; Tay, F.R. Intrafibrillar Collagen Mineralization Produced by Biomimetic Hierarchical Nanoapatite Assembly. Adv. Mater. 2011, 23, 975–980. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Mou, C.-Y.; Chan, J.C.C. Solid-State NMR Study of the Transformation of Octacalcium Phosphate to Hydroxyapatite: A Mechanistic Model for Central Dark Line Formation. J Am Chem Soc 2006, 128, 6909–6918. [Google Scholar] [CrossRef]
- Robin, M.; von Euw, S.; Renaudin, G.; Gomes, S.; Krafft, J.-M.; Nassif, N.; Azaïs, T.; Costentin, G. Insights into OCP Identification and Quantification in the Context of Apatite Biomineralization. CrystEngComm 2020, 22, 2728–2742. [Google Scholar] [CrossRef]
- Hamai, R.; Tsuchiya, K.; Suzuki, O. Adsorption of Serum Albumin onto Octacalcium Phosphate in Supersaturated Solutions Regarding Calcium Phosphate Phases. Materials 2019, 12, 2333. [Google Scholar] [CrossRef]
- Moriishi, T.; Ozasa, R.; Ishimoto, T.; Nakano, T.; Hasegawa, T.; Miyazaki, T.; Liu, W.; Fukuyama, R.; Wang, Y.; Komori, H.; et al. Osteocalcin Is Necessary for the Alignment of Apatite Crystallites, but Not Glucose Metabolism, Testosterone Synthesis, or Muscle Mass. PLOS Genet. 2020, 16, e1008586. [Google Scholar] [CrossRef]
- Simon, P.; Grüner, D.; Worch, H.; Pompe, W.; Lichte, H.; el Khassawna, T.; Heiss, C.; Wenisch, S.; Kniep, R. First Evidence of Octacalcium Phosphate@osteocalcin Nanocomplex as Skeletal Bone Component Directing Collagen Triple–Helix Nanofibril Mineralization. Sci. Rep. 2018, 8, 13696. [Google Scholar] [CrossRef]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; de With, G.; Sommerdijk, N.A.J.M. The Role of Collagen in Bone Apatite Formation in the Presence of Hydroxyapatite Nucleation Inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef]
- Tavoni, M.; Dapporto, M.; Tampieri, A.; Sprio, S. Bioactive Calcium Phosphate-Based Composites for Bone Regeneration. J. Compos. Sci. 2021, 5, 227. [Google Scholar] [CrossRef]
- Tampieri, A.; Iafisco, M.; Sprio, S.; Ruffini, A.; Panseri, S.; Montesi, M.; Adamiano, A.; Sandri, M. Hydroxyapatite: From Nanocrystals to Hybrid Nanocomposites for Regenerative Medicine. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-Dimensional Cell Culture Systems as an In Vitro Platform for Cancer and Stem Cell Modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Ffrench-Constant, C. Chapter 25-Integrins. Myelin Biol. Disord. 2004, 1, 609–632. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Bone Matrix Non-Collagenous Proteins in Tissue Engineering: Creating New Bone by Mimicking the Extracellular Matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef]
- Henriksen, K.; Sørensen, M.G.; Jensen, V.K.; Dziegiel, M.H.; Nosjean, O.; Karsdal, M.A. Ion Transporters Involved in Acidification of the Resorption Lacuna in Osteoclasts. Calcif. Tissue Int. 2008, 83, 230–242. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), 131–139. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-Β1-Induced Migration of Bone Mesenchymal Stem Cells Couples Bone Resorption with Formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef]
- Burr, D.B.; Allen, M.R. Basic and Applied Bone Biology, 2nd ed.; Academic Press: London, UK, 2013. [Google Scholar]
- Aubin, J.E. Mesenchymal Stem Cells and Osteoblast Differentiation. In Principles of Bone Biology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 85–107. [Google Scholar] [CrossRef]
- Seeman, E. Bone Modeling and Remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. The Cellular Basis of Bone Remodeling: The Quantum Concept Reexamined in Light of Recent Advances in the Cell Biology of Bone. Calcif. Tissue Int. 1984, 36, S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.L.; Abdelgawad, M.E.; Kristensen, H.B.; Hauge, E.M.; Rolighed, L.; Bollerslev, J.; Kjærsgaard-Andersen, P.; Delaisse, J.-M. Understanding Coupling between Bone Resorption and Formation. Am. J. Pathol. 2013, 183, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Neutzsky-Wulff, A.V.; Bonewald, L.F.; Karsdal, M.A. Local Communication on and within Bone Controls Bone Remodeling. Bone 2009, 44, 1026–1033. [Google Scholar] [CrossRef]
- Lu, J.; Yu, H.; Chen, C. Biological Properties of Calcium Phosphate Biomaterials for Bone Repair: A Review. RSC Advan. 2018, 8, 2015–2033. [Google Scholar] [CrossRef]
- Ishack, S.; Mediero, A.; Wilder, T.; Ricci, J.L.; Cronstein, B.N. Bone Regeneration in Critical Bone Defects Using Three-Dimensionally Printed β-Tricalcium Phosphate/Hydroxyapatite Scaffolds Is Enhanced by Coating Scaffolds with Either Dipyridamole or BMP-2. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2017, 105, 366–375. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, T.; Zhu, J.; Cai, P. Osteoinduction of Calcium Phosphate Ceramics in Four Kinds of Animals for 1 Year: Dog, Rabbit, Rat, and Mouse. In Transplantation Proceedings; Elsevier: New York, NY, USA, 2016; Volume 48, pp. 1309–1314. [Google Scholar] [CrossRef]
- Yuan, H.; van den Doel, M.; Li, S.; van Blitterswijk, C.A.; de Groot, K.; de Bruijn, J.D. A Comparison of the Osteoinductive Potential of Two Calcium Phosphate Ceramics Implanted Intramuscularly in Goats. J. Mater. Sci. Mater. Med. 2002, 13, 1271–1275. [Google Scholar] [CrossRef]
- Yuan, H.; van Blitterswijk, C.A.; de Groot, K.; de Bruijn, J.D. Cross-Species Comparison of Ectopic Bone Formation in Biphasic Calcium Phosphate (BCP) and Hydroxyapatite (HA) Scaffolds. Tissue Eng. 2006, 12, 1607–1615. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, F.; Yang, R.; Lu, X.; Shi, Y.; Li, L.; Fan, H.; Bu, H. Osteoinduction of Hydroxyapatite/β-Tricalcium Phosphate Bioceramics in Mice with a Fractured Fibula. Acta Biomater. 2010, 6, 1569–1574. [Google Scholar] [CrossRef]
- Baldonedo, J.; Fernández, J.R.; Segade, A. Numerical Analysis of an Osseointegration Model. Mathematics 2020, 8, 87. [Google Scholar] [CrossRef]
- Fernández, J.R.; García-Aznar, J.M.; Masid, M. Numerical Analysis of an Osteoconduction Model Arising in Bone-Implant Integration. ZAMM Z. Fur Angew. Math. Mech. 2017, 97, 1050–1063. [Google Scholar] [CrossRef]
- Frost, H.M. The Biology of Fracture Healing. An Overview for Clinicians. Clin. Orthop. Rel. Res. 1989, 1, 283–293. [Google Scholar]
- Trippel, S.B. Growth Factors as Therapeutic Agents. Instr. Course Lect. 1997, 46, 473–476. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, Osteoconduction and Osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T. The Healing of Autologous Bone Grafts after Varying Degrees of Surgical Trauma. A Microscopic and Histochemical Study in the Rabbit. J. Bone Jt. Surgery. Br. Vol. 1980, 62-B, 403–410. [Google Scholar] [CrossRef]
- Weiss, P.; Layrolle, P.; Clergeau, L.P.; Enckel, B.; Pilet, P.; Amouriq, Y.; Daculsi, G.; Giumelli, B. The Safety and Efficacy of an Injectable Bone Substitute in Dental Sockets Demonstrated in a Human Clinical Trial. Biomaterials 2007, 28, 3295–3305. [Google Scholar] [CrossRef] [PubMed]
- Vivanco, J.; Araneda, A.; Ploeg, H.-L. Effect of Sintering Temperature on Microstructural Properties of Bioceramic Bone. Biomater. Sci. Process. Prop. Appl. II 2012, 237, 101–109. [Google Scholar]
- Pramanik, S.; Agarwal, A.K.; Rai, K.N.; Garg, A. Development of High Strength Hydroxyapatite by Solid-State-Sintering Process. Ceram. Int. 2007, 33, 419–426. [Google Scholar] [CrossRef]
- Scalera, F.; Palazzo, B.; Barca, A.; Gervaso, F. Sintering of Magnesium-Strontium Doped Hydroxyapatite Nanocrystals: Towards the Production of 3D Biomimetic Bone Scaffolds. J. Biomed. Mater. Res. Part A 2020, 108, 633–644. [Google Scholar] [CrossRef]
- Landi, E.; Guizzardi, S.; Papa, E.; Galli, C. Mg,Sr-Cosubstituted Hydroxyapatite with Improved Structural Properties. Appl. Sci. 2021, 11, 4930. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Wu, B.; Huang, W. Effects of Microwave Sintering on the Properties of Porous Hydroxyapatite Scaffolds. Ceram. Int. 2013, 39, 2389–2395. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Haghiashtiani, G.; Hübscher, T.; Kelly, D.J.; Lee, J.M.; Lutolf, M.; McAlpine, M.C.; Yeong, W.Y.; Zenobi-Wong, M.; Malda, J. 3D Extrusion Bioprinting. Nat. Rev. Methods Primers 2021, 1, 75. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D Printed Bone Tissue Regenerative PLA/HA Scaffolds with Comprehensive Performance Optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Dukle, A.; Murugan, D.; Nathanael, A.J.; Rangasamy, L.; Oh, T.-H. Can 3D-Printed Bioactive Glasses Be the Future of Bone Tissue Engineering? Polymers 2022, 14, 1627. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D Printing of PLA/n-HA Composite Scaffolds with Customized Mechanical Properties and Biological Functions for Bone Tissue Engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Zhao, L.; Li, M.; Han, Y.; Wang, L.; Zhang, Z.; Li, J.; Zhou, C.; Liu, L. Fabrication and Properties of PLA/Nano-HA Composite Scaffolds with Balanced Mechanical Properties and Biological Functions for Bone Tissue Engineering Application. Nanotechnol. Rev. 2021, 10, 1359–1373. [Google Scholar] [CrossRef]
- Šupová, M. Problem of Hydroxyapatite Dispersion in Polymer Matrices: A Review. J. Mater. Sci. Mater. Med. 2009, 20, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-S.; Lee, S.; Lee, D.; Jho, J.Y. Mechanical Properties and Bioactivity of Poly(Lactic Acid) Composites Containing Poly(Glycolic Acid) Fiber and Hydroxyapatite Particles. Nanomaterials 2021, 11, 249. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Vakhnovetsky, J.; Vakhnovetsky, A.; Ghobrial, M.; Nath, D.; Morgano, S.M. Functional Role of Inorganic Trace Elements in Dentin Apatite Tissue—Part 1: Mg, Sr, Zn, and Fe. J. Trace Elem. Med. Biol. 2022, 71, 126932. [Google Scholar] [CrossRef]
- Sprio, S.; Preti, L.; Montesi, M.; Panseri, S.; Adamiano, A.; Vandini, A.; Pugno, N.M.; Tampieri, A. Surface Phenomena Enhancing the Antibacterial and Osteogenic Ability of Nanocrystalline Hydroxyapatite, Activated by Multiple-Ion Doping. ACS Biomater. Sci. Eng. 2019, 5, 5947–5959. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into Biological Apatite: Physiochemical Properties and Preparation Approaches. BioMed Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef]
- Landi, E.; Logroscino, G.; Proietti, L.; Tampieri, A.; Sandri, M.; Sprio, S. Biomimetic Mg-Substituted Hydroxyapatite: From Synthesis to in Vivo Behaviour. J. Mater. Sci. Mater. Med. 2008, 19, 239–247. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Mattioli-Belmonte, M.; Celotti, G.; Sandri, M.; Gigante, A.; Fava, P.; Biagini, G. Biomimetic Mg- and Mg,CO3-Substituted Hydroxyapatites: Synthesis Characterization and in Vitro Behaviour. J. Eur. Ceram. Soc. 2006, 26, 2593–2601. [Google Scholar] [CrossRef]
- Landi, E.; Sprio, S.; Sandri, M.; Celotti, G.; Tampieri, A. Development of Sr and CO3 Co-Substituted Hydroxyapatites for Biomedical Applications. Acta Biomater. 2008, 4, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Cazalbou, S.; Combes, C.; Rey, C. Biomimetic Approach for Strontium-Containing Ca-P Bioceramics with Enhanced Biological Activity. Key Eng. Mater. 2001, 192–195, 147–150. [Google Scholar] [CrossRef]
- Sprio, S.; Tampieri, A.; Landi, E.; Sandri, M.; Martorana, S.; Celotti, G.; Logroscino, G. Physico-Chemical Properties and Solubility Behaviour of Multi-Substituted Hydroxyapatite Powders Containing Silicon. Mater. Sci. Eng. C 2008, 28, 179–187. [Google Scholar] [CrossRef]
- Botelho, C.M.; Lopes, M.A.; Gibson, I.R.; Best, S.M.; Santos, J.D. Structural Analysis of Si-Substituted Hydroxyapatite: Zeta Potential and X-Ray Photoelectron Spectroscopy. J. Mater. Sci. Mater. Med. 2002, 13, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, K.S.; Chang, J.S.; Cho, W.S.; Kim, Y.; Kim, S.R.; Kim, Y.T. Biocompatibility of Si-Substituted Hydroxyapatite. Key Eng. Mater. 2004, 254–256, 135–138. [Google Scholar] [CrossRef]
- Rey, C.; Renugopalakrishman, V.; Collins, B.; Glimcher, M.J. Fourier Transform Infrared Spectroscopic Study of the Carbonate Ions in Bone Mineral during Aging. Calcif. Tissue Int. 1991, 49, 251–258. [Google Scholar] [CrossRef]
- Kannan, S.; Rebelo, A.; Ferreira, J.M.F. Novel Synthesis and Structural Characterization of Fluorine and Chlorine Co-Substituted Hydroxyapatites. J. Inorg. Biochem. 2006, 100, 1692–1697. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W.; Betancourt, T. Synthesis, Bioactivity and Zeta Potential Investigations of Chlorine and Fluorine Substituted Hydroxyapatite. Mater. Sci. Eng. C 2016, 59, 78–85. [Google Scholar] [CrossRef]
- Iafisco, M.; Degli Esposti, L.; Ramírez-Rodríguez, G.B.; Carella, F.; Gómez-Morales, J.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Delgado-López, J.M. Fluoride-Doped Amorphous Calcium Phosphate Nanoparticles as a Promising Biomimetic Material for Dental Remineralization. Sci. Rep. 2018, 8, 17016. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, L.; Adamiano, A.; Tampieri, A.; Ramirez-Rodriguez, G.B.; Siliqi, D.; Giannini, C.; Ivanchenko, P.; Martra, G.; Lin, F.-H.; Delgado-López, J.M.; et al. Combined Effect of Citrate and Fluoride Ions on Hydroxyapatite Nanoparticles. Cryst. Growth Des. 2020, 20, 3163–3172. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Ionescu, A.C.; Carella, F.; Adamiano, A.; Brambilla, E.; Iafisco, M. Antimicrobial Activity of Remineralizing Ion-Doped Amorphous Calcium Phosphates for Preventive Dentistry. Front. Mater. 2022, 9, 846130. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Shellis, R.P.R.; Featherstone, J.D.B.; Lussi, A. Understanding the Chemistry of Dental Erosion. Erosive Tooth Wear Diagn. Ther. 2012, 25, 163–179. [Google Scholar] [CrossRef]
- Porter, A.; Patel, N.; Brooks, R.; Best, S.; Rushton, N.; Bonfield, W. Effect of Carbonate Substitution on the Ultrastructural Characteristics of Hydroxyapatite Implants. J. Mater. Sci. Mater. Med. 2005, 16, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Spence, G.; Patel, N.; Brooks, R.; Rushton, N. Carbonate Substituted Hydroxyapatite: Resorption by Osteoclasts Modifies the Osteoblastic Response. J. Biomed. Mater. Res. Part A 2009, 90A, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.M.; Tavares, D.d.S.; dos Santos, E.A. Isolating the Effects of Mg2+, Mn2+ and Sr2+ Ions on Osteoblast Behavior from Those Caused by Hydroxyapatite Transformation. Mater. Res. 2020, 23, e20200083. [Google Scholar] [CrossRef]
- Bose, S.; Vu, A.A.; Emshadi, K.; Bandyopadhyay, A. Effects of Polycaprolactone on Alendronate Drug Release from Mg-Doped Hydroxyapatite Coating on Titanium. Mater. Sci. Eng. C 2018, 88, 166–171. [Google Scholar] [CrossRef]
- Bertinetti, L.; Drouet, C.; Combes, C.; Rey, C.; Tampieri, A.; Coluccia, S.; Martra, G. Surface Characteristics of Nanocrystalline Apatites: Effect of Mg Surface Enrichment on Morphology, Surface Hydration Species, and Cationic Environments. Langmuir 2009, 25, 5647–5654. [Google Scholar] [CrossRef] [PubMed]
- Brett, E.; Flacco, J.; Blackshear, C.; Longaker, M.T.; Wan, D.C. Biomimetics of Bone Implants: The Regenerative Road. BioRes. Open Access 2017, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Verberckmoes, S.C.; Behets, G.J.; Oste, L.; Bervoets, A.R.; Lamberts, L.V.; Drakopoulos, M.; Somogyi, A.; Cool, P.; Dorriné, W.; de Broe, M.E.; et al. Effects of Strontium on the Physicochemical Characteristics of Hydroxyapatite. Calcif. Tissue Int. 2004, 75, 405–415. [Google Scholar] [CrossRef]
- Kourkoumelis, N. Osteoporosis and Strontium-Substituted Hydroxyapatites. Ann. Transl. Med. 2016, 4, S10. [Google Scholar] [CrossRef]
- Curran, D.J.; Fleming, T.J.; Towler, M.R.; Hampshire, S. Mechanical Parameters of Strontium Doped Hydroxyapatite Sintered Using Microwave and Conventional Methods. J. Mech. Behav. Biomed. Mater. 2011, 4, 2063–2073. [Google Scholar] [CrossRef]
- Ullah, I.; Siddiqui, M.A.; Liu, H.; Kolawole, S.K.; Zhang, J.; Zhang, S.; Ren, L.; Yang, K. Mechanical, Biological, and Antibacterial Characteristics of Plasma-Sprayed (Sr,Zn) Substituted Hydroxyapatite Coating. ACS Biomater. Sci. Eng. 2020, 6, 1355–1366. [Google Scholar] [CrossRef]
- Chetty, A.; du Preez, I.; Marei, M.; Kamary, Y.E.; Moussa, R.M. Synthesis, Properties and Applications of Hydroxyapatite. In Hydroxyapatite: Synthesis, Properties and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 91–132. [Google Scholar]
- Saeri, M.R.; Afshar, A.; Ghorbani, M.; Ehsani, N.; Sorrell, C.C. The Wet Precipitation Process of Hydroxyapatite. Mater. Lett. 2003, 57, 4064–4069. [Google Scholar] [CrossRef]
- Afshar, A.; Ghorbani, M.; Ehsani, N.; Saeri, M.R.; Sorrell, C.C. Some Important Factors in the Wet Precipitation Process of Hydroxyapatite. Mater. Des. 2003, 24, 197–202. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Evolution of Bioceramics within the Field of Biomaterials. Comptes Rendus Chim. 2010, 13, 174–185. [Google Scholar] [CrossRef]
- Jillavenkatesa, A. Sol–Gel Processing of Hydroxyapatite. J. Mater. Sci. 1998, 33, 4111–4119. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Chen, X.; Ren, L.; Lai, C.; He, W.; Zhang, Q. A Simple Sol-Gel Technique for Synthesis of Nanostructured Hydroxyapatite, Tricalcium Phosphate and Biphasic Powders. Mater. Lett. 2011, 65, 1923–1926. [Google Scholar] [CrossRef]
- Liu, D.; Savino, K.; Yates, M.Z. Coating of Hydroxyapatite Films on Metal Substrates by Seeded Hydrothermal Deposition. Surf. Coat. Technol. 2011, 205, 3975–3986. [Google Scholar] [CrossRef]
- Liu, J.; Ye, X.; Wang, H.; Zhu, M.; Wang, B.; Yan, H. The Influence of PH and Temperature on the Morphology of Hydroxyapatite Synthesized by Hydrothermal Method. Ceram. Int. 2003, 29, 629–633. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A Review of Syntheses, Structure and Applications in Heterogeneous Catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Dissolution Mechanism of Calcium Apatites in Acids: A Review of Literature. World J. Methodol. 2012, 2, 1. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Inorganic Chemistry of the Dissolution Phenomenon: The Dissolution Mechanism of Calcium Apatites at the Atomic (Ionic) Level. Comments Inorg. Chem. 1999, 20, 285–299. [Google Scholar] [CrossRef]
- Wu, L.; Forsling, W.; Schindler, P.W. Surface Complexation of Calcium Minerals in Aqueous Solution. J. Colloid Interface Sci. 1991, 147, 178–185. [Google Scholar] [CrossRef]
- Vučinić, D.R.; Radulović, D.S.; Deušić, S.Đ. Electrokinetic Properties of Hydroxyapatite under Flotation Conditions. J. Colloid Interface Sci. 2010, 343, 239–245. [Google Scholar] [CrossRef]
- Christoffersen, J. Kinetics of Dissolution of Calcium Hydroxypatite. J. Cryst. Growth 1980, 49, 29–44. [Google Scholar] [CrossRef]
- Skartsila, K.; Spanos, N. Surface Characterization of Hydroxyapatite: Potentiometric Titrations Coupled with Solubility Measurements. J. Colloid Interface Sci. 2007, 308, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nancollas, G.H. Unexpected PH Dependence of Dissolution Kinetics of Dicalcium Phosphate Dihydrate. J. Phys. Chem. 1994, 98, 1689–1694. [Google Scholar] [CrossRef]
- Tampieri, A.; Celotti, G.C.; Landi, E.; Sandri, M. Magnesium Doped Hydroxyapatite: Synthesis and Characterization. Key Eng. Mater. 2004, 264–268, 2051–2054. [Google Scholar] [CrossRef]
- Ziani, S.; Meski, S.; Khireddine, H. Characterization of Magnesium-Doped Hydroxyapatite Prepared by Sol-Gel Process. Int. J. Appl. Ceram. Technol. 2014, 11, 83–91. [Google Scholar] [CrossRef]
- Arul, K.T.; Ramya, J.R.; Bhalerao, G.M.; Kalkura, S.N. Physicochemical Characterization of the Superhydrophilic, Magnesium and Silver Ions Co-Incorporated Nanocrystalline Hydroxyapatite, Synthesized by Microwave Processing. Ceram. Int. 2014, 40, 13771–13779. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, D.; Sun, L.; Li, H.; Hanaor, D.A.H.; Schmidt, F.; Xu, K. Nanostructural Insights into the Dissolution Behavior of Sr-Doped Hydroxyapatite. J. Eur. Ceram. Soc. 2018, 38, 5554–5562. [Google Scholar] [CrossRef]
- Vukomanovic, M.; Gazvoda, L.; Anicic, N.; Rubert, M.; Suvorov, D.; Müller, R.; Hofmann, S. Multi-Doped Apatite: Strontium, Magnesium, Gallium and Zinc Ions Synergistically Affect Osteogenic Stimulation in Human Mesenchymal Cells Important for Bone Tissue Engineering. Biomater. Adv. 2022, 140, 213051. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Ruffini, A.; Adamiano, A.; Sprio, S.; Tampieri, A. Biomimetic Magnesium–Carbonate-Apatite Nanocrystals Endowed with Strontium Ions as Anti-Osteoporotic Trigger. Mater. Sci. Eng. C 2014, 35, 212–219. [Google Scholar] [CrossRef]
- Ballardini, A.; Montesi, M.; Panseri, S.; Vandini, A.; Balboni, P.G.; Tampieri, A.; Sprio, S. New Hydroxyapatite Nanophases with Enhanced Osteogenic and Anti-Bacterial Activity. J. Biomed. Mater. Res. Part A 2018, 106, 521–530. [Google Scholar] [CrossRef]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic Magnetism and Hyperthermia in Bioactive Fe-Doped Hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- Reynaud, C.; Thomas, C.; Costentin, G. On the Comprehensive Precipitation of Hydroxyapatites Unraveled by a Combined Kinetic–Thermodynamic Approach. Inorg. Chem. 2022, 61, 3296–3308. [Google Scholar] [CrossRef]
- di Luca, A.; Longoni, A.; Criscenti, G.; Mota, C.; van Blitterswijk, C.; Moroni, L. Toward Mimicking the Bone Structure: Design of Novel Hierarchical Scaffolds with a Tailored Radial Porosity Gradient. Biofabrication 2016, 8, 045007. [Google Scholar] [CrossRef]
- Chang, B.-S.; Lee, C.-K.; Hong, K.-S.; Youn, H.-J.; Ryu, H.-S.; Chung, S.-S.; Park, K.-W. Osteoconduction at Porous Hydroxyapatite with Various Pore Configurations. Biomaterials 2000, 21, 1291–1298. [Google Scholar] [CrossRef]
- Elsheikh, M.; Kishida, R.; Hayashi, K.; Tsuchiya, A.; Shimabukuro, M.; Ishikawa, K. Effects of Pore Interconnectivity on Bone Regeneration in Carbonate Apatite Blocks. Regen. Biomater. 2022, 9, rbac010. [Google Scholar] [CrossRef]
- Chu, T.-M.G.; Orton, D.G.; Hollister, S.J.; Feinberg, S.E.; Halloran, J.W. Mechanical and in Vivo Performance of Hydroxyapatite Implants with Controlled Architectures. Biomaterials 2002, 23, 1283–1293. [Google Scholar] [CrossRef]
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, Definition, Overview. In Stem Cells and Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–98. [Google Scholar] [CrossRef]
- Rigo, E.C.S.; Boschi, A.O.; Yoshimoto, M.; Allegrini, S.; Konig, B.; Carbonari, M.J. Evaluation in Vitro and in Vivo of Biomimetic Hydroxyapatite Coated on Titanium Dental Implants. Mater. Sci. Eng. C 2004, 24, 647–651. [Google Scholar] [CrossRef]
- Branemark, P.-I. Osseointegration and Its Experimental Background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Sprio, S.; Fricia, M.; Maddalena, G.F.; Nataloni, A.; Tampieri, A. Osteointegration in Cranial Bone Reconstruction: A Goal to Achieve. J. Appl. Biomater. Funct. Mater. 2016, 14, 470–476. [Google Scholar] [CrossRef]
- Buckley, C.T.; O’Kelly, K.U. Fabrication and Characterization of a Porous Multidomain Hydroxyapatite Scaffold for Bone Tissue Engineering Investigations. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93B, 459–467. [Google Scholar] [CrossRef]
- Sprio, S.; Sandri, M.; Iafisco, M.; Panseri, S.; Filardo, G.; Kon, E.; Marcacci, M.; Tampieri, A. Composite Biomedical Foams for Engineering Bone Tissue. In Biomedical Foams for Tissue Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 249–280. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D. Fabrication Methods of Porous Metals for Use in Orthopaedic Applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Z.; Guo, H.; Sun, T.; Chen, A.; Zhou, Y.; He, Y. Function-Structure-Integrated Ti-HA Coatings on TiNbZr with Enhanced Mechanical Properties and Bioactivity Prepared by Spark Plasma Sintering. Vacuum 2021, 184, 109863. [Google Scholar] [CrossRef]
- Liao, S.S.; Guan, K.; Cui, F.Z.; Shi, S.S.; Sun, T.S. Lumbar Spinal Fusion with a Mineralized Collagen Matrix and RhBMP-2 in a Rabbit Model. Spine 2003, 28, 1954–1960. [Google Scholar] [CrossRef]
- Liao, S.S.; Cui, F.Z.; Zhang, W.; Feng, Q.L. Hierarchically Biomimetic Bone Scaffold Materials: Nano-HA/Collagen/PLA Composite. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2004, 69, 158–165. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Ai, X.; Zhang, S. Biomimetic Self-Assembly of Apatite Hybrid Materials: From a Single Molecular Template to Bi-/Multi-Molecular Templates. Biotechnol. Adv. 2014, 32, 744–760. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, S.; Zheng, W.; Chen, M.; Zhou, S.; Liao, M.; Huang, W.; Hu, Y.; Zhou, W. Formation of Poly(Ε-caprolactone)-embedded Bioactive Nanoparticles/Collagen Hierarchical Scaffolds with the Designed and Customized Porous Structures. J. Appl. Polym. Sci. 2022, 139, e52749. [Google Scholar] [CrossRef]
- Jang, C.H.; Kim, W.; Kim, G. Effects of Fibrous Collagen/CDHA/HUCS Biocomposites on Bone Tissue Regeneration. Int. J. Biol. Macromol. 2021, 176, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Peroglio, M.; Gremillard, L.; Gauthier, C.; Chazeau, L.; Verrier, S.; Alini, M.; Chevalier, J. Mechanical Properties and Cytocompatibility of Poly(ε-Caprolactone)-Infiltrated Biphasic Calcium Phosphate Scaffolds with Bimodal Pore Distribution. Acta Biomater. 2010, 6, 4369–4379. [Google Scholar] [CrossRef]
- Bai, F.; Wang, Z.; Lu, J.; Liu, J.; Chen, G.; Lv, R.; Wang, J.; Lin, K.; Zhang, J.; Huang, X. The Correlation between the Internal Structure and Vascularization of Controllable Porous Bioceramic Materials In Vivo: A Quantitative Study. Tissue Eng. Part A 2010, 16, 3791–3803. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Wang, Z.; Wang, Z.; Xie, Q.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. PEGylated Poly(Glycerol Sebacate)-Modified Calcium Phosphate Scaffolds with Desirable Mechanical Behavior and Enhanced Osteogenic Capacity. Acta Biomater. 2016, 44, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Ruther, F.; Salehi, S.; Boccaccini, A.R. Poly(Glycerol Sebacate) in Biomedical Applications—A Review of the Recent Literature. Adv. Healthc. Mater. 2021, 10, 2002026. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Wu, Z.; Zhang, J.; Ma, Y.; Yang, Z.; Yuan, Y. Development of Modified and Multifunctional Poly(Glycerol Sebacate) (PGS)-Based Biomaterials for Biomedical Applications. Eur. Polym. J. 2021, 161, 110830. [Google Scholar] [CrossRef]
- Rosenbalm, T.N.; Teruel, M.; Day, C.S.; Donati, G.L.; Morykwas, M.; Argenta, L.; Kuthirummal, N.; Levi-Polyachenko, N. Structural and Mechanical Characterization of Bioresorbable, Elastomeric Nanocomposites from Poly(Glycerol Sebacate)/Nanohydroxyapatite for Tissue Transport Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1366–1373. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, C.; Wang, Y.; Zhang, L.; Zhang, J.; Shi, J.; Si, J.; Yuan, Y.; Liu, C. Direct Three-Dimensional Printing of a Highly Customized Freestanding Hyperelastic Bioscaffold for Complex Craniomaxillofacial Reconstruction. Chem. Eng. J. 2021, 411, 128541. [Google Scholar] [CrossRef]
- Rodríguez, K.; Renneckar, S.; Gatenholm, P. Biomimetic Calcium Phosphate Crystal Mineralization on Electrospun Cellulose-Based Scaffolds. ACS Appl. Mater. Interfaces 2011, 3, 681–689. [Google Scholar] [CrossRef]
- Choi, M.-O.; Kim, Y.-J. Effect of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Gelatin Ratios on the Characteristics of Biomimetic Composite Nanofibrous Scaffolds. Colloid Polym. Sci. 2018, 296, 917–926. [Google Scholar] [CrossRef]
- Rajzer, I.; Menaszek, E.; Kwiatkowski, R.; Planell, J.A.; Castano, O. Electrospun Gelatin/Poly(ε-Caprolactone) Fibrous Scaffold Modified with Calcium Phosphate for Bone Tissue Engineering. Mater. Sci. Eng. C 2014, 44, 183–190. [Google Scholar] [CrossRef]
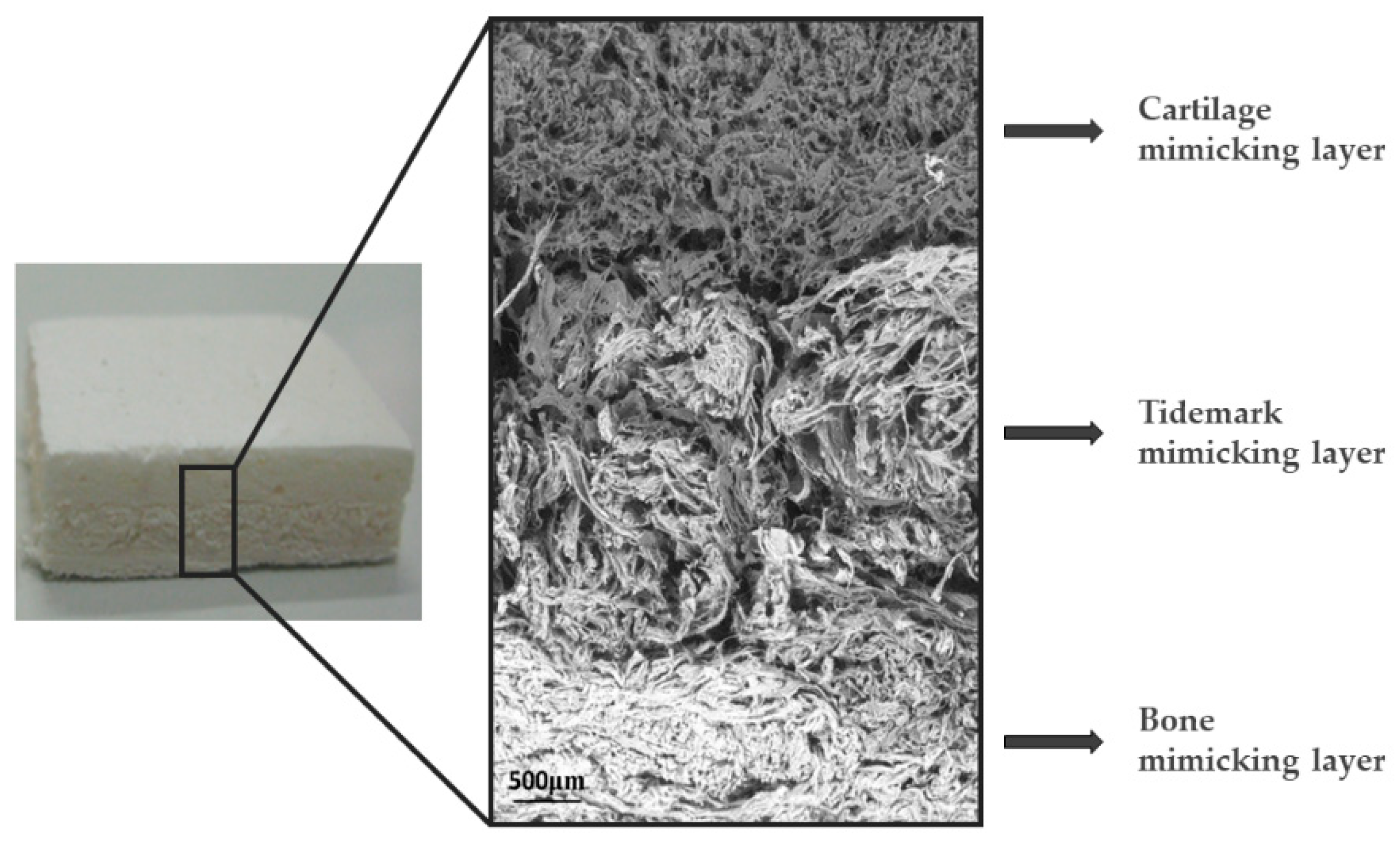
- Tampieri, A.; Sprio, S.; Sandri, M.; Valentini, F. Mimicking Natural Bio-Mineralization Processes: A New Tool for Osteochondral Scaffold Development. Trends Biotechnol. 2011, 29, 526–535. [Google Scholar] [CrossRef]
- Sprio, S.; Campodoni, E.; Sandri, M.; Preti, L.; Keppler, T.; Müller, F.; Pugno, N.; Tampieri, A. A Graded Multifunctional Hybrid Scaffold with Superparamagnetic Ability for Periodontal Regeneration. Int. J. Mol. Sci. 2018, 19, 3604. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.R.; Bradica, G.; Brekke, J.H.; Goldman, S.M.; Ieska, K.; Issack, P.; Bong, M.R.; Tian, H.; Gokhale, J.; Coutts, R.D.; et al. Regeneration of Articular Cartilage—Evaluation of Osteochondral Defect Repair in the Rabbit Using Multiphasic Implants. Osteoarthr. Cartil. 2005, 13, 798–807. [Google Scholar] [CrossRef]
- Schek, R.M.; Taboas, J.M.; Segvich, S.J.; Hollister, S.J.; Krebsbach, P.H. Engineered Osteochondral Grafts Using Biphasic Composite Solid Free-Form Fabricated Scaffolds. Tissue Eng. 2004, 10, 1376–1385. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural Origin Biodegradable Systems in Tissue Engineering and Regenerative Medicine: Present Status and Some Moving Trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Chiang, H.; Liao, C.-J.; Lin, Y.-J.; Kuo, T.-F.; Shieh, C.-S.; Huang, Y.-Y.; Tuan, R.S. Repair of Porcine Articular Cartilage Defect with a Biphasic Osteochondral Composite. J. Orthop. Res. 2007, 25, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-Polysaccharide Composite Scaffolds for 3D Cell Culture and Tissue Engineering: Towards Natural Therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Gostynska, N.; Dapporto, M.; Campodoni, E.; Montesi, M.; Panseri, S.; Tampieri, A.; Kon, E.; Marcacci, M.; Sprio, S.; et al. Evaluation of Different Crosslinking Agents on Hybrid Biomimetic Collagen-Hydroxyapatite Composites for Regenerative Medicine. Int. J. Biol. Macromol. 2018, 106, 739–748. [Google Scholar] [CrossRef]
- Gajjeraman, S.; Narayanan, K.; Hao, J.; Qin, C.; George, A. Matrix Macromolecules in Hard Tissues Control the Nucleation and Hierarchical Assembly of Hydroxyapatite. J. Biol. Chem. 2007, 282, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, X.; Zhou, Y.; Zhu, P.; Zhang, L.; Wei, S. In Vitro Growth of Bioactive Nanostructured Apatites via Agar-Gelatin Hybrid Hydrogel. J. Biomed. Nanotechnol. 2013, 9, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science. 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Delcogliano, M.; Filardo, G.; Fini, M.; Giavaresi, G.; Francioli, S.; Martin, I.; Pressato, D.; Arcangeli, E.; Quarto, R.; et al. Orderly Osteochondral Regeneration in a Sheep Model Using a Novel Nano-Composite Multilayered Biomaterial. J. Orthop. Res. 2009, 28, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; di Martino, A.; Busacca, M.; Moio, A.; Perdisa, F.; Marcacci, M. Clinical Results and MRI Evolution of a Nano-Composite Multilayered Biomaterial for Osteochondral Regeneration at 5 Years. Am. J. Sports Med. 2014, 42, 158–165. [Google Scholar] [CrossRef] [PubMed]
- di Martino, A.; Kon, E.; Perdisa, F.; Sessa, A.; Filardo, G.; Neri, M.P.; Bragonzoni, L.; Marcacci, M. Surgical Treatment of Early Knee Osteoarthritis with a Cell-Free Osteochondral Scaffold: Results at 24 Months of Follow-Up. Injury 2015, 46, S33–S38. [Google Scholar] [CrossRef]
- Berruto, M.; Delcogliano, M.; de Caro, F.; Carimati, G.; Uboldi, F.; Ferrua, P.; Ziveri, G.; de Biase, C.F. Treatment of Large Knee Osteochondral Lesions With a Biomimetic Scaffold. Am. J. Sports Med. 2014, 42, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; di Martino, A.; Busacca, M.; Altadonna, G.; Marcacci, M. Treatment of Knee Osteochondritis Dissecans with a Cell-Free Biomimetic Osteochondral Scaffold. Am. J. Sports Med. 2013, 41, 1786–1793. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ragel, V.; Salinas, A.J. Microreview Glasses with Medical Applications. Eur. J. Inorg. Chem. 2003, 2003, 1029–1042. [Google Scholar] [CrossRef]
- Gunawidjaja, P.N.; Lo, A.Y.H.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Stevensson, B.; Grins, J.; Vallet-Regí, M.; Edén, M. Biomimetic Apatite Mineralization Mechanisms of Mesoporous Bioactive Glasses as Probed by Multinuclear 31P, 29Si, 23Na and 13C Solid-State NMR. J. Phys. Chem. C 2010, 114, 19345–19356. [Google Scholar] [CrossRef]
- de Aza, P.N.; de Aza, A.H.; Pena, P.; de Aza, S. Bioactive Glasses and Glass-Ceramics. Bol. Soc. Esp. Ceram. Vidr. 2007, 46, 45–55. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Sumi, K.; Abe, T.; Kunimatsu, R.; Oki, N.; Tsuka, Y.; Awada, T.; Nakajima, K.; Ando, K.; Tanimoto, K. The Effect of Mesenchymal Stem Cells on Chemotaxis of Osteoclast Precursor Cells. J. Oral Sci. 2018, 60, 221–225. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Casarrubios, L.; Morales, I.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Effects of a Mesoporous Bioactive Glass on Osteoblasts, Osteoclasts and Macrophages. J. Colloid Interface Sci. 2018, 528, 309–320. [Google Scholar] [CrossRef]
- Łączka, M.; Cholewa-Kowalska, K.; Osyczka, A.M. Bioactivity and Osteoinductivity of Glasses and Glassceramics and Their Material Determinants. Ceram. Int. 2016, 42, 14313–14325. [Google Scholar] [CrossRef]
- Groh, D.; Döhler, F.; Brauer, D.S. Bioactive Glasses with Improved Processing. Part 1. Thermal Properties, Ion Release and Apatite Formation. Acta Biomater. 2014, 10, 4465–4473. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.E.L.; Piwowarczyk, W.; Pamula, E.; Liskova, J.; Schaubroeck, D.; Leeuwenburgh, S.C.G.; Brackman, G.; Balcaen, L.; Detsch, R.; Declercq, H.; et al. Injectable Self-Gelling Composites for Bone Tissue Engineering Based on Gellan Gum Hydrogel Enriched with Different Bioglasses. Biomed. Mater. 2014, 9, 045014. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Bikiaris, D. Biocompatible Nanobioglass Reinforced Poly(ε-Caprolactone) Composites Synthesized via In Situ Ring Opening Polymerization. Polymers 2018, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Srivastava, P. Design and Evaluation of Chitosan/Chondroitin Sulfate/Nano-Bioglass Based Composite Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jérôme, R. Porous Poly(α-Hydroxyacid)/Bioglass® Composite Scaffolds for Bone Tissue Engineering. I: Preparation and in Vitro Characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar] [CrossRef]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less Harmful Acidic Degradation of Poly(Lactic-Co-Glycolic Acid) Bone Tissue Engineering Scaffolds through Titania Nanoparticle Addition. Int. J. Nanomed. 2006, 1, 541–545. [Google Scholar] [CrossRef]
- Fernandez, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A.; Best, S.M. Production and Characterization of New Calcium Phosphate Bone Cements in the CaHPO4-Alpha-Ca3(PO4)2 System: PH, Workability and Setting Times. J. Mater. Sci. Mater. Med. 1999, 10, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium Phosphate Cements for Bone Substitution: Chemistry, Handling and Mechanical Properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Driessens, F.C.M.; Planell, J.A. Effect of the Particle Size on the Micro and Nanostructural Features of a Calcium Phosphate Cement: A Kinetic Analysis. Biomaterials 2004, 25, 3453–3462. [Google Scholar] [CrossRef]
- Shimogoryo, R.; Eguro, T.; Kimura, E.; Maruta, M.; Matsuya, S.; Ishikawa, K. Effects of Added Mannitol on the Setting Reaction and Mechanical Strength of Apatite Cement. Dent. Mater. J. 2009, 28, 627–633. [Google Scholar] [CrossRef]
- Lee, G.S.; Park, J.H.; Won, J.E.; Shin, U.S.; Kim, H.W. Alginate Combined Calcium Phosphate Cements: Mechanical Properties and in Vitro Rat Bone Marrow Stromal Cell Responses. J. Mater. Sci. Mater. Med. 2011, 22, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Shona Pek, Y.; Kurisawa, M.; Gao, S.; Chung, J.E.; Ying, J.Y. The Development of a Nanocrystalline Apatite Reinforced Crosslinked Hyaluronic Acid-Tyramine Composite as an Injectable Bone Cement. Biomaterials 2009, 30, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh-Asl, S.; Hesaraki, S.; Zamanian, A. Preparation and Characterisation of Calcium Phosphate–Hyaluronic Acid Nanocomposite Bone Cement. Adv. Appl. Ceram. 2011, 110, 340–345. [Google Scholar] [CrossRef]
- Rattanachan, S.; Boonphayak, P.; Lorprayoon, C. Development of Chitosan/Nanosized Apatite Composites for Bone Cements. Asian Biomed. 2011, 5, 499–506. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaître, J.; van Landuyt, P.; Zambelli, P.-Y.; Merkle, H.P.; Gander, B. Gentamicin-Loaded Hydraulic Calcium Phosphate Bone Cement as Antibiotic Delivery System. J. Pharm. Sci. 1997, 86, 565–572. [Google Scholar] [CrossRef]
- Liu, W.C.; Wong, C.T.; Fong, M.K.; Cheung, W.S.; Kao, R.Y.T.; Luk, K.D.K.; Lu, W.W. Gentamicin-Loaded Strontium-Containing Hydroxyapatite Bioactive Bone Cement-An Efficient Bioactive Antibiotic Drug Delivery System. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95B, 397–406. [Google Scholar] [CrossRef]
- Rabiee, S.M. Development of Hydroxyapatite Bone Cement for Controlled Drug Release via Tetracycline Hydrochloride. Bull. Mater. Sci. 2013, 36, 171–174. [Google Scholar] [CrossRef]
- Ratier, A.; Gibson, I.R.; Best, S.M.; Freche, M.; Lacout, J.L.; Rodriguez, F. Setting Characteristics and Mechanical Behaviour of a Calcium Phosphate Bone Cement Containing Tetracycline. Biomaterials 2001, 22, 897–901. [Google Scholar] [CrossRef]
- Tani, T.; Okada, K.; Takahashi, S.; Suzuki, N.; Shimada, Y.; Itoi, E. Doxorubicin-Loaded Calcium Phosphate Cement in the Management of Bone and Soft Tissue Tumors. In Vivo 2006, 20, 55–60. [Google Scholar]
- Tanzawa, Y.; Tsuchiya, H.; Shirai, T.; Nishida, H.; Hayashi, K.; Takeuchi, A.; Tomita, K.; Kawahara, M. Potentiation of the Antitumor Effect of Calcium Phosphate Cement Containing Anticancer Drug and Caffeine on Rat Osteosarcoma. J. Orthop. Sci. 2011, 16, 77–84. [Google Scholar] [CrossRef]
- Tahara, Y.; Ishii, Y. Apatite Cement Containing Cis-Diamminedichloroplatinum Implanted in Rabbit Femur for Sustained Release of the Anticancer Drug and Bone Formation. J. Orthop. Sci. 2001, 6, 556–565. [Google Scholar] [CrossRef]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef]
- Patil, S.; Montgomery, R. Management of Complex Tibial and Femoral Nonunion Using the Ilizarov Technique, and Its Cost Implications. J. Bone Jt. Surgery. Br. Vol. 2006, 88-B, 928–932. [Google Scholar] [CrossRef]
- Lozada-Gallegos, A.R.; Letechipia-Moreno, J.; Palma-Lara, I.; Montero, A.A.; Rodríguez, G.; Castro-Muñozledo, F.; Cornejo-Cortés, M.A.; Juárez-Mosqueda, M.L. Development of a Bone Nonunion in a Noncritical Segmental Tibia Defect Model in Sheep Utilizing Interlocking Nail as an Internal Fixation System. J. Surg. Res. 2013, 183, 620–628. [Google Scholar] [CrossRef]
- Pilia, M.; Guda, T.; Appleford, M. Development of Composite Scaffolds for Load-Bearing Segmental Bone Defects. BioMed Res. Int. 2013, 2013, 458253. [Google Scholar] [CrossRef]
- Sprio, S.; Panseri, S.; Montesi, M.; Dapporto, M.; Ruffini, A.; Dozio, S.M.; Cavuoto, R.; Misseroni, D.; Paggi, M.; Bigoni, D.; et al. Hierarchical Porosity Inherited by Natural Sources Affects the Mechanical and Biological Behaviour of Bone Scaffolds. J. Eur. Ceram. Soc. 2020, 40, 1717–1727. [Google Scholar] [CrossRef]
- Mercado-Pagán, Á.E.; Stahl, A.M.; Shanjani, Y.; Yang, Y. Vascularization in Bone Tissue Engineering Constructs. Ann. Biomed. Eng. 2015, 43, 718–729. [Google Scholar] [CrossRef]
- Baino, F.; Ferraris, M. Learning from Nature: Using Bioinspired Approaches and Natural Materials to Make Porous Bioceramics. Int. J. Appl. Ceram. Technol. 2017, 14, 507–520. [Google Scholar] [CrossRef]
- Fan, T.-X.; Chow, S.-K.; Zhang, D. Biomorphic Mineralization: From Biology to Materials. Prog. Mater. Sci. 2009, 54, 542–659. [Google Scholar] [CrossRef]
- White, R.A.; Weber, J.N.; White, E.W. Replamineform: A New Process for Preparing Porous Ceramic, Metal, and Polymer Prosthetic Materials. Science 1972, 176, 922–924. [Google Scholar] [CrossRef]
- Tampieri, A.; Sprio, S.; Ruffini, A.; Celotti, G.; Lesci, I.G.; Roveri, N. From Wood to Bone: Multi-Step Process to Convert Wood Hierarchical Structures into Biomimetic Hydroxyapatite Scaffolds for Bone Tissue Engineering. J. Mater. Chem. 2009, 19, 4973–4980. [Google Scholar] [CrossRef]
- Eichenseer, C.; Will, J.; Rampf, M.; Wend, S.; Greil, P. Biomorphous Porous Hydroxyapatite-Ceramics from Rattan (Calamus Rotang). J. Mater. Sci. Mater. Med. 2010, 21, 131–137. [Google Scholar] [CrossRef]
- Qian, J.; Kang, Y.; Zhang, W.; Li, Z. Fabrication, Chemical Composition Change and Phase Evolution of Biomorphic Hydroxyapatite. J. Mater. Sci. Mater. Med. 2008, 19, 3373–3383. [Google Scholar] [CrossRef]
- Rambo, C.R.; Andrade, T.; Fey, T.; Sieber, H.; Martinelli, A.E.; Greil, P. Microcellular Al2O3 Ceramics from Wood for Filter Applications. J. Am. Ceram. Soc. 2008, 91, 852–859. [Google Scholar] [CrossRef]
- Bigoni, D.; Cavuoto, R.; Misseroni, D.; Paggi, M.; Ruffini, A.; Sprio, S.; Tampieri, A. Ceramics with the Signature of Wood: A Mechanical Insight. Mater. Today Bio 2020, 5, 100032. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Salamanna, F.; Filardo, G.; di Matteo, B.; Shabshin, N.; Shani, J.; Fini, M.; Perdisa, F.; Parrilli, A.; Sprio, S.; et al. Bone Regeneration in Load-Bearing Segmental Defects, Guided by Biomorphic, Hierarchically Structured Apatitic Scaffold. Front. Bioeng. Biotechnol. 2021, 9, 734486. [Google Scholar] [CrossRef] [PubMed]
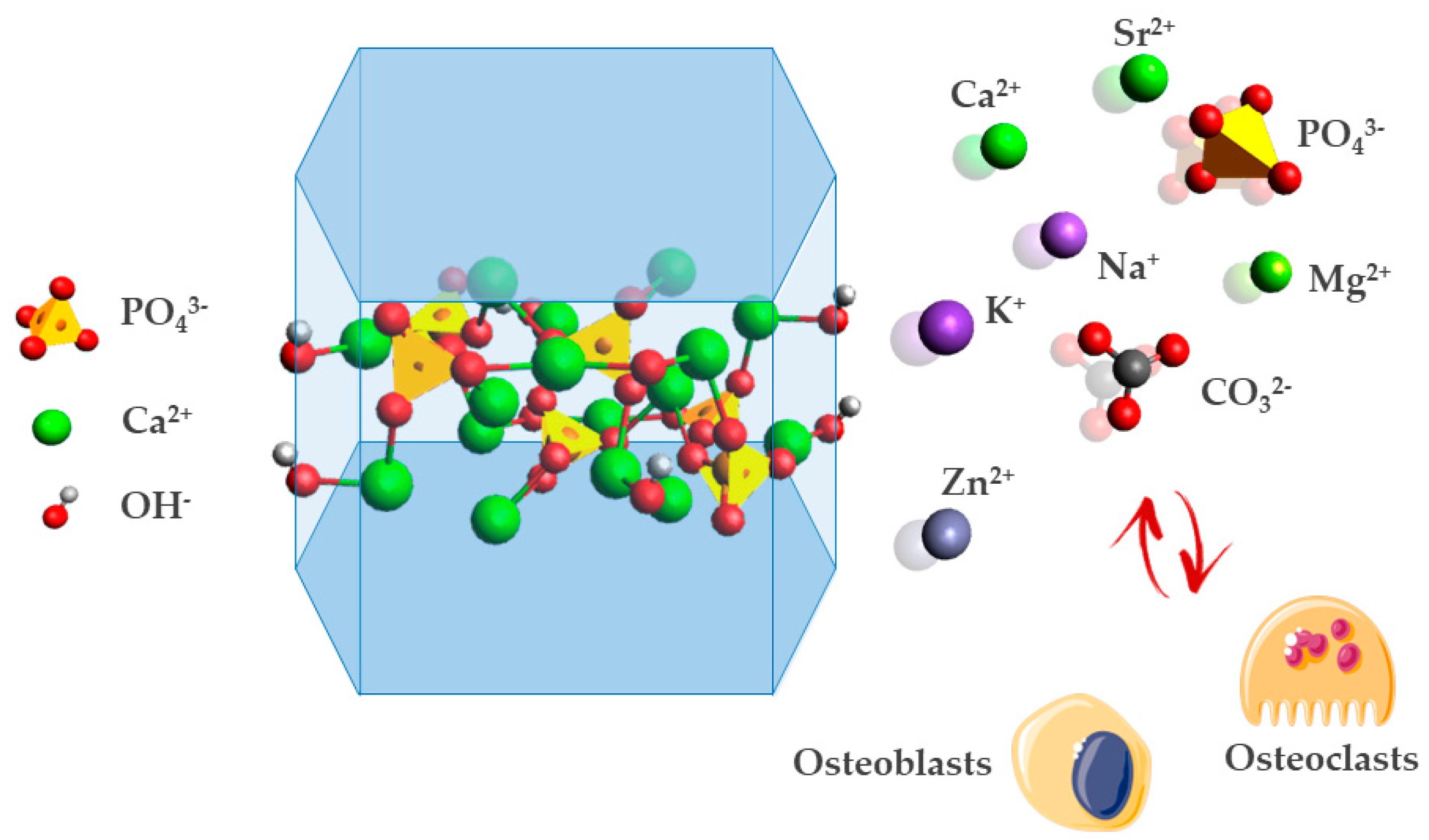

| Ion Substitution Site | Doping Ion | Main Effects |
|---|---|---|
| Ca2+ | Mg2+ | Magnesium ion is quantitatively the most important, typically amounting to around 6 mol%. In biological environment, magnesium boosts skeletal metabolism and bone growth, and its deficiency adversely affects all stages of skeletal metabolism, causing a decrease in osteoblastic and osteoclastic activities, osteopenia, and bone fragility [88,89]. |
| Sr2+ | Strontium ion increases bone formation, the number of bone-forming sites, and bone mineral density, and reduces bone resorption, leading to a gain in bone mass and improved bone mechanical properties in animals and humans [90]. Strontium increases osteoblast activity and decreases osteoclast resorption, making it suitable for the treatment of osteoporosis [91]. | |
| Zn2+ | Zinc ion stimulates osteoblastic activity in vitro and inhibits bone resorption in vivo [45]. In addition, doping induces the segregation of bioactive ions on the material surface which makes them available for exchange with physiological fluids, preventing bacterial antibiotic resistance in hospitals during the postoperative period [86]. | |
| PO43− | SiO44− | Silicates are among the trace elements in HA involved in biological processes. SiO44− substitution of phosphate ions site charge difference causes the formation of a Ca2+ partial vacancy for the equilibration of charge neutrality. Silicates enhance osteoblast cell proliferation compared with the pure HA phase and its depletion is often related to the deterioration in the proliferation and function of osteoblast due to osteoporosis and osteopenia [92,93,94]. |
| CO32− | The substitution of phosphate groups with carbonate ions is called B-type carbonation. B-type carbonation is present in young bone, which is subjected to remodeling processes, resulting in higher solubility [89,95]. | |
| OH− | Cl− | Chlorine ions in HA structure provide an acidic environment on the surface of bone that stimulates osteoclasts in the bone resorption process. Accordingly, this incorporation may be essential in the expansion of low pH to solubilize the alkaline salts of bone minerals and to digest the organic matrix by acid hydrolases, which are secreted by osteoclasts [96,97]. |
| F− | Fluorine ions substitution provides higher chemical and thermal stability. Moreover, the fluorine ion itself is known to suppress dental caries and stimulate the proliferation and differentiation of bone cells [97,98,99,100]. | |
| CO32− | The carbonation of the hydroxyl site is called A-type substitution. Studies have found that biological apatites, such as dentin, phytolith, and dental calculus have an A–B mixed type carbonation (B > A), and kidney stones may be both A–B mixed and B-type [101]. In bone apatites, A-type carbonation concentration progressively increases with age and maturation as the resulting apatite is less soluble and less subjected to remodeling processes [89,95]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pupilli, F.; Ruffini, A.; Dapporto, M.; Tavoni, M.; Tampieri, A.; Sprio, S. Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration. Biomimetics 2022, 7, 112. https://doi.org/10.3390/biomimetics7030112
Pupilli F, Ruffini A, Dapporto M, Tavoni M, Tampieri A, Sprio S. Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration. Biomimetics. 2022; 7(3):112. https://doi.org/10.3390/biomimetics7030112
Chicago/Turabian StylePupilli, Federico, Andrea Ruffini, Massimiliano Dapporto, Marta Tavoni, Anna Tampieri, and Simone Sprio. 2022. "Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration" Biomimetics 7, no. 3: 112. https://doi.org/10.3390/biomimetics7030112
APA StylePupilli, F., Ruffini, A., Dapporto, M., Tavoni, M., Tampieri, A., & Sprio, S. (2022). Design Strategies and Biomimetic Approaches for Calcium Phosphate Scaffolds in Bone Tissue Regeneration. Biomimetics, 7(3), 112. https://doi.org/10.3390/biomimetics7030112






