Biomimetic Mineralization of Tooth Enamel Using Nanocrystalline Hydroxyapatite under Various Dental Surface Pretreatment Conditions
Abstract
:1. Introduction
2. Methods of Production and Study of the Samples
2.1. Obtaining Enamel Samples
2.2. Sample Preparation
2.3. Sample Groups Treatment
2.3.1. N1 Samples
2.3.2. N2 Samples
2.3.3. N3 Samples
2.3.4. N4 Samples
2.3.5. N5 Samples
2.4. Mineralization
2.5. Experimental Design
2.6. Materials Used
2.6.1. Ca(OH)2 Solution
2.6.2. NcHAp Solution
2.6.3. Amino Acid Booster (AA)
2.7. Experimental Set-Up and Parameters
2.7.1. Optical Microscopy
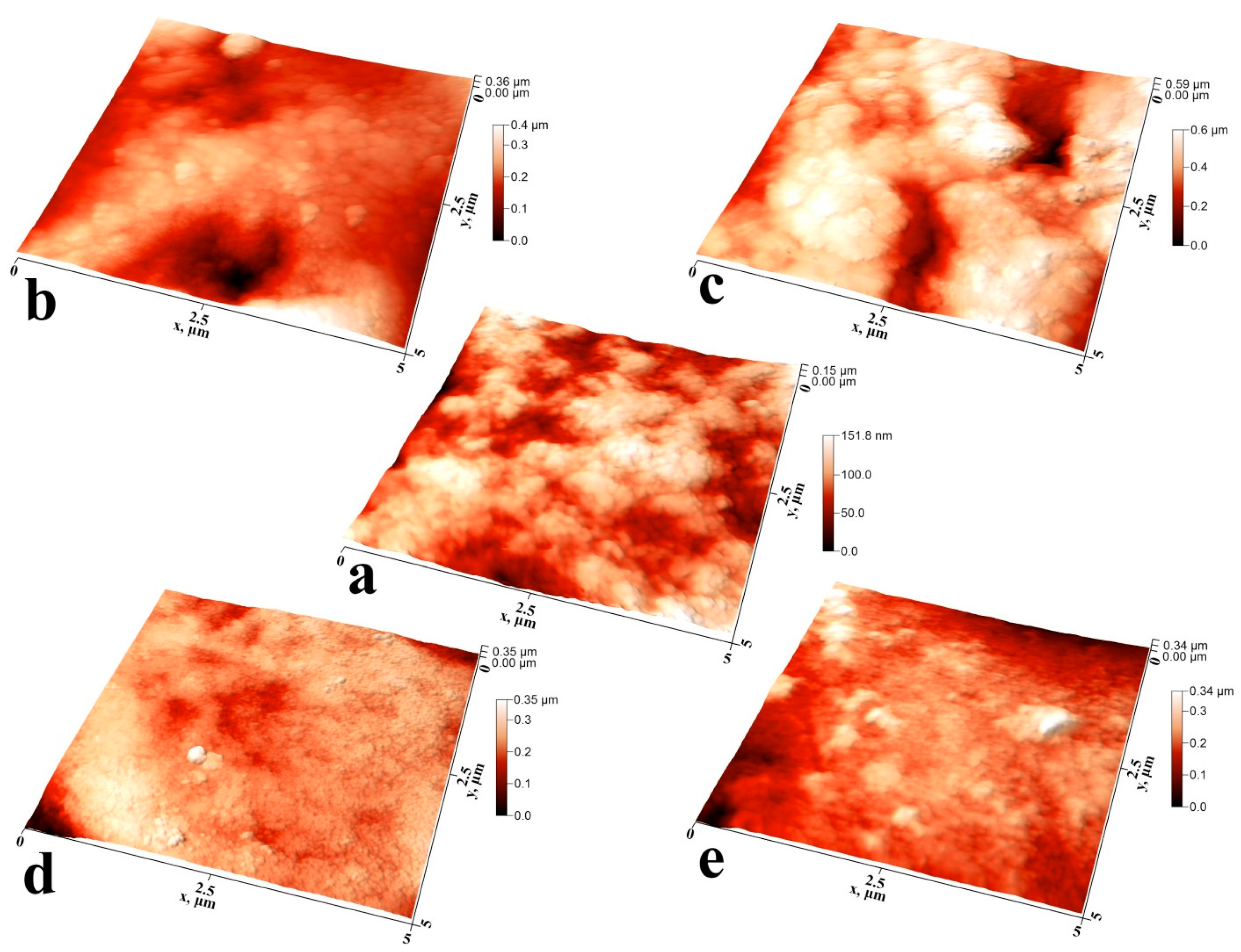
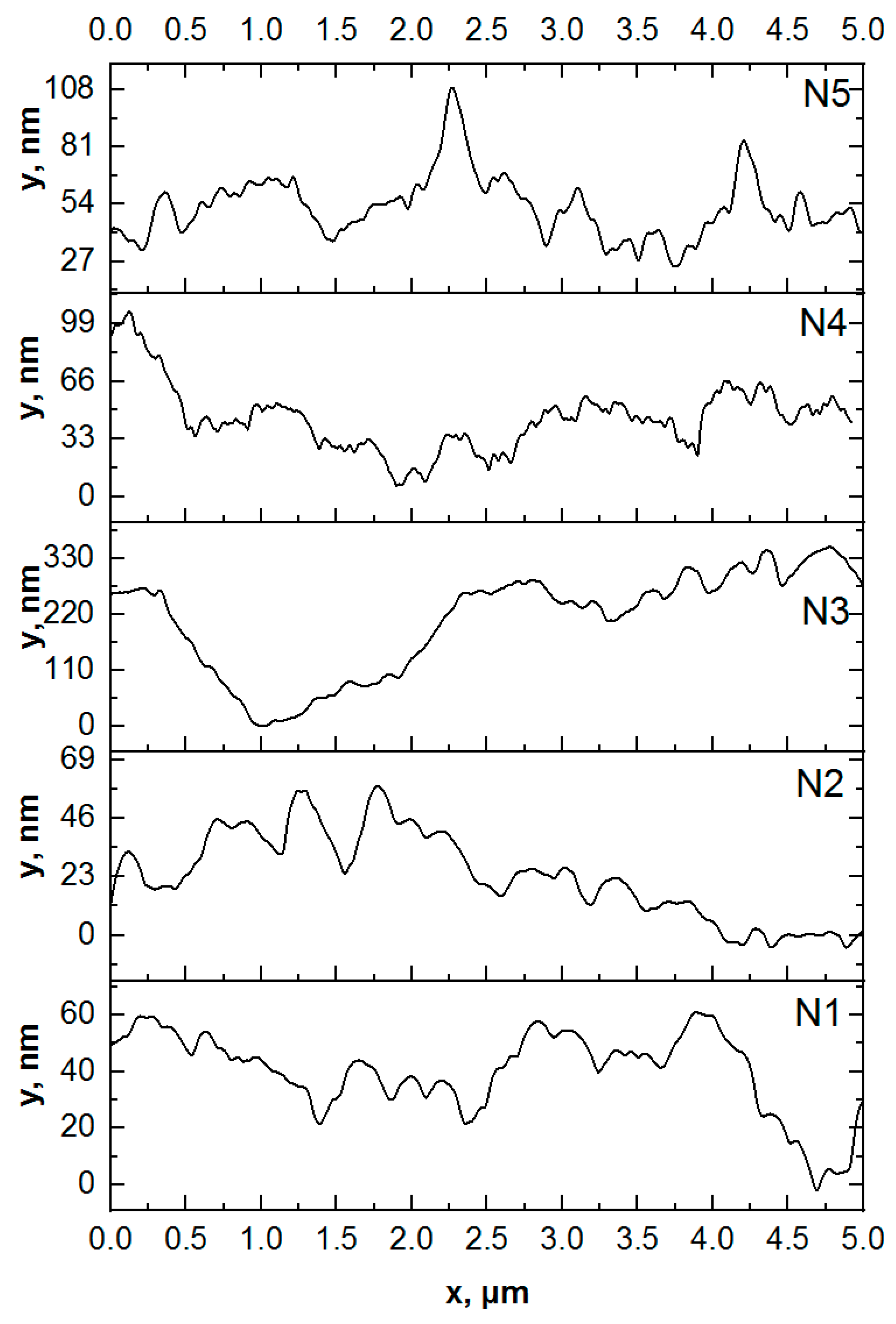
2.7.2. Atomic Force Microscopy
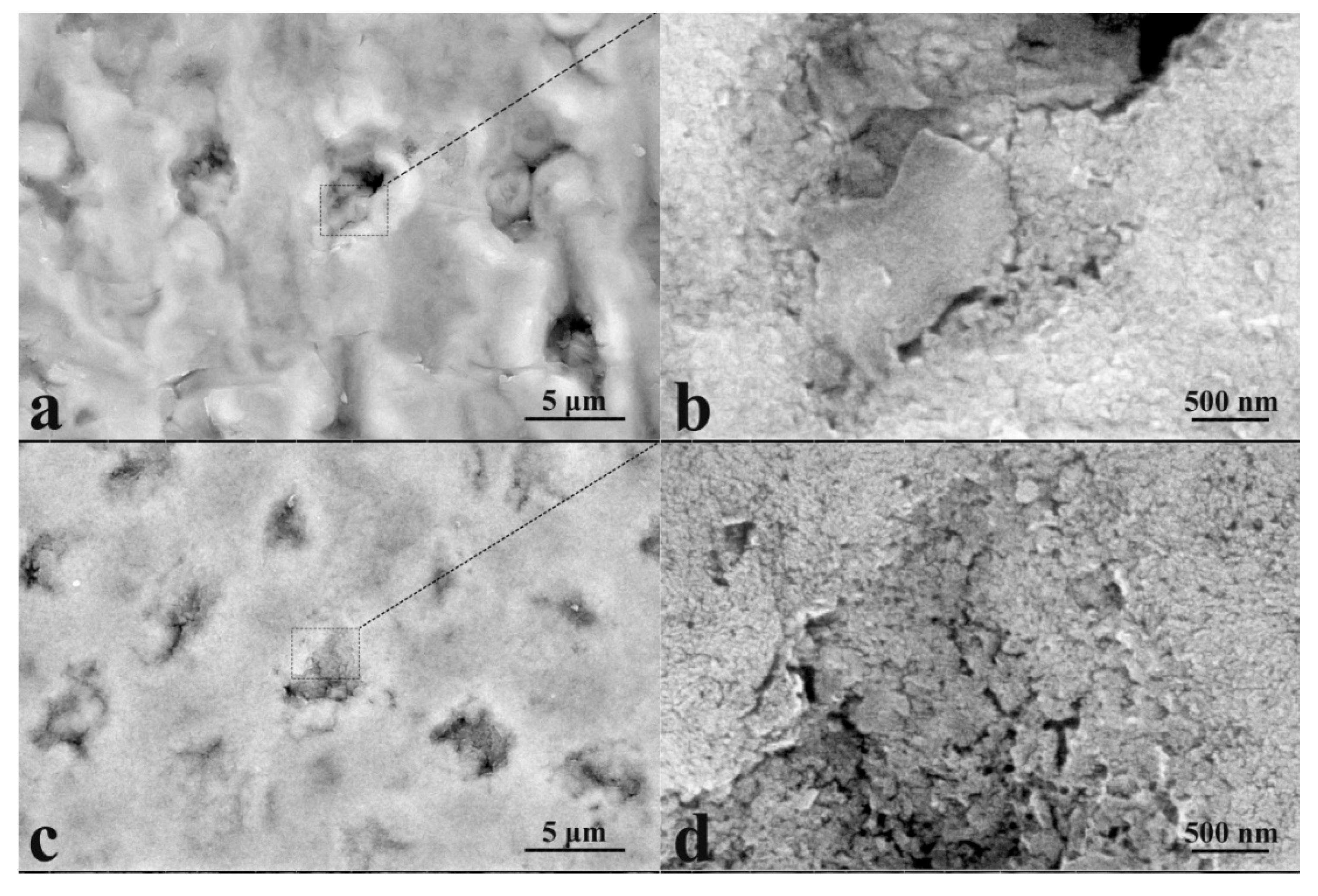
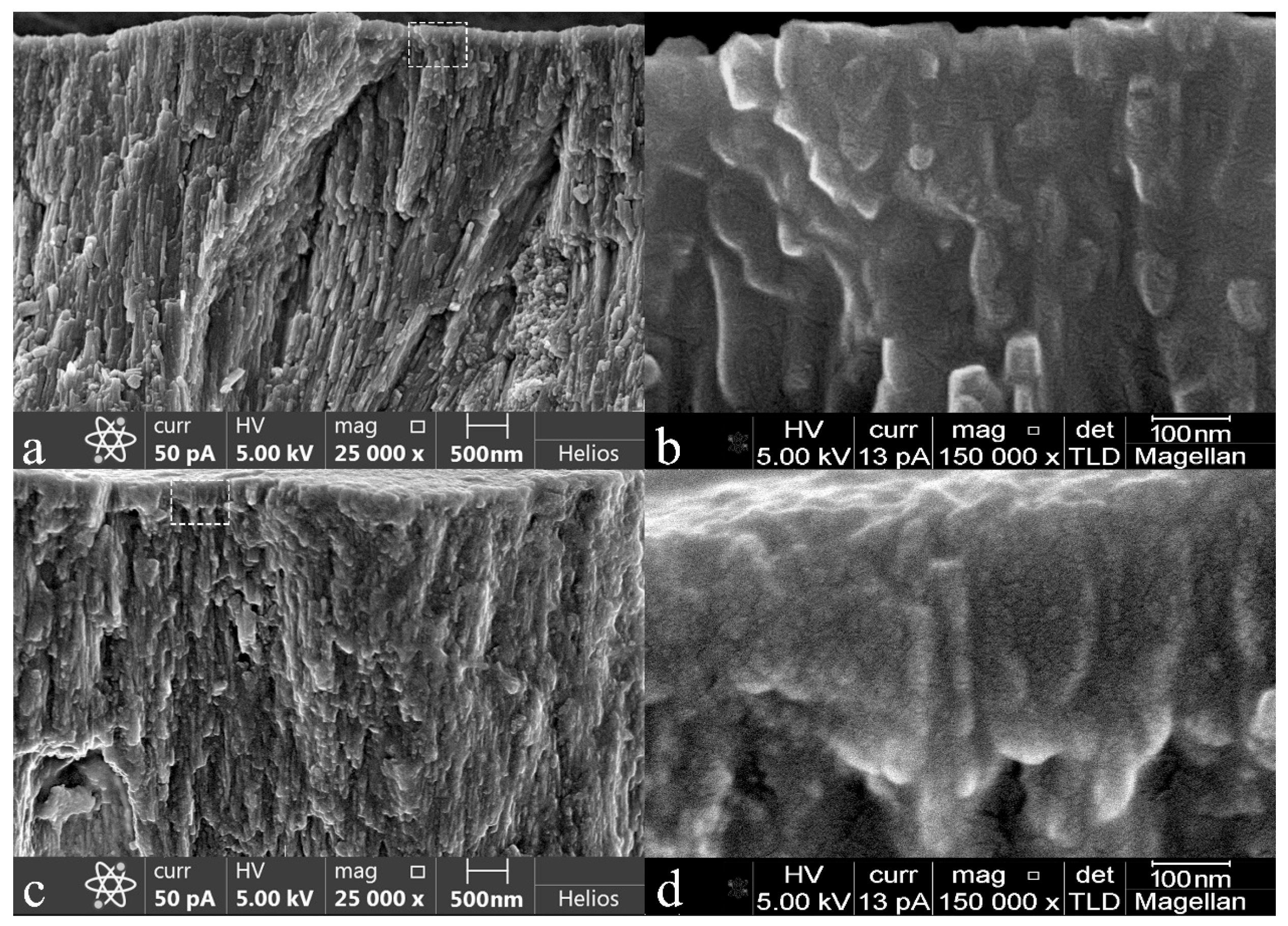
2.7.3. Electron Microscopy
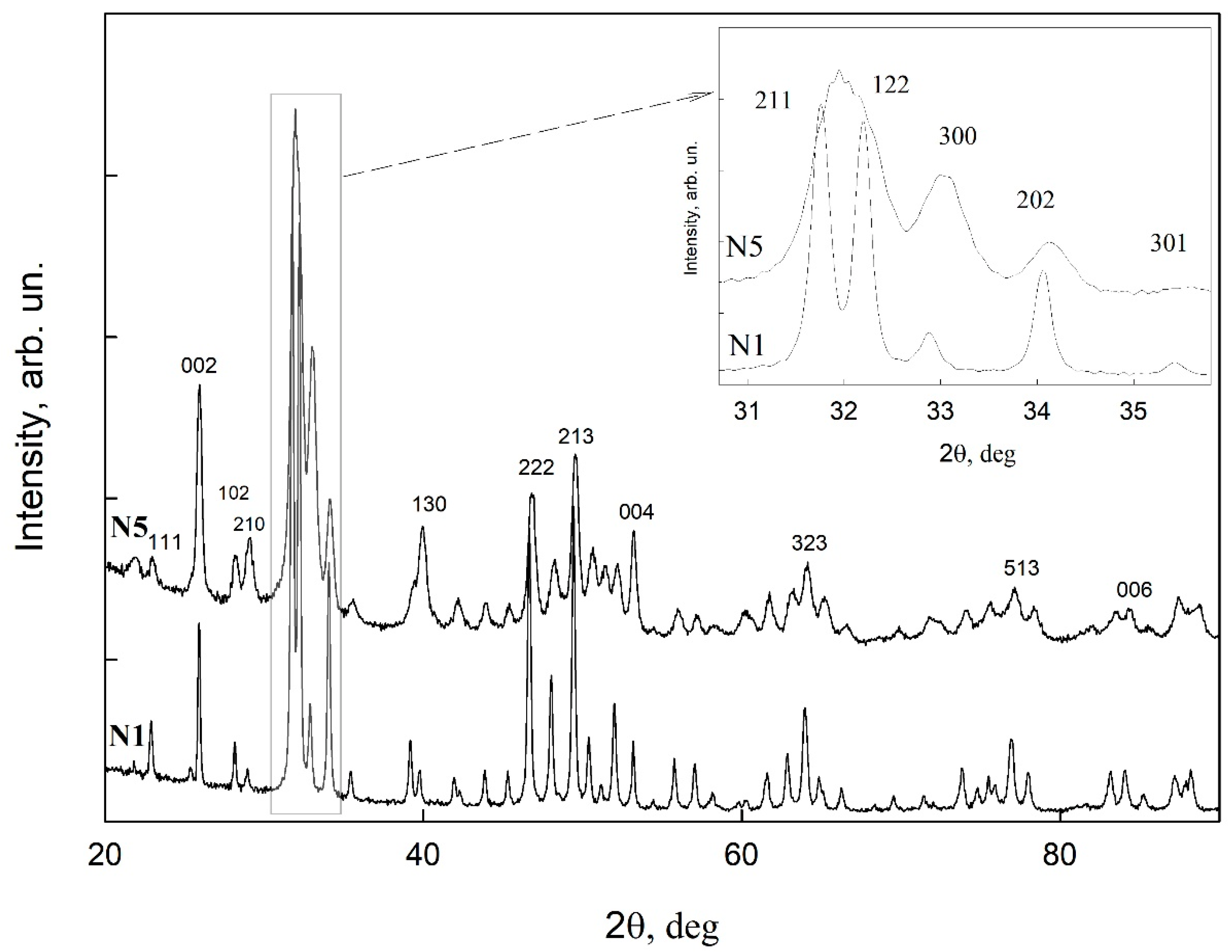
2.7.4. X-ray Diffraction
2.7.5. Raman Microspectroscopy
2.7.6. Data Collection and Spectral Processing
2.7.7. Measuring Nanohardness
3. Results and Discussion
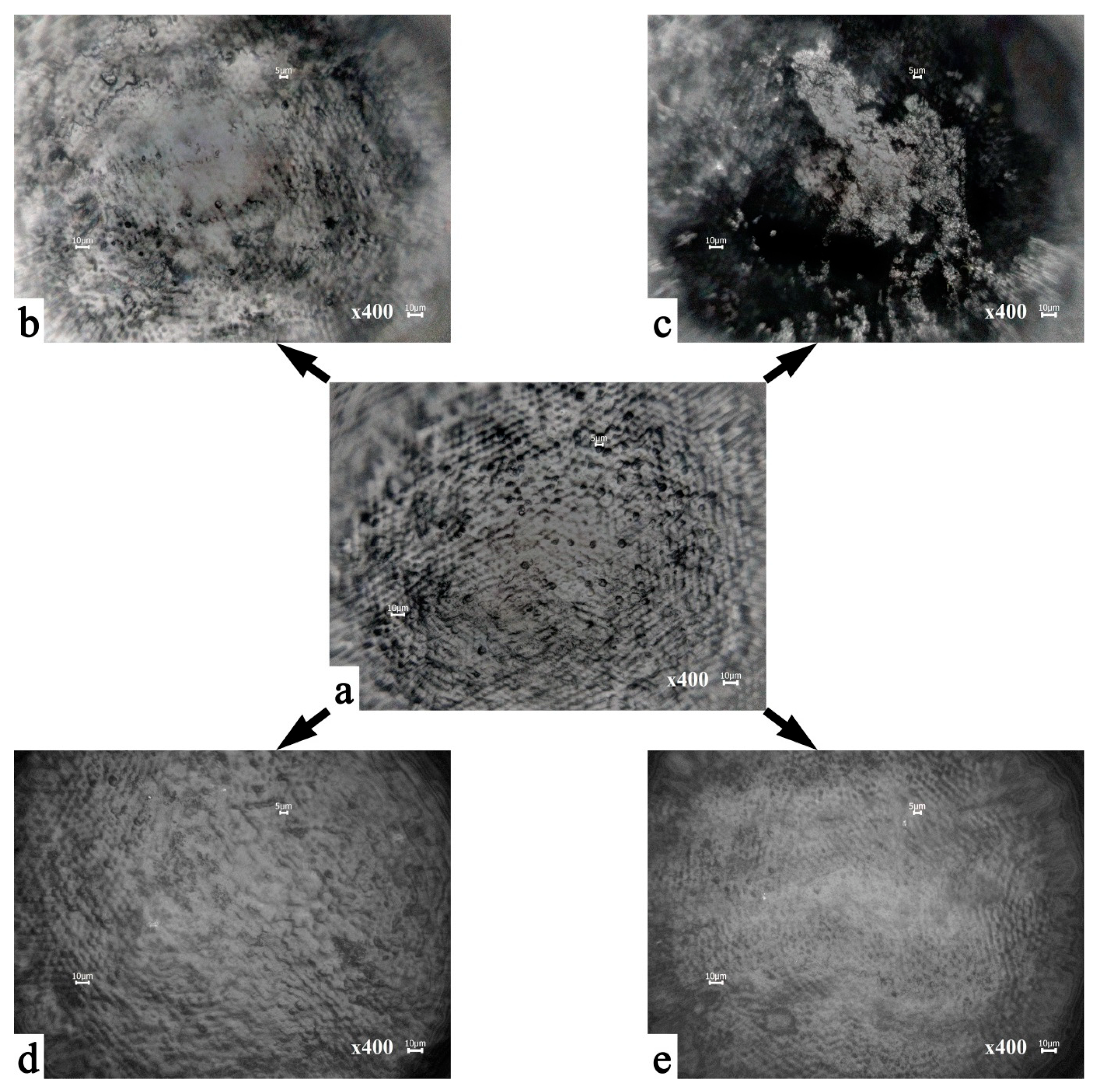
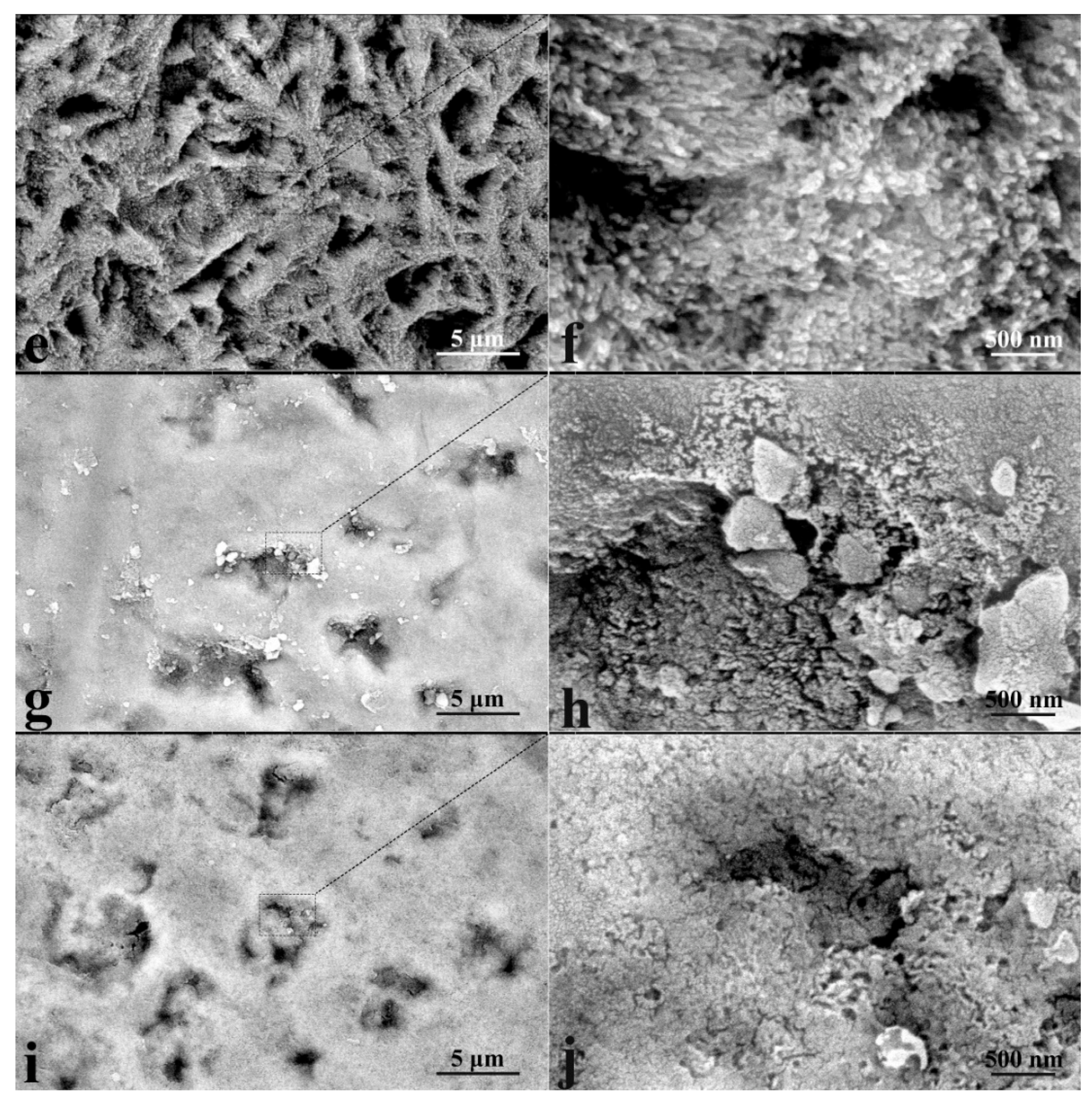
3.1. Microscopic Examinations
3.2. Raman Microspectroscopy
3.3. Measuring Nanohardness
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Liu, Z.; Ren, B.; Wang, Q.; Wu, J.; Yang, N.; Sui, X.; Li, L.; Li, M.; Zhang, X.; et al. Biomimetic Mineralisation Systems for in Situ Enamel Restoration Inspired by Amelogenesis. J. Mater. Sci. Mater. Med. 2021, 32, 115. [Google Scholar] [CrossRef] [PubMed]
- Clift, F. Artificial Methods for the Remineralization of Hydroxyapatite in Enamel. Mater. Today Chem. 2021, 21, 100498. [Google Scholar] [CrossRef]
- El Gezawi, M.; Wölfle, U.C.; Haridy, R.; Fliefel, R.; Kaisarly, D. Remineralization, Regeneration, and Repair of Natural Tooth Structure: Influences on the Future of Restorative Dentistry Practice. ACS Biomater. Sci. Eng. 2019, 5, 4899–4919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Li, Q.L.; Wong, H.M. Cell-Free Biomimetic Mineralization Strategies to Regenerate the Enamel Microstructure. Crystals 2021, 11, 1385. [Google Scholar] [CrossRef]
- Teaford, M.F.; Smith, M.M.; Ferguson, M.W.J. Development, Function and Evolution of Teeth; Cambridge University Press: Cambridge, UK, 2007; ISBN 978-1-139-42922-1. [Google Scholar]
- Wang, Y.; Lin, K.; Wu, C.; Liu, X.; Chang, J. Preparation of Hierarchical Enamel-like Structures from Nano- to Macro-Scale, Regulated by Inorganic Templates Derived from Enamel. J. Mater. Chem. B 2015, 3, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of Tooth Enamel by a Biomimetic Mineralization Frontier Ensuring Epitaxial Growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, Z.; Yang, J.; Lu, D.; Kishen, A.; Li, Y.; Chen, Z.; Que, K.; Zhang, Q.; Deng, X.; et al. Oriented and Ordered Biomimetic Remineralization of the Surface of Demineralized Dental Enamel Using HAP@ACP Nanoparticles Guided by Glycine. Sci. Rep. 2017, 7, 40701. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, H.; Tang, B.; Liang, K.; Li, J. Biomimetic Remineralization of Human Enamel in the Presence of Polyamidoamine Dendrimers in Vitro. Caries Res. 2015, 49, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Han, Q.; Zhang, J.; Han, Z.; Niu, S.; Ren, L. Advanced Bio-Inspired Structural Materials: Local Properties Determine Overall Performance. Mater. Today 2020, 41, 177–199. [Google Scholar] [CrossRef]
- Yao, S.; Jin, B.; Liu, Z.; Shao, C.; Zhao, R.; Wang, X.; Tang, R. Biomineralization: From Material Tactics to Biological Strategy. Adv. Mater. 2017, 29, 111815. [Google Scholar] [CrossRef]
- Šupová, M. The Significance and Utilisation of Biomimetic and Bioinspired Strategies in the Field of Biomedical Material Engineering: The Case of Calcium Phosphat—Protein Template Constructs. Materials 2020, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Erceg, I.; Maltar-Strmečki, N.; Jurašin, D.D.; Strasser, V.; Ćurlin, M.; Lyons, D.M.; Radatović, B.; Mlinarić, N.M.; Kralj, D.; Sikirić, M.D. Comparison of the Effect of the Amino Acids on Spontaneous Formation and Transformation of Calcium Phosphates. Crystals 2021, 11, 792. [Google Scholar] [CrossRef]
- Goloshchapov, D.; Kashkarov, V.; Nikitkov, K.; Seredin, P. Investigation of the Effect of Nanocrystalline Calcium Carbonate-Substituted Hydroxyapatite and L-Lysine and L-Arginine Surface Interactions on the Molecular Properties of Dental Biomimetic Composites. Biomimetics 2021, 6, 70. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Q.; Cui, J.; Hu, D.; Tian, T.; He, T.; Wang, K.; Jiang, W.; Zhang, L. Comparing the Efficacy of Hydroxyapatite Nucleation Regulated by Amino Acids, Poly-Amino Acids and an Amelogenin-Derived Peptide. CrystEngComm 2020, 22, 3814–3823. [Google Scholar] [CrossRef]
- Goloshchapov, D.L.; Ippolitov, Y.A.; Seredin, P.V. Mechanism of Interaction among Nanocrystalline Carbonate-Substituted Hydroxyapatite and Polar Amino-Acids for the Biomimetic Composite Technology: Spectroscopic and Structural Study. Results Phys. 2020, 18, 103277. [Google Scholar] [CrossRef]
- Yanyan, S.; Guangxin, W.; Guoqing, S.; Yaming, W.; Wuhui, L.; Osaka, A. Effects of Amino Acids on Conversion of Calcium Carbonate to Hydroxyapatite. RSC Adv. 2020, 10, 37005–37013. [Google Scholar] [CrossRef]
- McGuire, J.D.; Walker, M.P.; Dusevich, V.; Wang, Y.; Gorski, J.P. Enamel Organic Matrix: Potential Structural Role in Enamel and Relationship to Residual Basement Membrane Constituents at the Dentin Enamel Junction. Connect. Tissue Res. 2014, 55, 33–37. [Google Scholar] [CrossRef]
- Saranya, S.; Justin, S.J.S.; Vijay Solomon, R.; Wilson, P. L-Arginine Directed and Ultrasonically Aided Growth of Nanocrystalline Hydroxyapatite Particles with Tunable Morphology. Colloids Surf. Physicochem. Eng. Asp. 2018, 538, 270–279. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The Role of Amino Acids in Hydroxyapatite Mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef]
- Uskoković, V. Biomineralization and Biomimicry of Tooth Enamel. In Non-Metallic Biomaterials for Tooth Repair and Replacement; Elsevier: Amsterdam, The Netherlands, 2013; pp. 20–44. ISBN 978-0-85709-244-1. [Google Scholar]
- Contaldo, M.; Di Stasio, D.; della Vella, F.; Lauritano, D.; Serpico, R.; Santoro, R.; Lucchese, A. Real Time In Vivo Confocal Microscopic Analysis of the Enamel Remineralization by Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP): A Clinical Proof-of-Concept Study. Appl. Sci. 2020, 10, 4155. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Litman, A.; Margolis, H.C.; Yamakoshi, Y.; Simmer, J.P. Biomimetic Enamel Regeneration Mediated by Leucine-Rich Amelogenin Peptide. J. Dent. Res. 2017, 96, 524–530. [Google Scholar] [CrossRef]
- Mukherjee, K.; Ruan, Q.; Nutt, S.; Tao, J.; De Yoreo, J.J.; Moradian-Oldak, J. Peptide-Based Bioinspired Approach to Regrowing Multilayered Aprismatic Enamel. ACS Omega 2018, 3, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mao, C.; Wang, J.; Xu, X.; Pan, H.; Deng, Y.; Gu, X.; Tang, R. Bio-Inspired Enamel Repair via Glu-Directed Assembly of Apatite Nanoparticles: An Approach to Biomaterials with Optimal Characteristics. Adv. Mater. 2011, 23, 4695–4701. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, X.; Li, Y.; Yang, T.; Yan, X.; Wang, K. In Situ Synthesis Carbonated Hydroxyapatite Layers on Enamel Slices with Acidic Amino Acids by a Novel Two-Step Method. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 150–157. [Google Scholar] [CrossRef]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Taube, F.; Ylmén, R.; Shchukarev, A.; Nietzsche, S.; Norén, J.G. Morphological and Chemical Characterization of Tooth Enamel Exposed to Alkaline Agents. J. Dent. 2010, 38, 72–81. [Google Scholar] [CrossRef]
- Yamazaki, H.; Margolis, H.C. Enhanced Enamel Remineralization under Acidic Conditions in Vitro. J. Dent. Res. 2008, 87, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Goloshchapov, D.L.; Lenshin, A.S.; Savchenko, D.V.; Seredin, P.V. Importance of Defect Nanocrystalline Calcium Hydroxyapatite Characteristics for Developing the Dental Biomimetic Composites. Results Phys. 2019, 13, 102158. [Google Scholar] [CrossRef]
- Seredin, P.V.; Goloshchapov, D.L.; Prutskij, T.; Ippolitov, Y.A. Fabrication and Characterisation of Composites Materials Similar Optically and in Composition to Native Dental Tissues. Results Phys. 2017, 7, 1086–1094. [Google Scholar] [CrossRef]
- Memarpour, M.; Shafiei, F.; Rafiee, A.; Soltani, M.; Dashti, M.H. Effect of Hydroxyapatite Nanoparticles on Enamel Remineralization and Estimation of Fissure Sealant Bond Strength to Remineralized Tooth Surfaces: An in Vitro Study. BMC Oral Health 2019, 19, 92. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Ippolitov, Y.; Vongsvivut, J. Engineering of a Biomimetic Interface between a Native Dental Tissue and Restorative Composite and Its Study Using Synchrotron FTIR Microscopic Mapping. Int. J. Mol. Sci. 2021, 22, 6510. [Google Scholar] [CrossRef] [PubMed]
- Goloshchapov, D.; Buylov, N.; Emelyanova, A.; Ippolitov, I.; Ippolitov, Y.; Kashkarov, V.; Khudyakov, Y.; Nikitkov, K.; Seredin, P. Raman and XANES Spectroscopic Study of the Influence of Coordination Atomic and Molecular Environments in Biomimetic Composite Materials Integrated with Dental Tissue. Nanomaterials 2021, 11, 3099. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Ippolitov, I.; Vongsvivut, J. To the Question on the Use of Multivariate Analysis and 2D Visualisation of Synchrotron ATR-FTIR Chemical Imaging Spectral Data in the Diagnostics of Biomimetic Sound Dentin/Dental Composite Interface. Diagnostics 2021, 11, 1294. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Phase Transformations in a Human Tooth Tissue at the Initial Stage of Caries. PLoS ONE 2015, 10, e0124008. [Google Scholar] [CrossRef] [PubMed]
- Goloshchapov, D.L.; Kashkarov, V.M.; Ippolitov, Y.A.; Prutskij, T.; Seredin, P.V. Early Screening of Dentin Caries Using the Methods of Micro-Raman and Laser-Induced Fluorescence Spectroscopy. Results Phys. 2018, 10, 346–347. [Google Scholar] [CrossRef]
- Buddhachat, K.; Klinhom, S.; Siengdee, P.; Brown, J.L.; Nomsiri, R.; Kaewmong, P.; Thitaram, C.; Mahakkanukrauh, P.; Nganvongpanit, K. Elemental Analysis of Bone, Teeth, Horn and Antler in Different Animal Species Using Non-Invasive Handheld X-Ray Fluorescence. PLoS ONE 2016, 11, e0155458. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, T.; Gao, J.; Li, C.; Jiang, S.; Zhang, X. Building an Aprismatic Enamel-like Layer on a Demineralized Enamel Surface by Using Carboxymethyl Chitosan and Lysozyme-Encapsulated Amorphous Calcium Phosphate Nanogels. J. Dent. 2021, 107, 103599. [Google Scholar] [CrossRef]
- Besnard, C.; Harper, R.A.; Salvati, E.; Moxham, T.E.J.; Romano Brandt, L.; Landini, G.; Shelton, R.M.; Korsunsky, A.M. Analysis of in Vitro Demineralised Human Enamel Using Multi-Scale Correlative Optical and Scanning Electron Microscopy, and High-Resolution Synchrotron Wide-Angle X-ray Scattering. Mater. Des. 2021, 206, 109739. [Google Scholar] [CrossRef]
- Tanaka, O.; Camargo, E.; Cerci, B.; Guariza-Filho, O.; Roman, L. Dental Enamel Roughness with Different Acid Etching Times: Atomic Force Microscopy Study. Eur. J. Gen. Dent. 2012, 1, 187–191. [Google Scholar] [CrossRef]
- Tavafoghi Jahromi, M.; Cerruti, M. Amino Acid/Ion Aggregate Formation and Their Role in Hydroxyapatite Precipitation. Cryst. Growth Des. 2015, 15, 1096–1104. [Google Scholar] [CrossRef]
- Goloshchapov, D.L.; Kashkarov, V.M.; Rumyantseva, N.A.; Seredin, P.V.; Lenshin, A.S.; Agapov, B.L.; Domashevskaya, E.P. Synthesis of Nanocrystalline Hydroxyapatite by Precipitation Using Hen’s Eggshell. Ceram. Int. 2013, 39, 4539–4549. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR Studies of Synthetic and Natural Apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Boffelli, M.; Adachi, T.; Ichioka, H.; Yamamoto, T.; Marunaka, Y.; Kanamura, N. Vibrational Algorithms for Quantitative Crystallographic Analyses of Hydroxyapatite-Based Biomaterials: I, Theoretical Foundations. Anal. Bioanal. Chem. 2015, 407, 3325–3342. [Google Scholar] [CrossRef]
- Wang, P.; Anderson, E.J.D.; Muller, E.A.; Gao, F.; Zhong, Y.; Raschke, M.B. Hyper-Spectral Raman Imaging Correlating Chemical Substitution and Crystallinity in Biogenic Hydroxyapatite: Dentin and Enamel in Normal and Hypoplastic Human Teeth. J. Raman Spectrosc. 2018, 49, 1559–1567. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, G.; Rey, C.; Bres, E. MicroRaman Spectral Study of the PO4 and CO3 Vibrational Modes in Synthetic and Biological Apatites. Calcif. Tissue Int. 1998, 63, 475–481. [Google Scholar] [CrossRef]
- Mihály, J.; Gombás, V.; Afishah, A.; Mink, J. FT-Raman Investigation of Human Dental Enamel Surfaces. J. Raman Spectrosc. 2009, 40, 898–902. [Google Scholar] [CrossRef]
- Spizzirri, P.G.; Cochrane, N.J.; Prawer, S.; Reynolds, E.C. A Comparative Study of Carbonate Determination in Human Teeth Using Raman Spectroscopy. Caries Res. 2012, 46, 353–360. [Google Scholar] [CrossRef]
- Zelic, K.; Milovanovic, P.; Rakocevic, Z.; Askrabic, S.; Potocnik, J.; Popovic, M.; Djuric, M. Nano-Structural and Compositional Basis of Devitalized Tooth Fragility. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014, 30, 476–486. [Google Scholar] [CrossRef]
- Bērziņš, K.; Sutton, J.J.; Loch, C.; Beckett, D.; Wheeler, B.J.; Drummond, B.K.; Fraser-Miller, S.J.; Gordon, K.C. Application of Low-wavenumber Raman Spectroscopy to the Analysis of Human Teeth. J. Raman Spectrosc. 2019, 50, 1375–1387. [Google Scholar] [CrossRef]
- Calderín, L.; Dunfield, D.; Stott, M.J. Shell-Model Study of the Lattice Dynamics of Hydroxyapatite. Phys. Rev. B 2005, 72, 224304. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and Comprehensive Characterization of a Calcium Hydroxyapatite Reference Material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553. [Google Scholar] [CrossRef] [PubMed]
- Sowa, M.G.; Popescu, D.P.; Werner, J.; Hewko, M.; Ko, A.C.-T.; Payette, J.; Dong, C.C.S.; Cleghorn, B.; Choo-Smith, L.-P. Precision of Raman Depolarization and Optical Attenuation Measurements of Sound Tooth Enamel. Anal. Bioanal. Chem. 2006, 387, 1613–1619. [Google Scholar] [CrossRef]
- Anwar Alebrahim, M.; Krafft, C.; Sekhaneh, W.; Sigusch, B.; Popp, J. ATR-FTIR and Raman Spectroscopy of Primary and Permanent Teeth. Biomed. Spectrosc. Imaging 2014, 3, 15–27. [Google Scholar] [CrossRef]
- Robin, M.; Euw, S.V.; Renaudin, G.; Gomes, S.; Krafft, J.-M.; Nassif, N.; Azaïs, T.; Costentin, G. Insights into OCP Identification and Quantification in the Context of Apatite Biomineralization. CrystEngComm 2020, 22, 2728–2742. [Google Scholar] [CrossRef]
- Jegova, G.; Titorenkova, R.; Rashkova, M.; Mihailova, B. Raman and IR Reflection Micro-Spectroscopic Study of Er:YAG Laser Treated Permanent and Deciduous Human Teeth. J. Raman Spectrosc. 2013, 44, 1483–1490. [Google Scholar] [CrossRef]
- Awonusi, A.; Morris, M.D.; Tecklenburg, M.M.J. Carbonate Assignment and Calibration in the Raman Spectrum of Apatite. Calcif. Tissue Int. 2007, 81, 46–52. [Google Scholar] [CrossRef]
- Taylor, E.A.; Mileti, C.J.; Ganesan, S.; Kim, J.H.; Donnelly, E. Measures of Bone Mineral Carbonate Content and Mineral Maturity/Crystallinity for FT-IR and Raman Spectroscopic Imaging Differentially Relate to Physical–Chemical Properties of Carbonate-Substituted Hydroxyapatite. Calcif. Tissue Int. 2021, 109, 77–91. [Google Scholar] [CrossRef]
- Sadyrin, E.; Swain, M.; Mitrin, B.; Rzhepakovsky, I.; Nikolaev, A.; Irkha, V.; Yogina, D.; Lyanguzov, N.; Maksyukov, S.; Aizikovich, S. Characterization of Enamel and Dentine about a White Spot Lesion: Mechanical Properties, Mineral Density, Microstructure and Molecular Composition. Nanomaterials 2020, 10, 1889. [Google Scholar] [CrossRef]
- Choo-Smith, L.-P.; Hewko, M.; Sowa, M.G. Emerging Dental Applications of Raman Spectroscopy. In Emerging Raman Applications and Techniques in Biomedical and Pharmaceutical Fields; Biological and Medical Physics, Biomedical Engineering; Matousek, P., Morris, M.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 263–284. ISBN 978-3-642-02648-5. [Google Scholar]
- Taylor, E.A.; Donnelly, E. Raman and Fourier Transform Infrared Imaging for Characterization of Bone Material Properties. Bone 2020, 139, 115490. [Google Scholar] [CrossRef]
- Calzolari, A.; Pavan, B.; Curtarolo, S.; Buongiorno Nardelli, M.; Fornari, M. Vibrational Spectral Fingerprinting for Chemical Recognition of Biominerals. Chem. Phys. Chem. 2020, 21, 770–778. [Google Scholar] [CrossRef]
- Shen, L.; Barbosa de Sousa, F.; Tay, N.; Lang, T.S.; Kaixin, V.L.; Han, J.; Kilpatrick-Liverman, L.; Wang, W.; Lavender, S.; Pilch, S.; et al. Deformation Behavior of Normal Human Enamel: A Study by Nanoindentation. J. Mech. Behav. Biomed. Mater. 2020, 108, 103799. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-F.; Xuan, F.-Z. Anisotropic Fatigue Behavior of Human Enamel Characterized by Multi-Cycling Nanoindentation. J. Mech. Behav. Biomed. Mater. 2012, 16, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Comeau, P.; Willett, T. Impact of Side Chain Polarity on Non-Stoichiometric Nano-Hydroxyapatite Surface Functionalization with Amino Acids. Sci. Rep. 2018, 8, 12700. [Google Scholar] [CrossRef] [PubMed]
- Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Ippolitov, Y.; Vongsvivut, J. The Molecular and Mechanical Characteristics of Biomimetic Composite Dental Materials Composed of Nanocrystalline Hydroxyapatite and Light-Cured Adhesive. Biomimetics 2022, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Hu, D.Y.; Li, X.; Fan, X.; Zhang, Y.P.; Pretty, I.A.; Mateo, L.R.; Cummins, D.; Ellwood, R.P. The Anti-Caries Efficacy of a Dentifrice Containing 1.5% Arginine and 1450 Ppm Fluoride as Sodium Monofluorophosphate Assessed Using Quantitative Light-Induced Fluorescence (QLF). J. Dent. 2013, 41, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Rege, A.; Corby, P.; Klaczany, G.; Allen, K.; Hershkowitz, D.; Goldder, B.; Wolff, M. Evaluation of a Dentifrice Containing 8% Arginine, Calcium Carbonate, and Sodium Monofluorophosphate to Repair Acid-Softened Enamel Using an Intra-Oral Remineralization Model. J. Clin. Dent. 2014, 25, A14–A19. [Google Scholar]

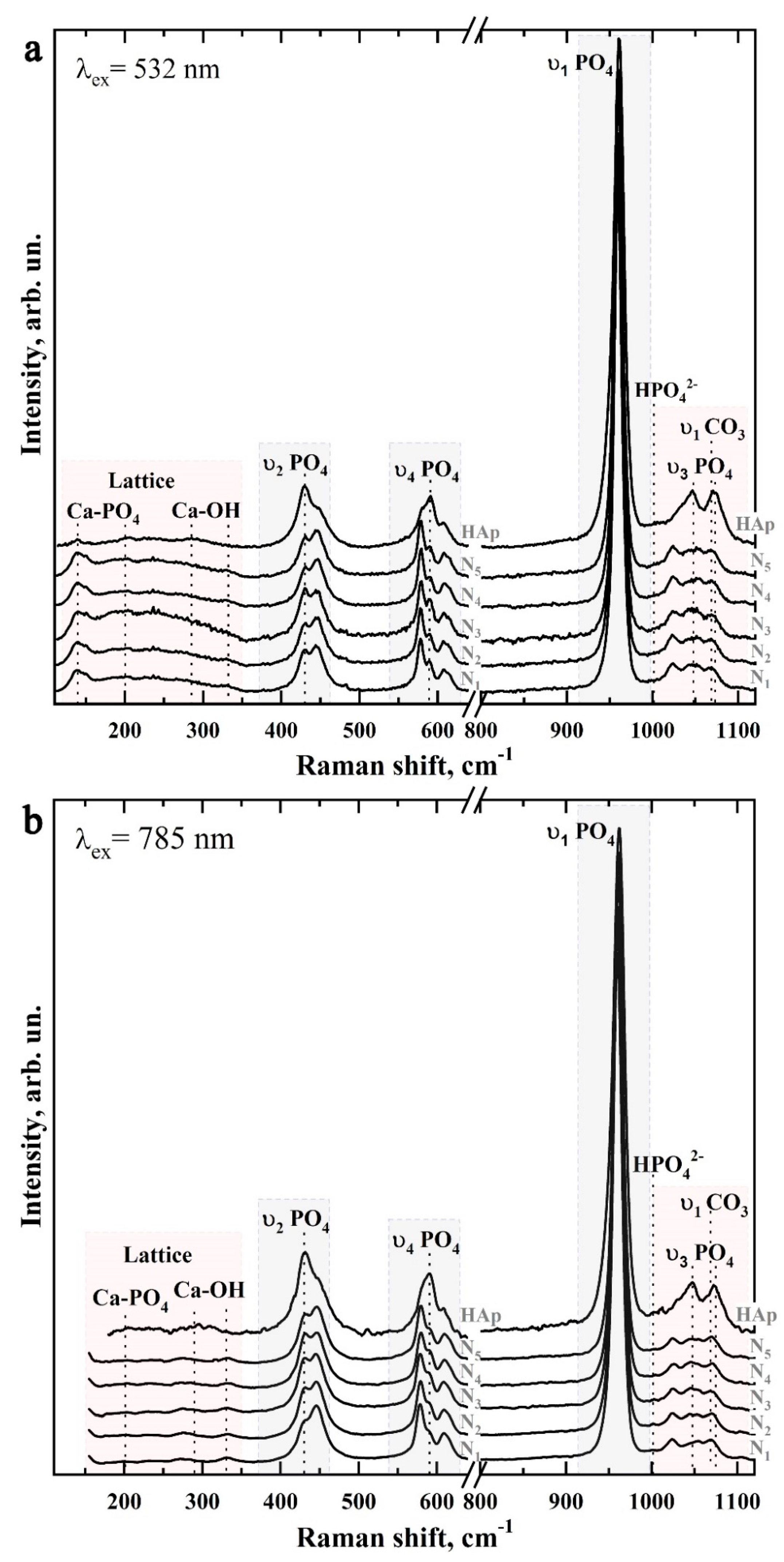

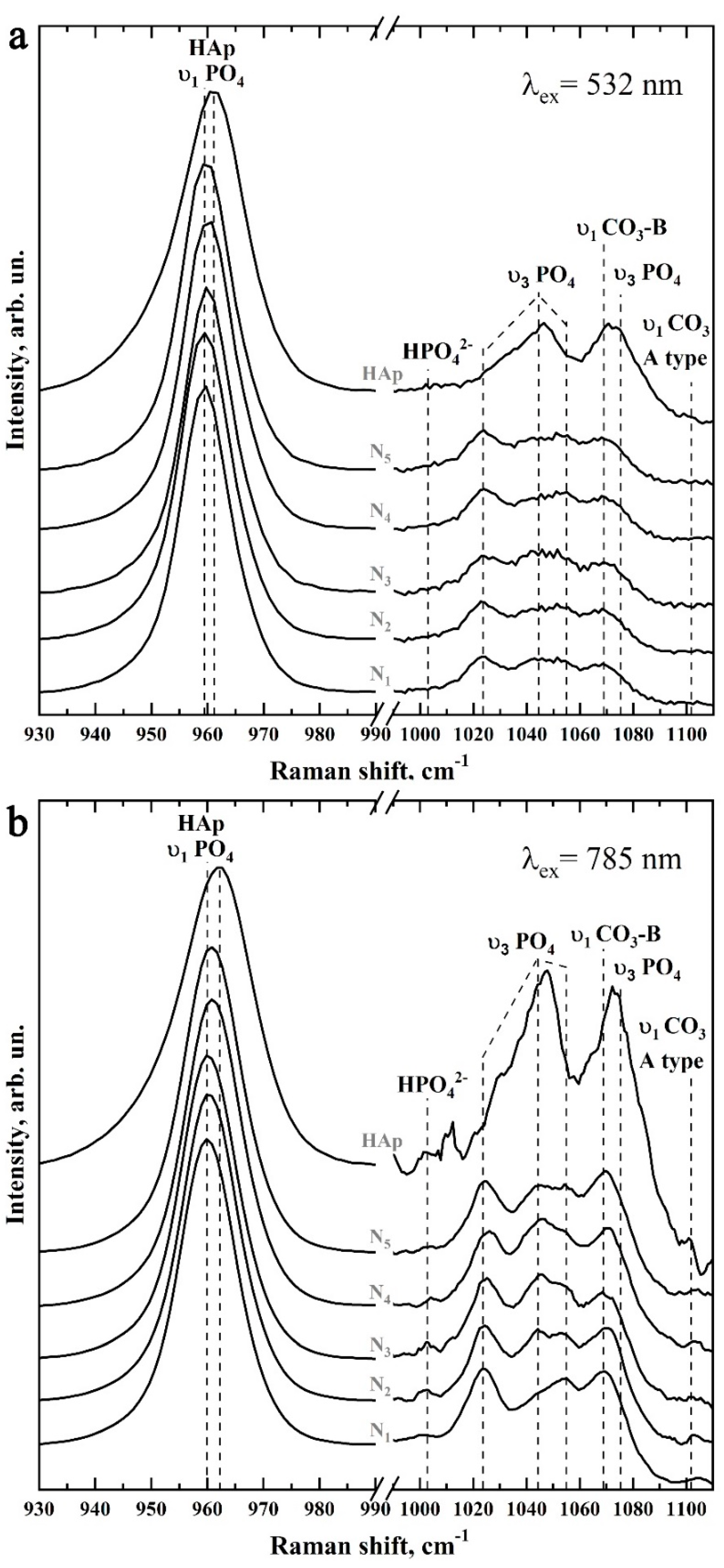

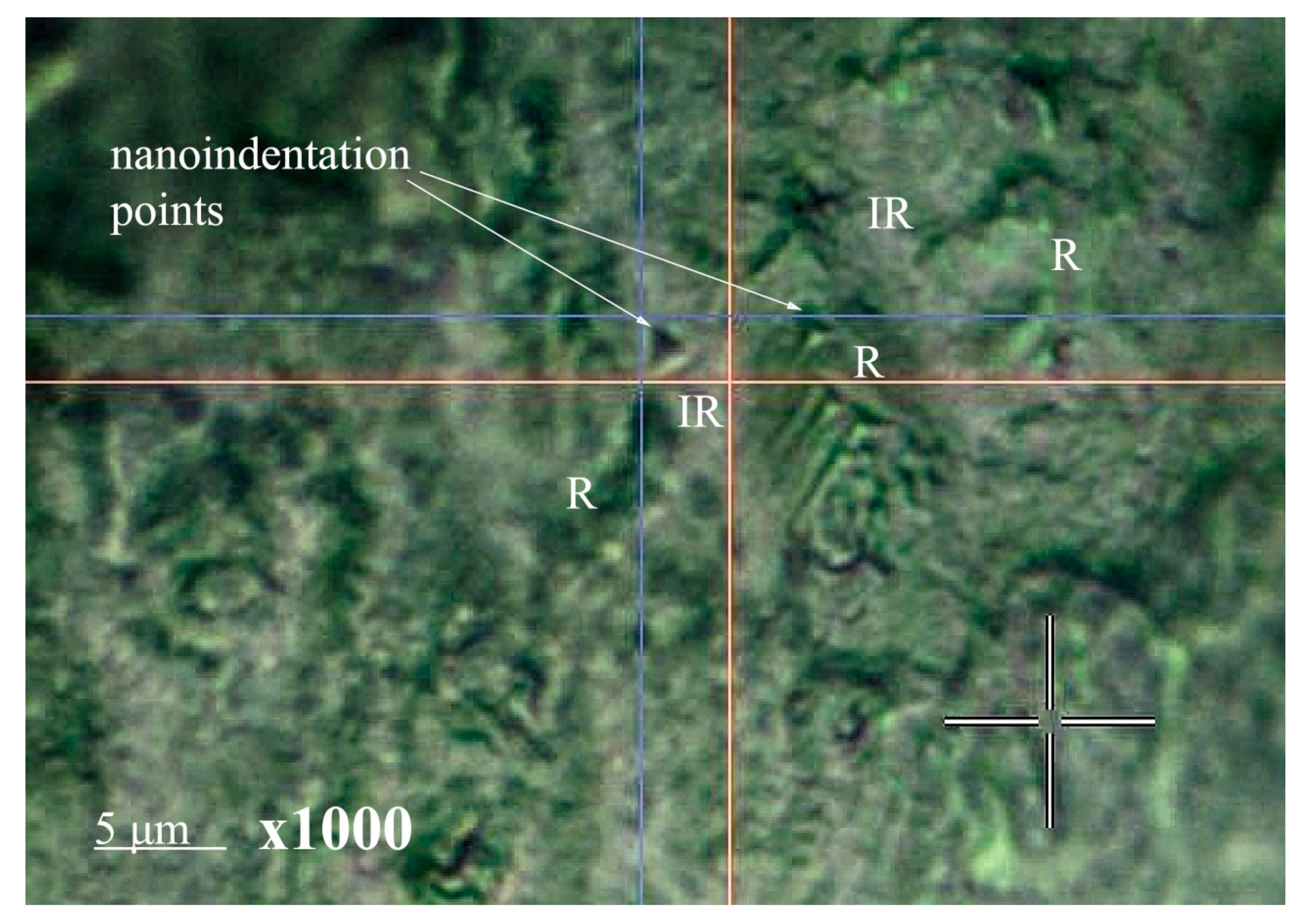
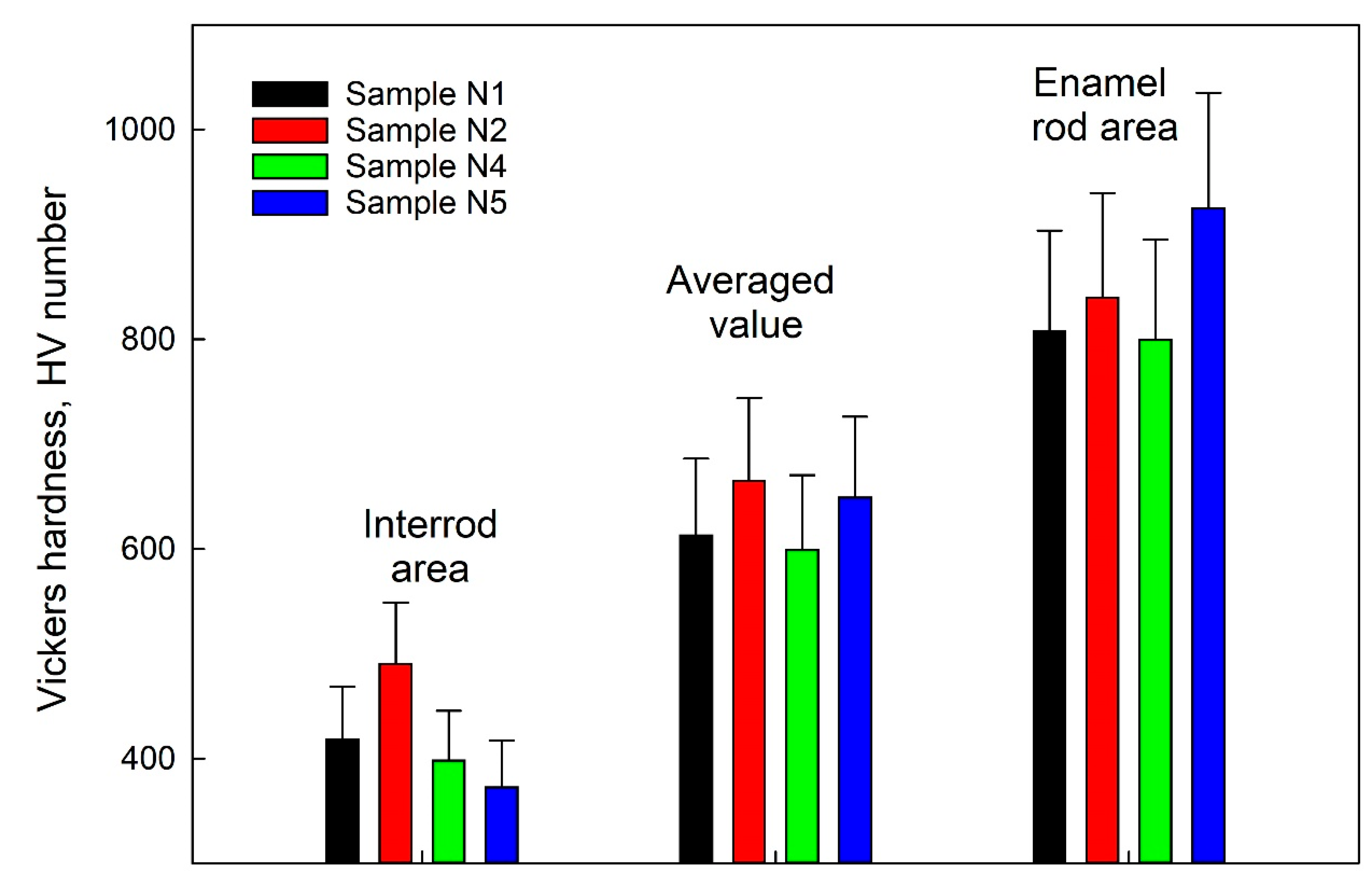
| Bond | Wavenumber, cm−1 | Assignment | References |
|---|---|---|---|
| Ca–PO43− | 139–140 | Lattice | [44] |
| CaII–OH | 186–191 | Lattice | [44] |
| Ca–PO43− | 201–205 | Lattice | [44,51,52] |
| Ca–PO43− | 231–234 | Lattice | [44,51,52] |
| Ca–PO43− | 263–265 | Lattice | [44,51,52] |
| CaII–OH | 272–274 | Translation | [44,51,52] |
| Ca–PO43− | 283–285 | Libration | [44,51,52] |
| CaII–OH | 309–315 | Translation | [44,51,52,53] |
| CaII–OH | 329–330 | Translation | [51,53] |
| υ2 PO43− | 430–432 | O-P-O bending, υ2 | [45,47,48,50,51,54] |
| υ2 PO43− | 445–446 | O-P-O bending, υ2 | [45,47,48,50,51,54] |
| υ4 PO43− | 578–580 | O-P-O bending, υ4 | [45,47,48,50,51,54] |
| υ4 PO43− | 589–591 | O-P-O bending, υ4 | [45,47,48,50,51,54] |
| υ4 PO43− | 608–610 | O-P-O bending, υ4 | [45,47,48,50,51,54] |
| υ4 PO43− | 615–617 | O-P-O bending, υ4 | [45,47,48,50,51,54] |
| υ1 PO43− | 959–962 | P-O stretching, enamel, synthetic hydroxyapatite | [45,47,48,50,51,54,55] |
| HPO42− | 1002–1004 | phosphate Sym stretching | [47,56] |
| υ3 PO43− | 1024–1026 (1030 Hap) | P-O asymmetric stretching, enamel, synthetic hydroxyapatite | [47,49,51,57,58,59] |
| υ3 PO43− | 1041–1042 | P-O asymmetric stretching | [47,49,51,57,58,59] |
| υ3 PO43− | 1044–1046 (1047 Hap) | P-O asymmetric stretching, enamel, synthetic hydroxyapatite | [47,49,51,57,58,59] |
| υ3 PO43− | 1055–1057 | P-O asymmetric stretching | [47,49,51,57,58,59] |
| υ1 CO3 B-type | 1068–1070 | PO43− by CO3 substitution | [47,49,51,57,58,59] |
| υ3 PO43− | 1075–1078 HAp | P-O asymmetric stretching, enamel, synthetic hydroxyapatite | [47,49,51,57,58,59] |
| υ1 CO3 A-type | 1101–1104 | OH by CO3 substitution | [47,49,50] |
| Sample | The Value of the R-Ratio | Vickers HV Hardness | ||
|---|---|---|---|---|
| IR | R | IR | R | |
| N1 | 0.64 | 0.49 | 418.9 ± 49.7 | 807.7 ± 95.9 |
| N2 | 0.69 | 0.42 | 490.4 ± 58.2 | 839.8 ± 99.7 |
| N4 | 0.56 | 0.76 | 498.3 ± 47.3 | 800.1 ± 95.0 |
| N5 | 0.55 | 0.75 | 373.1 ± 44.1 | 925.2 ± 109.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seredin, P.; Goloshchapov, D.; Kashkarov, V.; Emelyanova, A.; Buylov, N.; Barkov, K.; Ippolitov, Y.; Khmelevskaia, T.; Mahdy, I.A.; Mahdy, M.A.; et al. Biomimetic Mineralization of Tooth Enamel Using Nanocrystalline Hydroxyapatite under Various Dental Surface Pretreatment Conditions. Biomimetics 2022, 7, 111. https://doi.org/10.3390/biomimetics7030111
Seredin P, Goloshchapov D, Kashkarov V, Emelyanova A, Buylov N, Barkov K, Ippolitov Y, Khmelevskaia T, Mahdy IA, Mahdy MA, et al. Biomimetic Mineralization of Tooth Enamel Using Nanocrystalline Hydroxyapatite under Various Dental Surface Pretreatment Conditions. Biomimetics. 2022; 7(3):111. https://doi.org/10.3390/biomimetics7030111
Chicago/Turabian StyleSeredin, Pavel, Dmitry Goloshchapov, Vladimir Kashkarov, Anna Emelyanova, Nikita Buylov, Konstantin Barkov, Yuri Ippolitov, Tatiana Khmelevskaia, Iman A. Mahdy, Manal A. Mahdy, and et al. 2022. "Biomimetic Mineralization of Tooth Enamel Using Nanocrystalline Hydroxyapatite under Various Dental Surface Pretreatment Conditions" Biomimetics 7, no. 3: 111. https://doi.org/10.3390/biomimetics7030111
APA StyleSeredin, P., Goloshchapov, D., Kashkarov, V., Emelyanova, A., Buylov, N., Barkov, K., Ippolitov, Y., Khmelevskaia, T., Mahdy, I. A., Mahdy, M. A., & Prutskij, T. (2022). Biomimetic Mineralization of Tooth Enamel Using Nanocrystalline Hydroxyapatite under Various Dental Surface Pretreatment Conditions. Biomimetics, 7(3), 111. https://doi.org/10.3390/biomimetics7030111









