Citric Acid Tunes the Formation of Antimicrobial Melanin-Like Nanostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
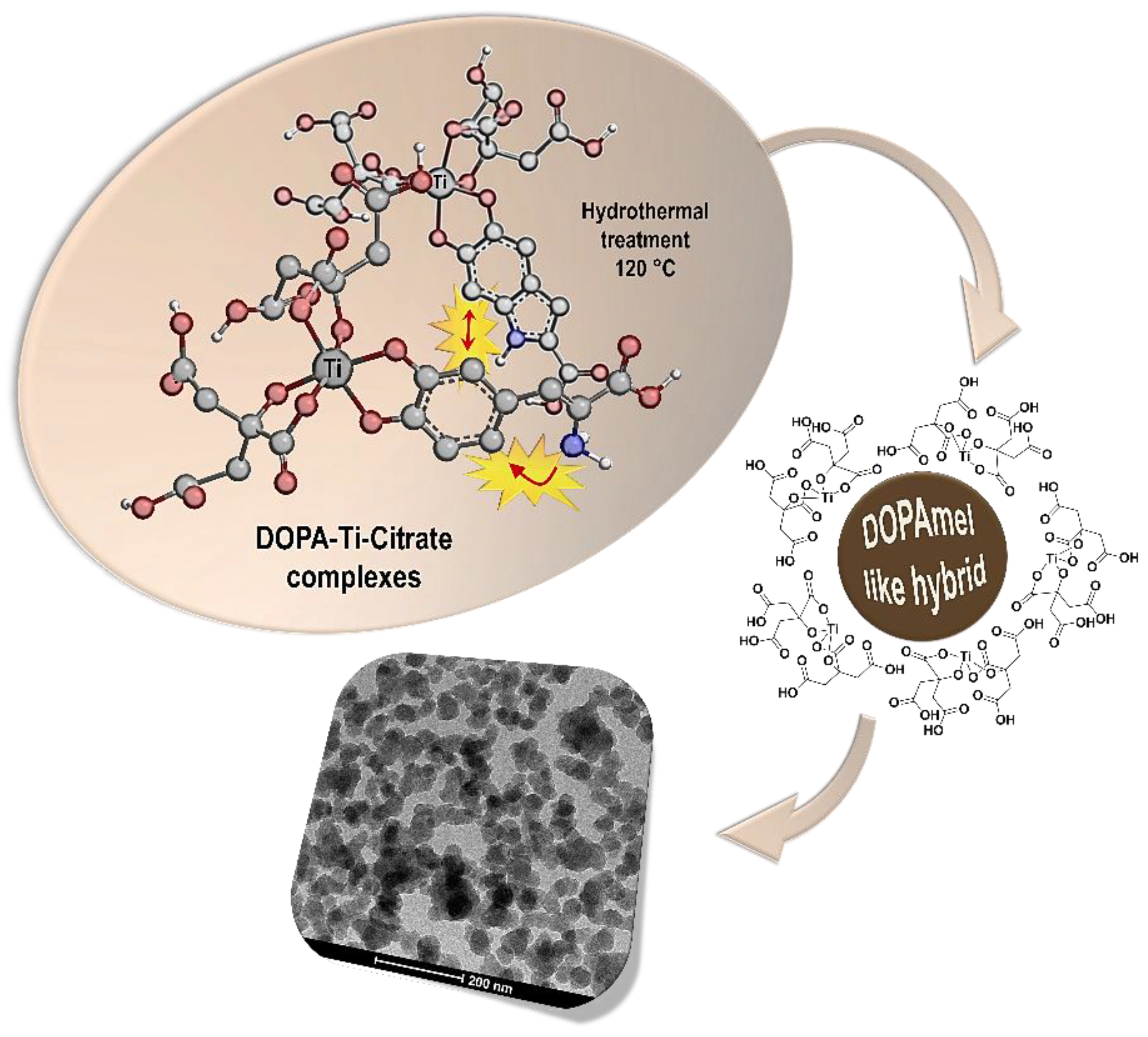
2.2. Synthesis of Hybrid Melanin-Like Nanostructures
2.3. Quantitative Determination of Melanin Content
2.4. Physico-Chemical Characterization
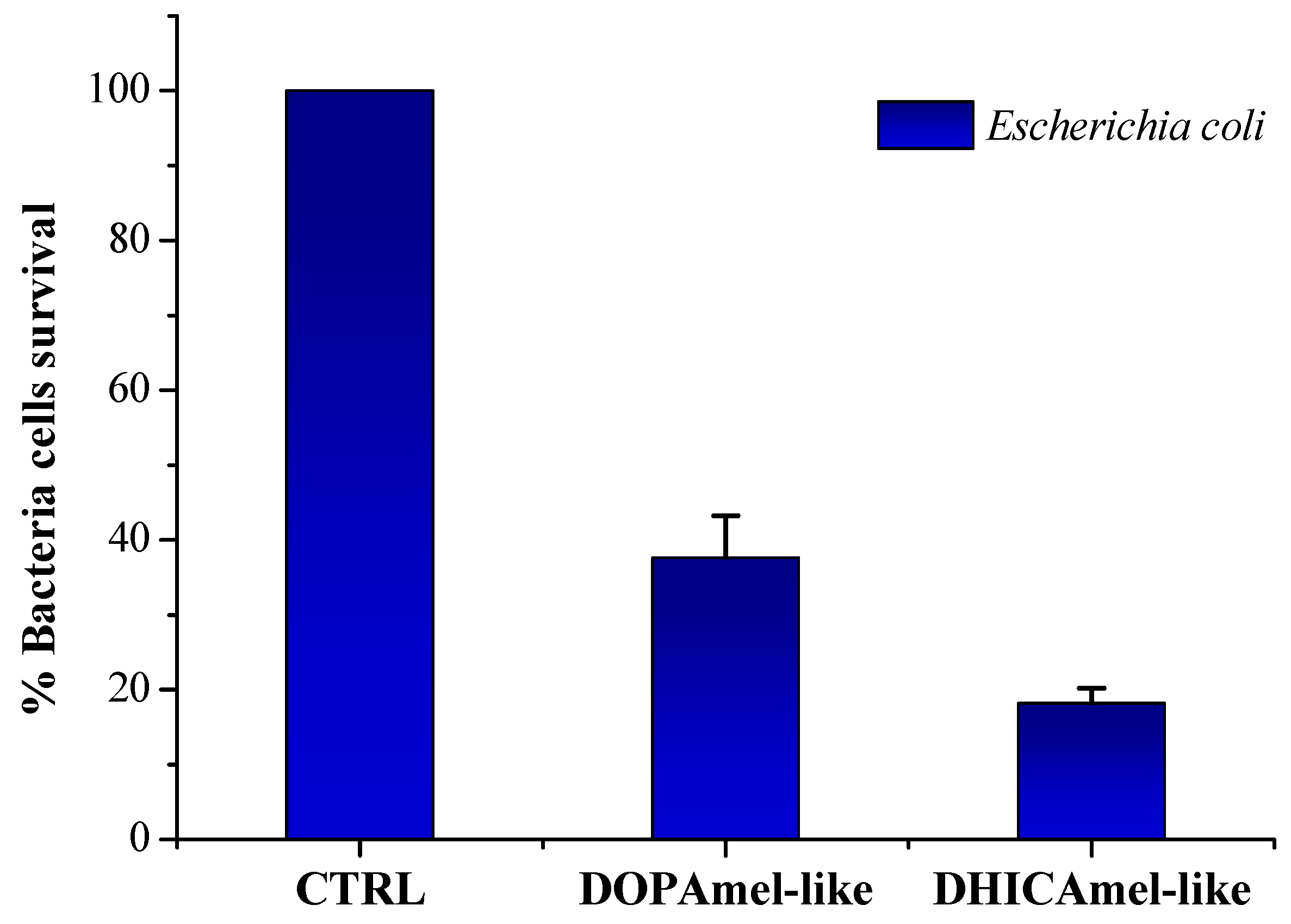
2.5. Antimicrobial Assays
3. Results and Discussion
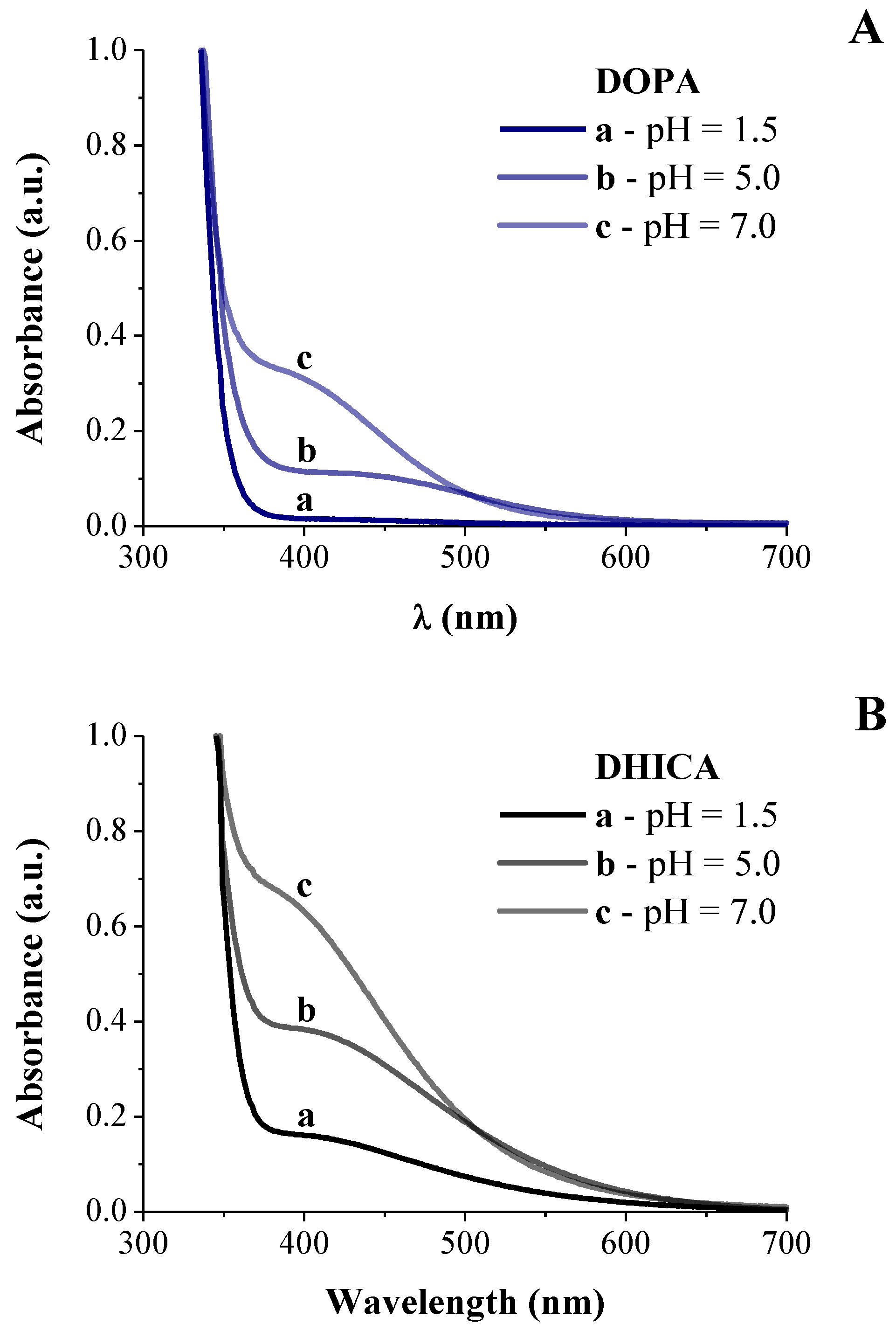
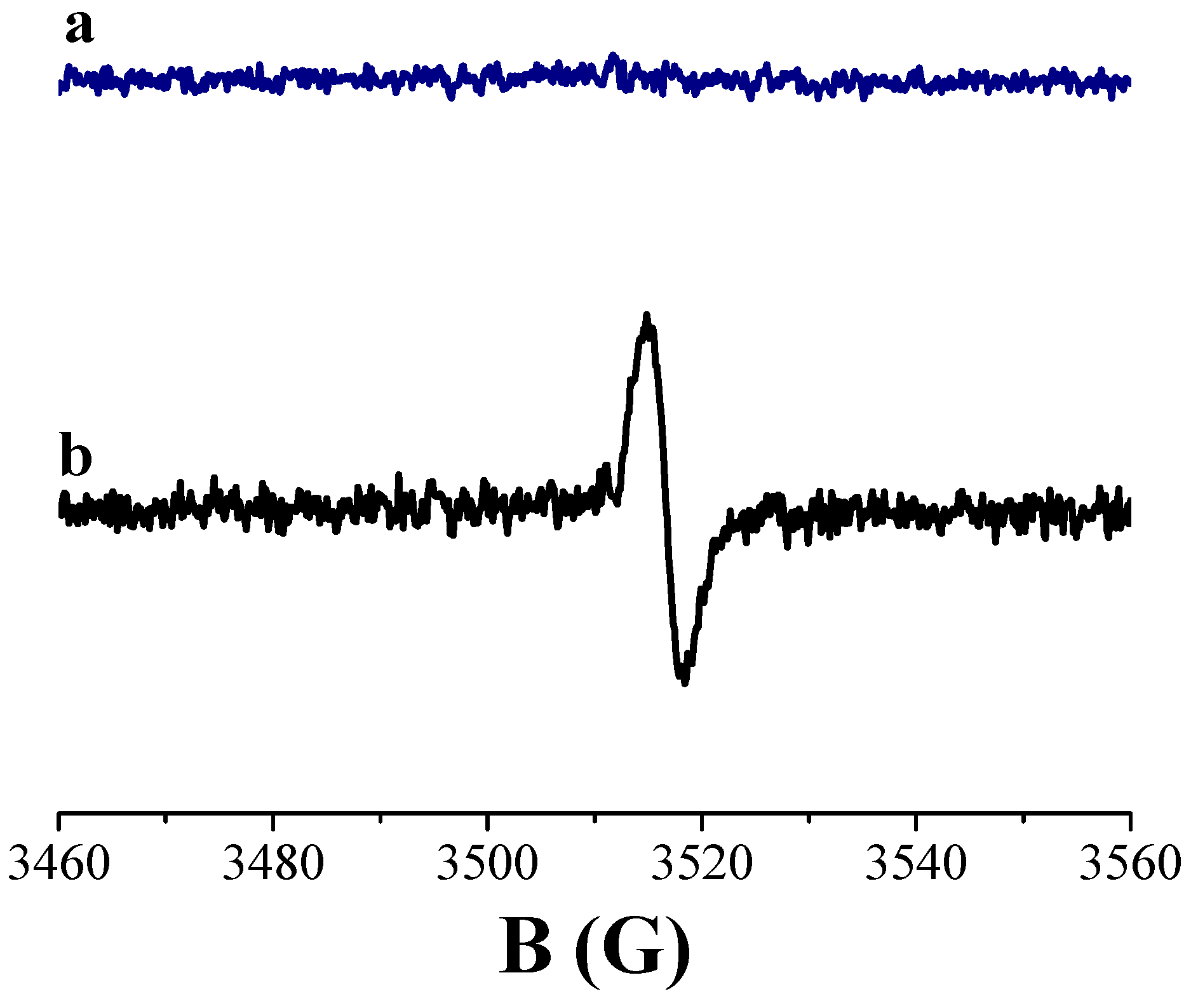
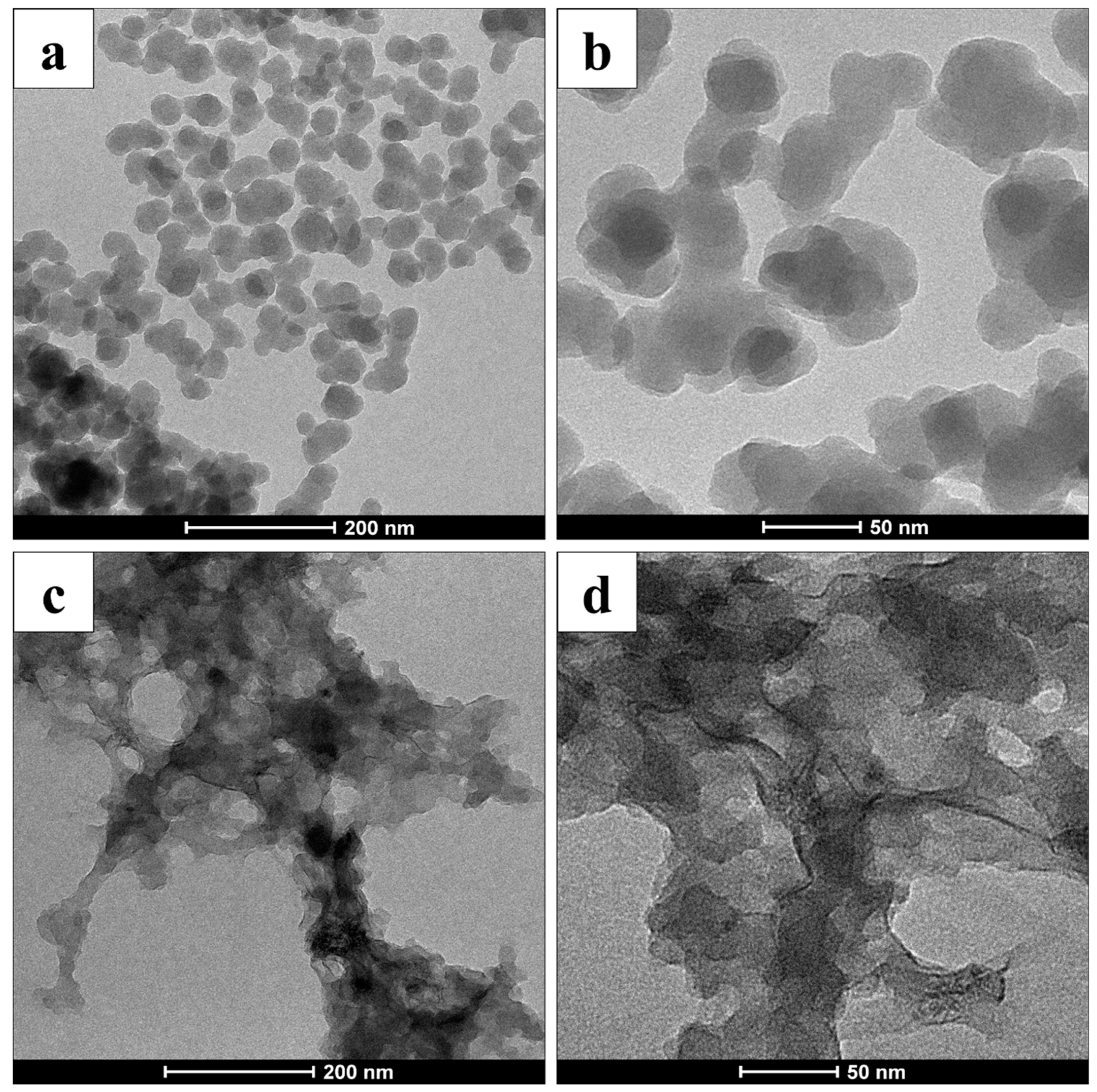
3.1. Spectroscopic Investigation on Hybrid Melanin-Like Nanostructures
3.2. Antimicrobial Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, H.; Nishida, J.; Ma, W.; Wu, H.; Kobayashi, M.; Otsuka, H.; Takahara, A. Competition between oxidation and coordination in cross-linking of polystyrene copolymer containing catechol groups. ACS Macro Lett. 2012, 1, 457–460. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Zhu, W.; Peck, Y.; Iqbal, J.; Wang, D.-A. A novel DOPA-albumin based tissue adhesive for internal medical applications. Biomaterials 2017, 147, 99–115. [Google Scholar] [CrossRef]
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G.; et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef]
- Ahn, B.K. Perspectives on Mussel-Inspired Wet Adhesion. J. Am. Chem. Soc. 2017, 139, 10166–10171. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Weng, H.; Gyawali, D.; Tang, L.; Yang, J.; Mehdizadeh, M.R. Injectable Citrate-Based Mussel-Inspired Tissue Bioadhesives With High Wet Strength for Sutureless Wound Closure. Biomaterials 2012, 33, 7972–7983. [Google Scholar] [CrossRef]
- Nighswander-Rempel, S.P.; Riesz, J.; Gilmore, J.; Bothma, J.P.; Meredith, P. Quantitative Fluorescence Excitation Spectra of Synthetic Eumelanin. J. Phys. Chem. B 2005, 109, 20629–20635. [Google Scholar] [CrossRef]
- Zhao, P.; Li, J.; Wang, Y.; Jiang, H. Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochem. Mol. Biol. 2007, 37, 952–959. [Google Scholar] [CrossRef]
- Zanfardino, A.; Costantini, A.; Luciani, G.; Silvestri, B.; Vitiello, G.; Pezzella, A.; Varcamonti, M.; Branda, F. Titania as a driving agent for DHICA polymerization: a novel strategy for the design of bioinspired antimicrobial nanomaterials. J. Mater. Chem. B 2015, 3, 2808–2815. [Google Scholar]
- Caputo, G.; Bonadies, I.; Migliaccio, L.; Caso, M.F.; Pezzella, A. Eumelanin Coating of Silica Aerogel by Supercritical Carbon Dioxide Deposition of a 5,6-Dihydroxyindole Thin Film. Materials 2018, 11, 1494. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Zanfardino, A.; Silvestri, B.; Giudicianni, P.; Costantini, A.; Varcamonti, M.; Branda, F.; Luciani, G. Antimicrobial activity of eumelanin-based hybrids: The role of TiO2 in modulating the structure and biological performance. Mater. Sci. Eng. C 2017, 75, 454–462. [Google Scholar] [CrossRef]
- Vitiello, G.; Zanfardino, A.; Tammaro, O.; Di Napoli, M.; Caso, M.F.; Pezzella, A.; Varcamonti, M.; Silvestri, B.; D’Errico, G.; Costantini, A.; et al. Bioinspired hybrid eumelanin–TiO2 antimicrobial nanostructures: The key role of organo–inorganic frameworks in tuning eumelanin’s biocide action mechanism through membrane interaction. RSC Adv. 2018, 8, 28275–28283. [Google Scholar] [CrossRef]
- Silvestri, B.; Vitiello, G.; Luciani, G.; Calcagno, V.; Costantini, A.; Gallo, M.; Parisi, S.; Paladino, S.; Iacomino, M.; D’Errico, G.; et al. Probing the Eumelanin–Silica Interface in Chemically Engineered Bulk Hybrid Nanoparticles for Targeted Subcellular Antioxidant Protection. ACS Appl. Mater. Interfaces 2017, 9, 37615–37622. [Google Scholar] [CrossRef]
- Vitiello, G.; Pezzella, A.; Calcagno, V.; Silvestri, B.; Raiola, L.; D’Errico, G.; Costantini, A.; Branda, F.; Luciani, G. 5,6-Dihydroxyindole-2-carboxylic Acid–TiO2 Charge Transfer Complexes in the Radical Polymerization of Melanogenic Precursor(s). J. Phys. Chem. C 2016, 120, 6262–6268. [Google Scholar] [CrossRef]
- Guo, J.; Wang, W.; Hu, J.; Xie, D.; Gerhard, E.; Nisic, M.; Shan, D.; Qian, G.; Zheng, S.; Yang, J. Synthesis and characterization of anti-bacterial and anti-fungal citrate-based mussel-inspired bioadhesives. Biomaterials 2016, 85, 204–217. [Google Scholar] [CrossRef]
- Yang, J.; Webb, A.R.; Ameer, G.A. Novel Citric Acid-Based Biodegradable Elastomers for Tissue Engineering. Adv. Mater. 2004, 16, 511–516. [Google Scholar] [CrossRef]
- Su, L.-C.; Xie, Z.; Zhang, Y.; Nguyen, K.T.; Yang, J. Study on the Antimicrobial Properties of Citrate-Based Biodegradable Polymers. Front. Bioeng. Biotechnol. 2014, 2, 23. [Google Scholar] [CrossRef]
- Pezzella, A.; Vogna, D.; Prota, G. Synthesis of optically active tetrameric melanin intermediates by oxidation of the melanogenic precursor 5,6-dihydroxyindole-2-carboxylic acid under biomimetic conditions. Tetrahedron Asymmetry 2003, 14, 1133–1140. [Google Scholar] [CrossRef]
- Edge, R.; D’Ischia, M.; Land, E.J.; Napolitano, A.; Navaratnam, S.; Panzella, L.; Pezzella, A.; Ramsden, C.A.; Riley, P.A. Dopaquinone redox exchange with dihydroxyindole and dihydroxyindole carboxylic acid. Pigment. Cell Res. 2006, 19, 443–450. [Google Scholar] [CrossRef]
- Pezzella, A.; Capelli, L.; Costantini, A.; Luciani, G.; Tescione, F.; Silvestri, B.; Vitiello, G.; Branda, F. Towards the development of a novel bioinspired functional material: Synthesis and characterization of hybrid TiO2/DHICA-melanin nanoparticles. Mater. Sci. Eng. C 2013, 33, 347–355. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Sannino, F.; Vitiello, G.; Pirozzi, D.; Minieri, L.; Aronne, A.; Pernice, P.; Pavone, M.; D’Errico, G. Origin and Electronic Features of Reactive Oxygen Species at Hybrid Zirconia-Acetylacetonate Interfaces. ACS Appl. Mater. Interfaces 2015, 7, 21662–21667. [Google Scholar] [CrossRef]
- Califano, V.; Sannino, F.; Costantini, A.; Avossa, J.; Cimino, S.; Aronne, A. Wrinkled Silica Nanoparticles: Efficient Matrix for β-Glucosidase Immobilization. J. Phys. Chem. C 2018, 122, 8373–8379. [Google Scholar] [CrossRef]
- Branda, F.; Malucelli, G.; Durante, M.; Piccolo, A.; Mazzei, P.; Costantini, A.; Silvestri, B.; Pennetta, M.; Bifulco, A.; Boudenne, A. Silica Treatments: A Fire Retardant Strategy for Hemp Fabric/Epoxy Composites. Polymers 2016, 8, 313. [Google Scholar] [CrossRef]
- Matzapetakis, M.; Raptopoulou, C.P.; Tsohos, A.; Papaefthymiou, V.; Moon, N.; Salifoglou, A. Synthesis, Spectroscopic and Structural Characterization of the First Mononuclear, Water Soluble Iron−Citrate Complex, (NH4)5Fe(C6H4O7)2⋅2H2O. J. Am. Chem. Soc. 1998, 120, 13266–13267. [Google Scholar] [CrossRef]
- Panzella, L.; D’Errico, G.; Vitiello, G.; Perfetti, M.; Alfieri, M.L.; Napolitano, A.; D’Ischia, M.; D’Errico, G.; D’Ischia, M. Disentangling structure-dependent antioxidant mechanisms in phenolic polymers by multiparametric EPR analysis. Chem. Commun. 2018, 54, 9426–9429. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Luchini, A.; D’Errico, G.; Szekely, N.K.; D’Ischia, M.; Napolitano, A.; Vitiello, G.; Paduano, L. Tris Buffer Modulates Polydopamine Growth, Aggregation, and Paramagnetic Properties. Langmuir 2014, 30, 9811–9818. [Google Scholar] [CrossRef]
- Panzella, L.; Gentile, G.; D’Errico, G.; Della Vecchia, N.F.; Errico, M.E.; Napolitano, A.; Carfagna, C.; D’Ischia, M.; Errico, M. Atypical Structural and π-Electron Features of a Melanin Polymer that Lead to Superior Free-Radical-Scavenging Properties. Angew. Chem. Int. Ed. 2013, 52, 12684–12687. [Google Scholar] [CrossRef]
- Cesareo, E.; Korkina, L.; D’Errico, G.; Vitiello, G.; Aguzzi, M.S.; Passarelli, F.; Pedersen, J.Z.; Facchiano, A. An Endogenous Electron Spin Resonance (ESR) Signal Discriminates Nevi from Melanomas in Human Specimens: A Step Forward in Its Diagnostic Application. PLoS ONE 2012, 7, e48849. [Google Scholar] [CrossRef][Green Version]
- Khade, G.V.; Suwarnkar, M.B.; Gavade, N.L.; Garadkar, K.M. Green synthesis of TiO2 and its photocatalytic activity. J. Mater. Sci. Mater. Med. 2015, 26, 3309–3315. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Ozeki, H. Spectrophotometric Assay of Eumelanin in Tissue Samples. Analyt. Biochem. 1993, 215, 273–277. [Google Scholar] [CrossRef]
- D’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; García-Borrón, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef]
- Panzella, L.; Szewczyk, G.; d’Ischia, M.; Napolitano, A.; Sarna, T. Zinc-induced structural effects enhance oxygen consumption and superoxide generation in synthetic pheomelanins on UVA/visible light irradiation. Photochem. Photobiol. 2010, 86, 757–764. [Google Scholar] [CrossRef]
- Mostert, A.B.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Powell, B.J.; Meredith, P. Hydration-Controlled X-Band EPR Spectroscopy: A Tool for Unravelling the Complexities of the Solid-State Free Radical in Eumelanin. J. Phys. Chem. B 2013, 117, 4965–4972. [Google Scholar] [CrossRef]
- Micillo, R.; Iacomino, M.; Perfetti, M.; Panzella, L.; Koike, K.; D’Errico, G.; D’Ischia, M.; Napolitano, A. Unexpected impact of esterification on the antioxidant activity and (photo)stability of a eumelanin from 5,6-dihydroxyindole-2-carboxylic acid. Pigment Cell Melanoma Res. 2018, 31, 475–483. [Google Scholar] [CrossRef]
- Zadlo, A.; Szewczyk, G.; Sarna, M.; Camenisch, T.G.; Sidabras, J.W.; Ito, S.; Wakamatsu, K.; Sagan, F.; Mitoraj, M.; Sarna, T. Photobleaching of pheomelanin increases its phototoxic potential: Physicochemical studies of synthetic pheomelanin subjected to aerobic photolysis. Pigment Cell Melanoma Res. 2019, 32, 359–372. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Grassian, V.H. Citric Acid Adsorption on TiO2 Nanoparticles in Aqueous Suspensions at Acidic and Circumneutral pH: Surface Coverage, Surface Speciation, and Its Impact on Nanoparticle−Nanoparticle Interactions. J. Am. Chem. Soc. 2010, 132, 14986–14994. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Kundu, S.; Naskar, M.K. Effect of organic acids on the physicochemical properties of titania and its photodegradation efficiency of methyl orange. J. Phys. Chem. Solids 2018, 121, 367–374. [Google Scholar] [CrossRef]
- Patra, A.K.; Dutta, A.; Bhaumik, A.; Patra, D.A.K. Self-assembled mesoporous γ-Al2O3 spherical nanoparticles and their efficiency for the removal of arsenic from water. J. Hazard. Mater. 2012, 201, 170–177. [Google Scholar] [CrossRef]
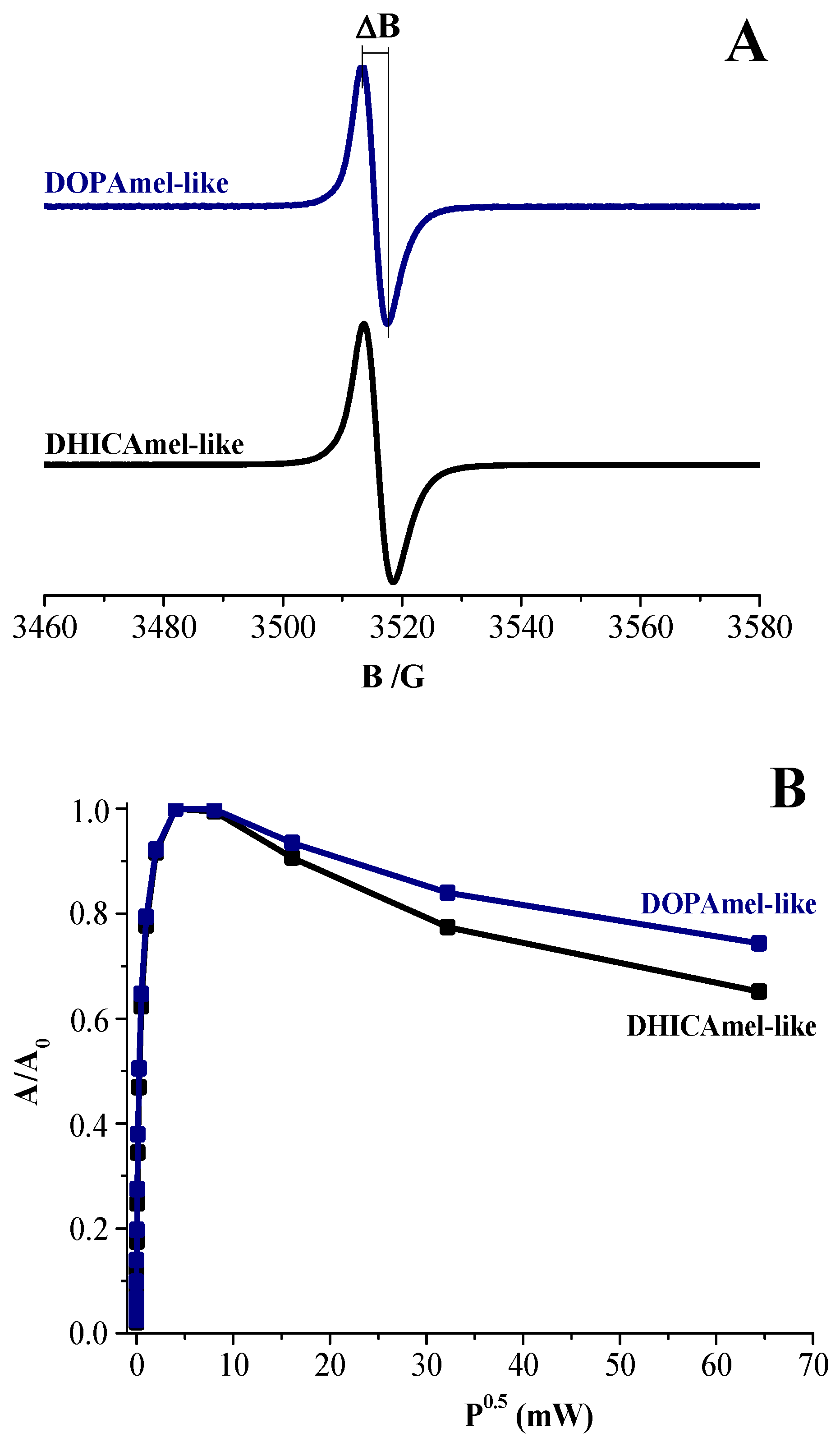
| Samples | g-Factor | ΔB | Spin-Density (×1019 spin/g) |
|---|---|---|---|
| DOPAmel-like | 2.0030 | 4.2 | 0.065 |
| DHICAmel-like | 2.0032 | 4.9 | 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melone, P.; Vitiello, G.; Di Napoli, M.; Zanfardino, A.; Caso, M.F.; Silvestri, B.; Varcamonti, M.; D’Errico, G.; Luciani, G. Citric Acid Tunes the Formation of Antimicrobial Melanin-Like Nanostructures. Biomimetics 2019, 4, 40. https://doi.org/10.3390/biomimetics4020040
Melone P, Vitiello G, Di Napoli M, Zanfardino A, Caso MF, Silvestri B, Varcamonti M, D’Errico G, Luciani G. Citric Acid Tunes the Formation of Antimicrobial Melanin-Like Nanostructures. Biomimetics. 2019; 4(2):40. https://doi.org/10.3390/biomimetics4020040
Chicago/Turabian StyleMelone, Pietro, Giuseppe Vitiello, Michela Di Napoli, Anna Zanfardino, Maria Federica Caso, Brigida Silvestri, Mario Varcamonti, Gerardino D’Errico, and Giuseppina Luciani. 2019. "Citric Acid Tunes the Formation of Antimicrobial Melanin-Like Nanostructures" Biomimetics 4, no. 2: 40. https://doi.org/10.3390/biomimetics4020040
APA StyleMelone, P., Vitiello, G., Di Napoli, M., Zanfardino, A., Caso, M. F., Silvestri, B., Varcamonti, M., D’Errico, G., & Luciani, G. (2019). Citric Acid Tunes the Formation of Antimicrobial Melanin-Like Nanostructures. Biomimetics, 4(2), 40. https://doi.org/10.3390/biomimetics4020040










