Mechanoregulation of Bone Remodeling and Healing as Inspiration for Self-Repair in Materials
Abstract
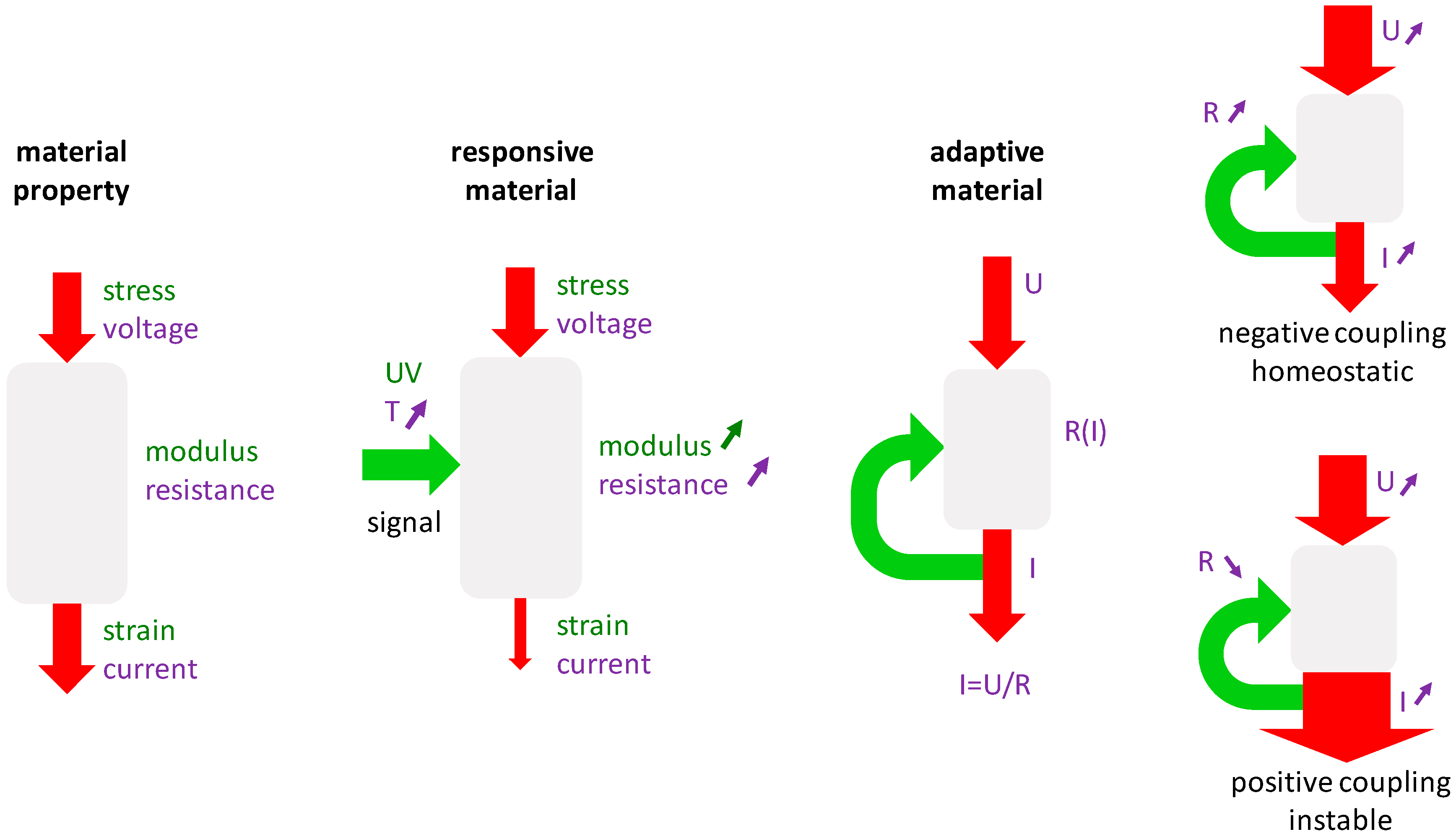
1. Introduction
2. Bone Remodeling
2.1. Biological Background
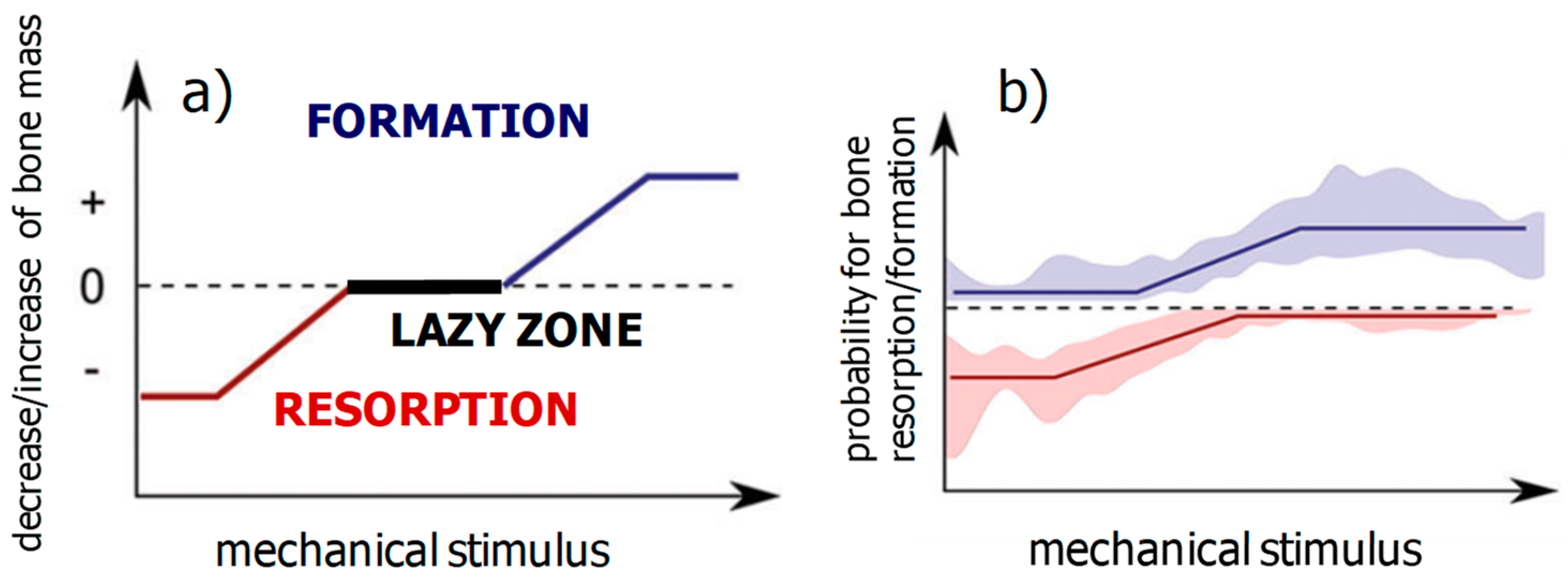
2.2. Theoretical and Experimental Results about the Mechanocontrol of Bone Remodeling
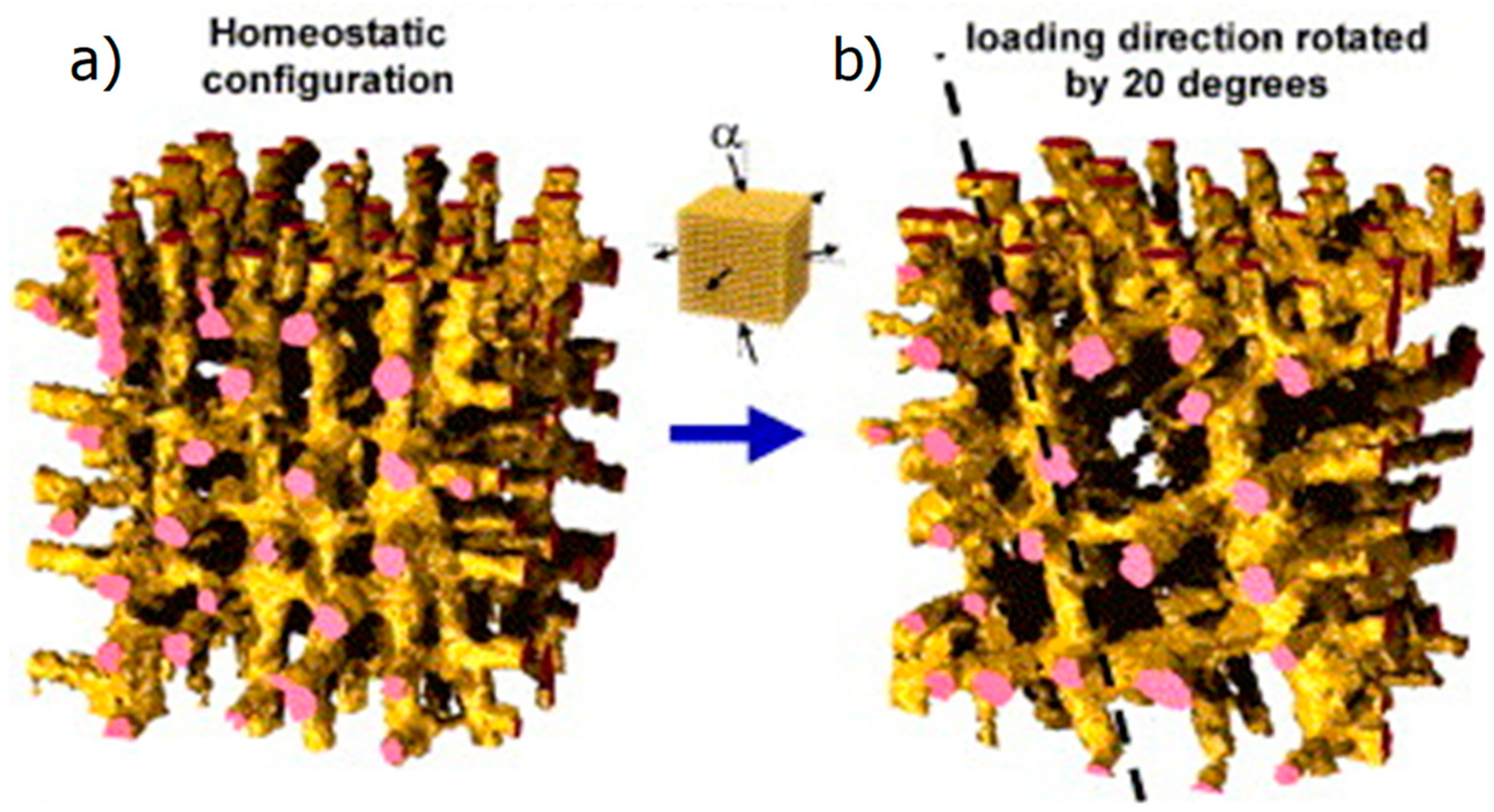
2.3. Computational Models of the Mechanoregulation of Remodeling
2.3.1. Mechanoregulated Formation and Resorption of Bone Material
2.3.2. Mechanoregulated Effective Stiffening/Softening of the Bone Material
2.3.3. Bone Remodeling as Damage Management
3. Bone Healing
3.1. Biological Background
3.2. Theoretical Considerations about the Mechanocontrol in Bone Healing
3.3. Models of the Mechanoregulation of Bone Healing
3.3.1. Mechanoregulated Models of Tissue Differentiation During Bone Healing
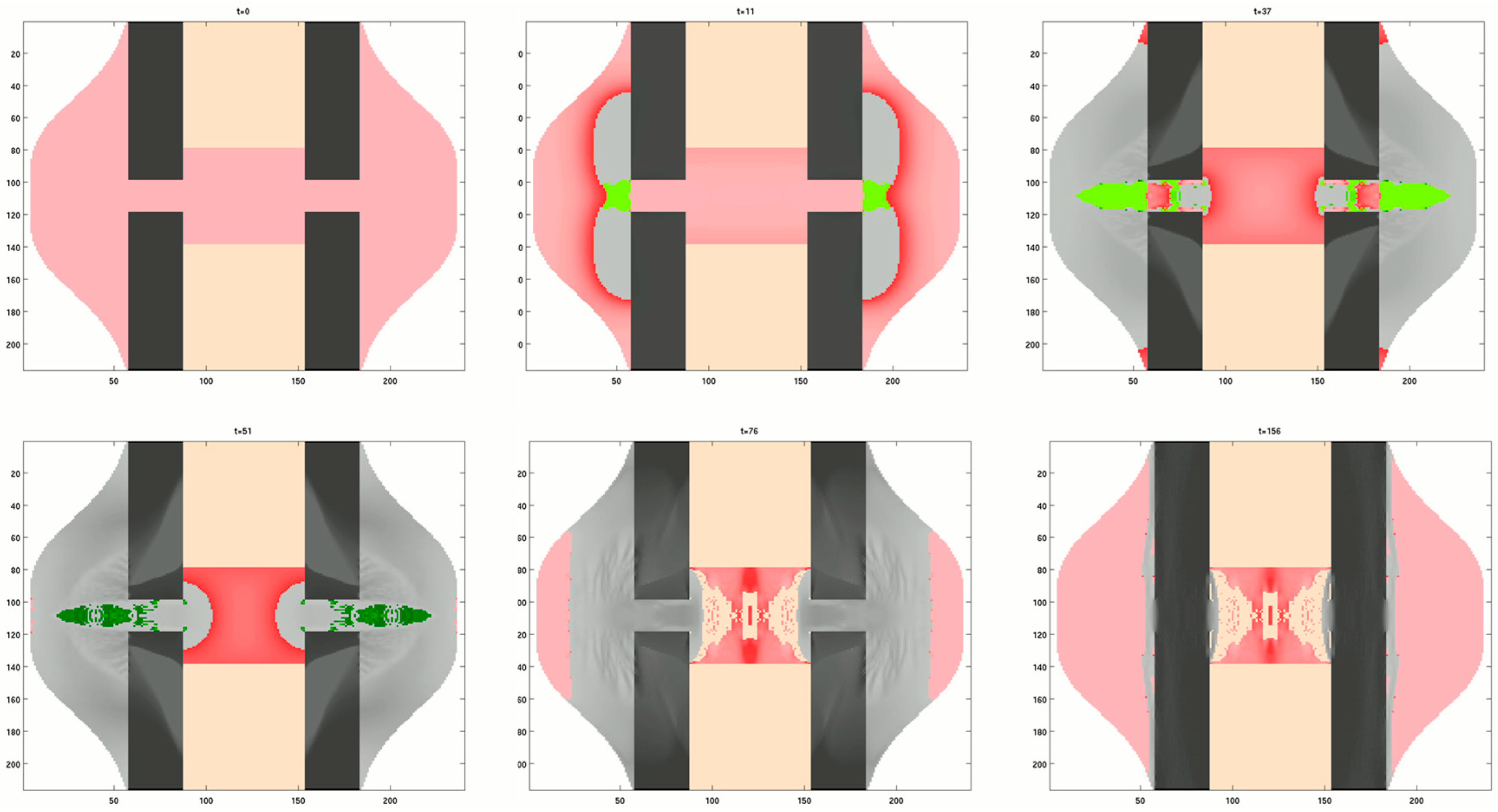
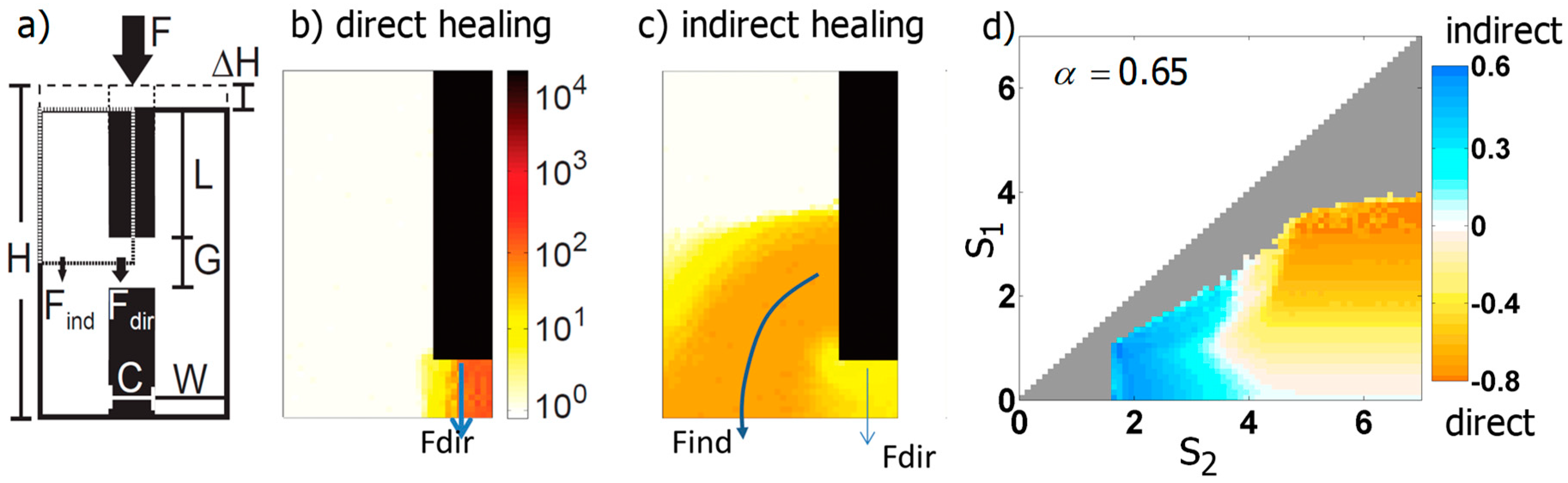
3.3.2. Generic Model of Self-Repair in a Mechanoresponsive Material
4. Conclusions and Implications for Synthetic Self-Healing Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKibbin, B. The biology of fracture healing in long bones. J. Bone Jt. Surg. 1978, 60, 150–162. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.E.; Moore-Lotridge, S.N.; Hysong, A.A.; Posey, S.L.; Robinette, J.P.; Blum, D.M.; Benvenuti, M.A.; Cole, H.A.; Egawa, S.; Okawa, A.; et al. Bone Fracture Acute Phase Response—A Unifying Theory of Fracture Repair: Clinical and Scientific Implications. Clin. Rev. Bone Miner. Metab. 2018, 16, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Speck, O.; Speck, T.; Wagner, S.; Weinkamer, R. Biological Archetypes for Self-Healing Materials. In Self-Healing Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 307–344. [Google Scholar]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Willie, B.; Duda, G.N.; Weinkamer, R. Bone structural adaptation and Wolff’s law. In Materials Design Inspired by Nature: Function through Inner Architecture; RSC Publishing: Cambridge, UK, 2013; pp. 17–45. [Google Scholar]
- Jee, W.S.S. Integrated Bone Tissue Physiology: Anatomy and Physiology. In Bone Mechanics Handbook; Cowin, S.C., Ed.; CRC Press: Boca Raton, FL, USA, 2001; p. I-1-68. [Google Scholar]
- Van der Meulen, M.C.; Huiskes, R. Why mechanobiology?: A survey article. J. Biomech. 2002, 35, 401–414. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Gerhard, F.A.; Webster, D.J.; van Lenthe, G.H.; Müller, R. In silico biology of bone modelling and remodelling: Adaptation. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 2011–2030. [Google Scholar] [CrossRef]
- Hart, R.T. Bone Modeling and Remodeling: Theories and Computation. In Bone Mechanics Handbook; Cowin, S.C., Ed.; CRC Press: Boca Raton, FL, USA, 2001; pp. 1–42. [Google Scholar]
- Adachi, T.; Kameo, Y. Computational Biomechanics of Bone Adaptation by Remodeling. In Multiscale Mechanobiology of Bone Remodeling and Adaptation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 231–257. [Google Scholar]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Pivonka, P.; Dunstan, C.R. Role of mathematical modeling in bone fracture healing. BoneKEy Rep. 2012, 1. [Google Scholar] [CrossRef]
- Doblaré, M.; Garcıa, J.; Gómez, M. Modelling bone tissue fracture and healing: A review. Eng. Fract. Mech. 2004, 71, 1809–1840. [Google Scholar] [CrossRef]
- Geris, L.; Sloten, J.V.; Van Oosterwyck, H. In silico biology of bone modelling and remodelling: Regeneration. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 2031–2053. [Google Scholar] [CrossRef]
- Ducher, G.; Courteix, D.; Même, S.; Magni, C.; Viala, J.F.; Benhamou, C.L. Bone geometry in response to long-term tennis playing and its relationship with muscle volume: A quantitative magnetic resonance imaging study in tennis players. Bone Rep. 2005, 37, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.; Harris, S.T.; Miller, P.D.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; Kendler, D.L.; Yuen, C.K.; Lewiecki, E.M. Bisphosphonate therapy for osteoporosis: Benefits, risks, and drug holiday. Am. J. Med. 2013, 126, 13–20. [Google Scholar] [CrossRef]
- Parfitt, A.M. Osteonal and hemi-osteonal remodeling: The spatial and temporal framework for signal traffic in adult human bone. J. Cell. Biochem. 1994, 55, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.; Melsen, F.; Sod, E.; Barton, I.; Chines, A. Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone Rep. 2002, 31, 620–625. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Cheung, W.-Y.; Majeska, R.; Kennedy, O. Osteocytes: Master Orchestrators of Bone. Calcif. Tissue Int. 2014, 94, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The Amazing Osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Fritton, S.P.; Weinbaum, S. Fluid and solute transport in bone: Flow-induced mechanotransduction. Annu. Rev. Fluid Mech. 2009, 41, 347–374. [Google Scholar] [CrossRef]
- Weinbaum, S.; Cowin, S.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Burger, E.H.; Klein-Nulend, J. Mechanotransduction in bone—Role of the lacuno-canalicular network. FASEB J. 1999, 13, S101–S112. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B. Remodeling and the repair of fatigue damage. Calcif. Tissue Int. 1993, 53, S75–S81. [Google Scholar] [CrossRef] [PubMed]
- Verborgt, O.; Gibson, G.J.; Schaffler, M.B. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue In Vivo. J. Bone Miner. Res. 2000, 15, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H. Homeostatic control of bone structure: An application of feedback theory. Bone Rep. 1991, 12, 203–217. [Google Scholar] [CrossRef]
- Lerebours, C.; Buenzli, P.R. Towards a cell-based mechanostat theory of bone: The need to account for osteocyte desensitisation and osteocyte replacement. J. Biomech. 2016, 49, 2600–2606. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R. If bone is the answer, then what is the question? J. Anat. 2000, 197, 145–156. [Google Scholar] [CrossRef]
- Waarsing, J.; Day, J.S.; van der Linden, J.C.; Ederveen, A.G.; Spanjers, C.; De Clerck, N.; Sasov, A.; Verhaar, J.A.N.; Weinans, H. Detecting and tracking local changes in the tibiae of individual rats: A novel method to analyse longitudinal in vivo micro-CT data. Bone 2004, 34, 163–169. [Google Scholar] [CrossRef]
- Birkhold, A.I.; Razi, H.; Weinkamer, R.; Duda, G.N.; Checa, S.; Willie, B.M. Monitoring in vivo (re) modeling: A computational approach using 4D microCT data to quantify bone surface movements. Bone 2015, 75, 210–221. [Google Scholar] [CrossRef]
- Schulte, F.A.; Ruffoni, D.; Lambers, M.; Christen, D.; Webster, D.J.; Kuhn, G.; Müller, R. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS ONE 2013, 8, e62172. [Google Scholar] [CrossRef]
- Razi, H.; Birkhold, A.I.; Weinkamer, R.; Duda, G.N.; Willie, B.M.; Checa, S. Aging leads to a dysregulation in mechanically driven bone formation and resorption. J. Bone Miner. Res. 2015, 30, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Weinkamer, R.; Hartmann, M.A.; Brechet, Y.; Fratzl, P. Stochastic lattice model for bone remodeling and aging. Phys. Rev. Lett. 2004, 93, 228102. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, J.W.C.; Hartmann, M.A.; Bréchet, Y.J.; Fratzl, P.; Weinkamer, R. New suggestions for the mechanical control of bone remodeling. Calcif. Tissue Int. 2009, 85, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Dunlop, J.W.C.; Bréchet, Y.J.M.; Fratzl, P.; Weinkamer, R. Trabecular bone remodelling simulated by a stochastic exchange of discrete bone packets from the surface. J. Mech. Behav. Biomed. Mater. 2011, 4, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Christen, P.; Ito, K.; Ellouz, R.; Boutroy, S.; Sornay-Rendu, E.; Chapurlat, R.D.; van Rietbergen, B. Bone remodelling in humans is load-driven but not lazy. Nat. Commun. 2014, 5, 4855. [Google Scholar] [CrossRef] [PubMed]
- Huiskes, R.; Ruimerman, R.; van Lenthe, G.H.; Janssen, J.D. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 2000, 405, 704. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Mullender, M.; Huiskes, R. Proposal for the regulatory mechanism of Wolff’s law. J. Orthop. Res. 1995, 13, 503–512. [Google Scholar] [CrossRef]
- Weinans, H.; Huiskes, R.; Grootenboer, H.J. The behavior of adaptive bone-remodeling simulation models. J. Biomech. 1992, 25, 1425–1441. [Google Scholar] [CrossRef]
- Ruimerman, R.; Hilbers, P.; van Rietbergen, B.; Huiskes, R. A theoretical framework for strain-related trabecular bone maintenance and adaptation. J. Biomech. 2005, 38, 931–941. [Google Scholar] [CrossRef]
- Martin, B. Mathematical model for repair of fatigue damage and stress fracture in osteonal bone. J. Orthop. Res. 1995, 13, 309–316. [Google Scholar] [CrossRef]
- Mori, S.; Burr, D. Increased intracortical remodeling following fatigue damage. Bone Rep. 1993, 14, 103–109. [Google Scholar] [CrossRef]
- Burr, D.B. 7 Bone, Exercise, and Stress Fractures. Exerc. Sport Sci. Rev. 1997, 25, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, C.; et al. Fracture repair. In Bone Regeneration and Repair; Springer: Berlin/Heidelberg, Germany, 2005; pp. 21–44. [Google Scholar]
- Schmidt-Bleek, K.; Kwee, B.J.; Mooney, D.J.; Duda, G.N. Boon and bane of inflammation in bone tissue regeneration and its link with angiogenesis. Tissue Eng. Part B Rev. 2015, 21, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, F. Grundriss einer Biomechanik der Frakturheilung. In Verhandlungen der Deutschen Orthopädischen Gesellschaft, Stuttgart: Ferdinand Enke Verlag; 1941, 62–108. Translated in: Biomechanics of fracture healing, in: Biomechanics of the locomotor apparatus; Springer-Verlag: Berlin, Germany, 1980. [Google Scholar]
- Perren, S.; Cordey, J. The concept of interfragmentary strain. In Current Concepts of Internal Fixation of Fractures; Springer: Berlin/Heidelberg, Germany, 1980; pp. 63–77. [Google Scholar]
- Lacroix, D.; Prendergast, P. A mechano-regulation model for tissue differentiation during fracture healing: Analysis of gap size and loading. J. Biomech. 2002, 35, 1163–1171. [Google Scholar] [CrossRef]
- Isaksson, H.; Wilson, W.; van Donkelaar, C.C.; Huiskes, R.; Ito, K. Comparison of biophysical stimuli for mechano-regulation of tissue differentiation during fracture healing. J. Biomech. 2006, 39, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Vetter, A.; Witt, F.; Sander, O.; Duda, G.N.; Weinkamer, R. The spatio-temporal arrangement of different tissues during bone healing as a result of simple mechanobiological rules. Biomech. Model. Mechanobiol. 2012, 11, 147–160. [Google Scholar] [CrossRef]
- Claes, L.; Heigele, C. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 1999, 32, 255–266. [Google Scholar] [CrossRef]
- Gomez-Benito, M.; García-Aznar, J.M.; Kuiper, J.H.; Doblaré, M. Influence of fracture gap size on the pattern of long bone healing: A computational study. J. Theor. Biol. 2005, 235, 105–119. [Google Scholar] [CrossRef]
- Lu, Y.; Lekszycki, T. Modelling of bone fracture healing: Influence of gap size and angiogenesis into bioresorbable bone substitute. Math. Mech. Solids 2017, 22, 1997–2010. [Google Scholar] [CrossRef]
- Isaksson, H.; Comas, O.; van Donkelaar, C.C.; Mediavilla, J.; Wilson, W.; Huiskes, R.; Ito, K. Bone regeneration during distraction osteogenesis: Mechano-regulation by shear strain and fluid velocity. J. Biomech. 2007, 40, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Repp, F.; Vetter, A.; Duda, G.N.; Weinkamer, R. The connection between cellular mechanoregulation and tissue patterns during bone healing. Med. Biol. Eng. Comput. 2015, 53, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Checa, S.; Prendergast, P.J.; Duda, G.N. Inter-species investigation of the mechano-regulation of bone healing: Comparison of secondary bone healing in sheep and rat. J. Biomech. 2011, 44, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Vetter, A.; Sander, O.; Duda, G.N.; Weinkamer, R. Healing of a mechano-responsive material. EPL 2014, 104, 68005. [Google Scholar] [CrossRef]
- Jefferson, T.; Javierre, E.; Freeman, B.; Zaoui, A.; Koenders, E.; Ferrara, L. Research Progress on Numerical Models for Self-Healing Cementitious Materials. Adv. Mater. Interfaces 2018, 5, 1701378. [Google Scholar] [CrossRef]
- Kadic, M.; Milton, G.W.; van Hecke, M.; Wegener, M. 3D metamaterials. Nat. Rev. Phys. 2019, 1, 198–210. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, J.; Liang, H.; Jiang, Z.; Wua, L. Mechanical metamaterials associated with stiffness, rigidity and compressibility: A brief review. Prog. Mater. Sci. 2018, 94, 114–173. [Google Scholar] [CrossRef]
- Coulais, C.; Teomy, E.; de Reus, K.; Shokef, Y.; van Hecke, M. Combinatorial design of textured mechanical metamaterials. Nature 2016, 535, 529. [Google Scholar] [CrossRef]
- Hawkes, E.; An, B.; Benbernou, N.M.; Tanaka, H.; Kim, S.; Demaine, E.D.; Rus, D.; Wood, R.J. Programmable matter by folding. Proc. Natl. Acad. Sci. USA 2010, 107, 12441–12445. [Google Scholar] [CrossRef]
- Florijn, B.; Coulais, C.; van Hecke, M. Programmable mechanical metamaterials. Phys. Rev. Lett. 2014, 113, 175503. [Google Scholar] [CrossRef]
- Berwind, M.F.; Kamas, A.; Eberl, C. A Hierarchical Programmable Mechanical Metamaterial Unit Cell Showing Metastable Shape Memory. Adv. Eng. Mater. 2018, 20, 1800771. [Google Scholar] [CrossRef]
- Brown, C.L.; Craig, S.L. Molecular engineering of mechanophore activity for stress-responsive polymeric materials. Chem. Sci. 2015, 6, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.L.B.; et al. Mechanochemical strengthening of a synthetic polymer in response to typically destructive shear forces. Nat. Chem. 2013, 5, 757. [Google Scholar] [CrossRef] [PubMed]
- Klukovich, H.M.; Kouznetsova, T.B.; Kean, Z.S.; Lenhardt, J.M.; Craig, S.L. A backbone lever-arm effect enhances polymer mechanochemistry. Nat. Chem. 2013, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Kawakami1, R.; Namba1, R.; Nakajima, T.; Gong, J.P. Mechanoresponsive self-growing hydrogels inspired by muscle training. Science 2019, 363, 504–508. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinkamer, R.; Eberl, C.; Fratzl, P. Mechanoregulation of Bone Remodeling and Healing as Inspiration for Self-Repair in Materials. Biomimetics 2019, 4, 46. https://doi.org/10.3390/biomimetics4030046
Weinkamer R, Eberl C, Fratzl P. Mechanoregulation of Bone Remodeling and Healing as Inspiration for Self-Repair in Materials. Biomimetics. 2019; 4(3):46. https://doi.org/10.3390/biomimetics4030046
Chicago/Turabian StyleWeinkamer, Richard, Christoph Eberl, and Peter Fratzl. 2019. "Mechanoregulation of Bone Remodeling and Healing as Inspiration for Self-Repair in Materials" Biomimetics 4, no. 3: 46. https://doi.org/10.3390/biomimetics4030046
APA StyleWeinkamer, R., Eberl, C., & Fratzl, P. (2019). Mechanoregulation of Bone Remodeling and Healing as Inspiration for Self-Repair in Materials. Biomimetics, 4(3), 46. https://doi.org/10.3390/biomimetics4030046




