2. Materials and Methods
2.1. AN-EXTRA-Push
AN-EXTRA-Push is a biomimetically inspired ankle exo intended for gait rehabilitation for people with neuromuscular impairments (e.g., post stroke), made to be used on a treadmill in a clinical environment. The concept for the device was first introduced in [
14], where we tested its feasibility in silico. It was later further iteratively developed in [
15,
16,
17]. Here, we give a brief overview of the latest version of our exo.
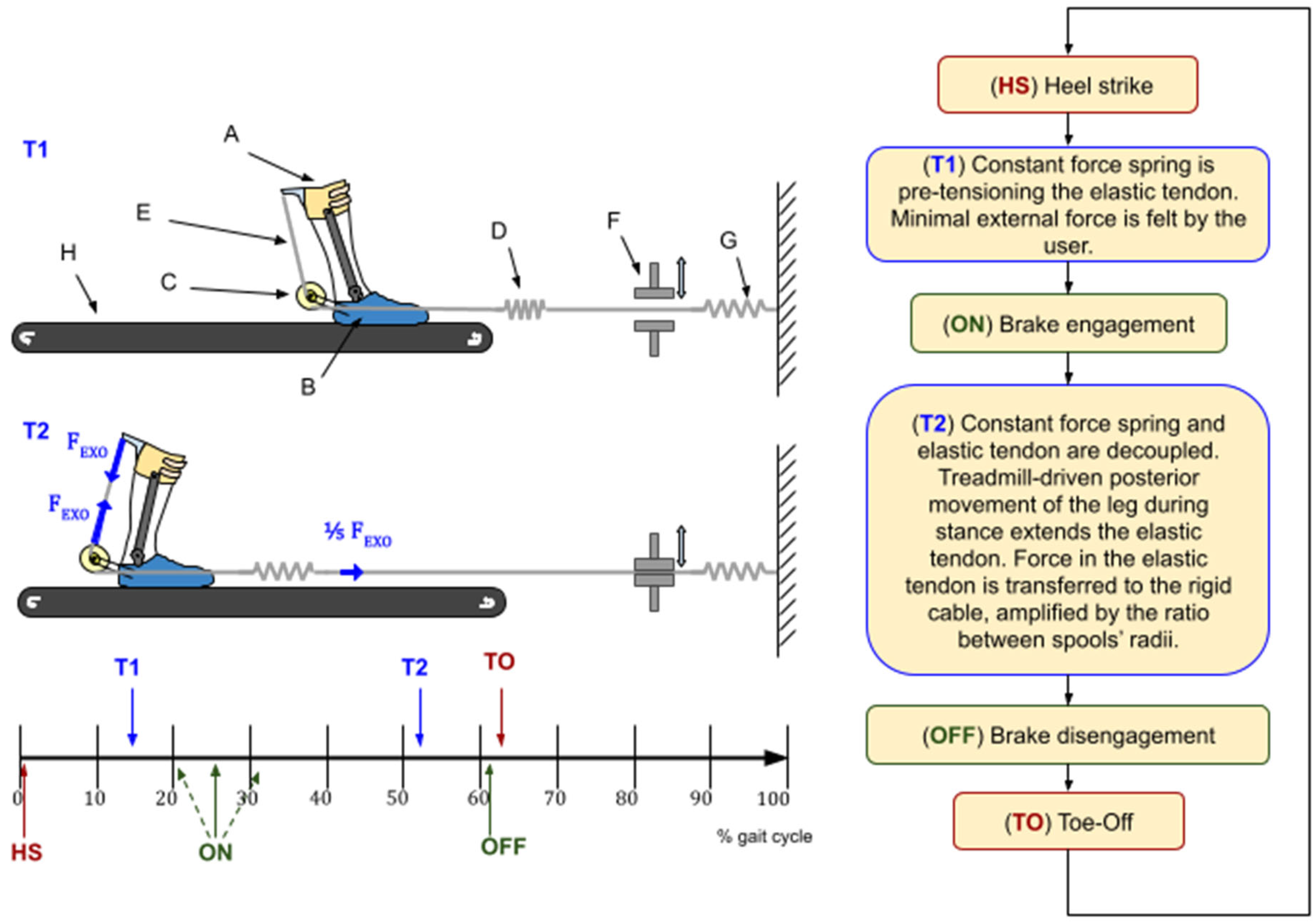
AN-EXTRA-Push consists of an orthosis (parts A, B, and C in
Figure 1) worn by the user, a braking and pretensioning mechanism (parts F and G in
Figure 1) located in front of the treadmill (H), and an elastic tendon (D and E in
Figure 1) connecting the two. The orthosis has one degree of freedom. Its rotary joint is aligned as best as possible with the biological ankle joint sagittal plane rotation axis.
On one end, the elastic tendon (D) is routed through an electromechanical brake (F) and connected to a wall with an additional constant force spring (G) in series that prevents the elastic tendon from going slack. The other end of the tendon is split in two and wound on the outer pair of a trio of concentric spools (C) at the rear end of the orthosis. The three spools form a rigid structure. They rotate around their common axis. A rigid cable is wound around the middle spool and connected to the shank part of the orthosis (A). A pulling force in the elastic tendon (D) is transferred to the rigid cable (E) via the concentric spools (C), causing the shank (A) and foot (B) parts of the orthosis to be pulled together, creating torque at the rotary joint. Spools in C have different radii, defining the force transmission ratio, explained in detail in [
16].
The total weight of the parts of the device that were worn by the user was 1.45 kg. For users weighing over 72.5 kg, this does not exceed the 2% body weight threshold given in the literature [
18,
19], which marks the point where the gait pattern is significantly affected by the added weight at the ankle.
The device was designed to imitate the combined function of the soleus muscle and the Achilles tendon [
16]. The normal AN-EXTRA-Push operation is summarized in a flowchart in
Figure 1. Two representative moments (T1 and T2) during the gait cycle are depicted in the top left portion of this figure. During the swing phase, the brake is disengaged, and only a compliant constant force spring pulls on the tendon to keep it taut. During stance, we engage the brake. Posterior movement of the leg by the treadmill combined with ankle dorsiflexion stretches the elastic tendon, storing elastic energy, building up force, and consequently building plantarflexion torque. Peak force in the tendon occurs right before push-off. After push-off initiation, the ankle plantarflexes rapidly, rapidly releasing the stored energy in a burst of power. The brake is then disengaged, making it ready for the next cycle. Details about when the brake engagement/disengagement should occur were explored in silico in [
14] and confirmed in preliminary experiments.
2.2. Study Participants
Twelve healthy adults (all male, age: 27.3 ± 0.9 years, height: 178.1 ± 5.1 cm, weight: 80.7 ± 7.9 kg (mean ± SD), 7 wore EU size 42 shoes, 5 wore EU size 44 shoes, all naïve subjects) participated in this study. All participants provided informed consent. This study was approved by the National Medical Ethics Committee of the Republic of Slovenia.
Due to a mechanical malfunction of the device, measurements recorded for Subject 2 were corrupted and thus excluded from the results.
2.3. Study Protocol
Participants wore AN-EXTRA-Push on their left leg. They walked under 11 different experimental conditions: (1) ZeroTorque—wearing only the additional weight of the exo while untethered, (2) Transparent—system pre-tensioned never engaged (exo connected to constant spring via inelastic rope), and (3–11) Active assistance conditions comprised of all combinations of three brake engagement timings (Early = 20.0% GC, Middle = 25.7% GC, Late = 31.4% GC) and three elastic tendon stiffnesses (k1 = 33 N/m, k2 = 67 N/m, k3 = 105 N/m), e.g., Middle_k3. The stiffness levels were the same for all participants and were not scaled to their body weight. During preliminary testing, walking without the exo was compared to the ZeroTorque condition. Only minor differences at the knee joint were present, which we could best explain just by the added weight at the ankle. An experimental condition of walking without the exo was therefore not tested in this study to avoid the additional donning/doffing time and differences in added weight between conditions.
Throughout the entire experiment, treadmill speed was set to 0.8 m/s. While substantially lower than the normal preferred walking speed [
20], it was chosen to allow for closer future comparisons to gaits of people with neuromuscular impairments (e.g., stroke [
21]).
The participants familiarized themselves with the setup, first by walking on a treadmill for 10 min without the exo, then 4 min in the ZeroTorque condition, 2 min in the Transparent condition, and finally 4 min with active assistance in the Middle_k2 condition (
Figure 2a).
Familiarization was followed immediately by measurements. Experimental conditions were distributed in a pseudo-random order (
Figure 2c). The ZeroTorque condition was always measured first and repeated again as the last condition. Other experimental conditions were randomly interspersed with conditions that feature the same tendon stiffness always grouped together to minimize interruptions, since the stiffness was adjusted with a manual intervention. Each experimental condition lasted 2 min, followed by 30 s of walking with the brake off (equal to the Transparent condition) to allow the participants to return to their usual gait pattern before continuing.
2.4. Biomechanics and EMG Measurements
Sixteen reflective markers were placed onto the participants’ bodies in accordance with the Plug-In Gait lower body protocol [
22]. Their position was tracked at 120 Hz using the Optitrack six-camera motion capture system (NaturalPoint, Inc., Corvallis, OR, USA). The raw data were first filtered using a 10 Hz low-pass filter in Motive (NaturalPoint, Inc., Corvallis, OR, USA) and then merged with the subjects’ anthropometric data in a custom Python 3.11 script to calculate direct kinematics.
During this study, subjects walked on a custom-built split-belt treadmill. The left belt, on which they walked with their leg wearing the exo, was instrumented using four triaxial force transducers (K3D120, ME Systeme GmbH, Hennigsdorf, Germany). Ground reaction force and center of pressure data were measured at 250 Hz. The right belt served as an auxiliary walking surface and was not instrumented. The custom treadmill setup carried certain limitations, further discussed in the Discussion section.
AN-EXTRA-Push was equipped with a U9C force sensor (HBM, Darmstadt, Germany). It measured the force in the rigid cable at the back of the orthosis, which equals five times the force in the elastic tendon. The force measurement was performed at 250 Hz. We used a Beckhoff CX5020 industrial computer (Beckhoff Automation, Verl, Germany) to record the force data from the treadmill and the back of AN-EXTRA-Push.
Muscle activity of m. soleus, m. gastrocnemius lateralis, m. tibialis anterior, m. vastus medialis, m. biceps femoris, and m. rectus femoris of both legs was measured using surface electromyography (EMG) (TelemyoMini 16, Neurodata, Vienna, Austria). We used 3M Red Dot 2560 (3M, Saint Paul, MN, USA) electrodes (interelectrode distance: 4 mm). EMG data were recorded at 1 kHz. An example of electrode and reflective marker placements can be seen in
Figure 2b.
2.5. Data Processing
The Newton–Euler inverse dynamics algorithm was used to calculate torques and powers at the ankle, knee, and hip of the left leg. The use of AN-EXTRA-Push was accounted for by recalculating the mass and inertia matrix of the shank and foot segments. The force measurement in the rigid cable at the back of the orthosis was used to calculate the additional external torque in the sagittal plane at the ankle joint and the external force of the elastic tendon pulling on the orthosis in the direction of the brake. The contributions of AN-EXTRA-Push towards the total kinetics were then subtracted from the total to calculate the biological contributions. Spatiotemporal parameters (e.g., stride length, stance phase time, etc.) were also calculated for every measurement.
Muscle excitation signals were calculated from raw EMG signals by first manually removing the movement artifacts and outliers, then filtering with a bandpass (20–140 Hz) and notch (49–51 Hz) filter, full-wave rectifying, and finally calculating the envelopes using moving average (window width: 150 ms). For each participant, signals were then normalized to the peak value of the average stride in the ZeroTorque condition for each muscle.
All data processing was performed in Python. We synced up the kinematics, kinetics, and muscle excitations, then segmented the data into gait cycles. Measurements from the ZeroTorque condition, which was the only condition we repeated, were merged into a single file so that the steps from each measurement instance alternated.
Only 20 strides (from the 20th to 39th) from each walking bout were used for further analysis to ensure only steady-state walking was considered.
2.6. Statistics
Biomechanically interesting scalar features were extracted from timeseries data for joint angles, torques, powers, and muscle excitations. Those included peak and trough values of time series, as well as signal integrals. When applicable, only parts of the gait cycle were used for feature extraction: 0 to 55% of GC is labeled stance phase, and 30 to 50% of GC is labeled midstance, even though the actual phase transitions vary stride-to-stride and subject-to-subject. Similarly, only positive or negative torque and power values are integrated where applicable (e.g., ankle power, where increases in both positive and negative power might cancel out over the GC).
To objectively report the synchrony between ankle exo assistance and the user’s own biological efforts, we defined a new metric. We calculated the time lag between the peak biological ankle torque and power and peak exo-assistive torque and power, respectively. For well-synchronized exo assistance and healthy-like shapes of the biological torque and power trajectories, the time lag should be close to zero. This metric does not consider the shape of the assistive torque and power; however, it still gives a quick and simple way to compare the success of synchronizing the exo to the biological musculotendon system. Despite the well-known importance of synchrony, we have not encountered any such measure in the literature.
We compared the characteristic scalar features across all eleven experimental conditions. We assessed differences using a one-way repeated measures ANOVA, with a significance threshold of α = 0.05.
To evaluate the sphericity assumption, we performed Mauchly’s test. If sphericity was violated, we applied the Greenhouse–Geisser correction to adjust the degrees of freedom and preserve the validity of the ANOVA results.
Whenever the ANOVA indicated a significant effect of condition, we conducted pairwise t-tests for all condition pairs. To mitigate the risk of false positives from multiple comparisons, we controlled the false discovery rate by adjusting p-values with a Benjamini–Hochberg correction.
Our highly anthropometrically homogenous study cohort is the consequence of shoe size limitations of AN-EXTRA-Push, which we address in detail in the limitations subsection of the Discussion. Additionally, the Intersubject Variability Subsection was added to the Results Section, where differences between the subjects’ gaits are objectively evaluated.
In the next section, only a selected portion of statistical results are presented. All the RM ANOVA and post hoc test results are compiled in the
Supplementary File S2.
When summarizing the results of post hoc tests in this text, we sometimes group the conditions with respect to their common stiffness level (k1, k2, or k3) or brake engagement timing (later abbreviated as “timing”; Early, Middle, or Late). References to groups are established for the sake of brevity of reporting—all conditions are treated as separate during statistical analysis.
4. Discussion
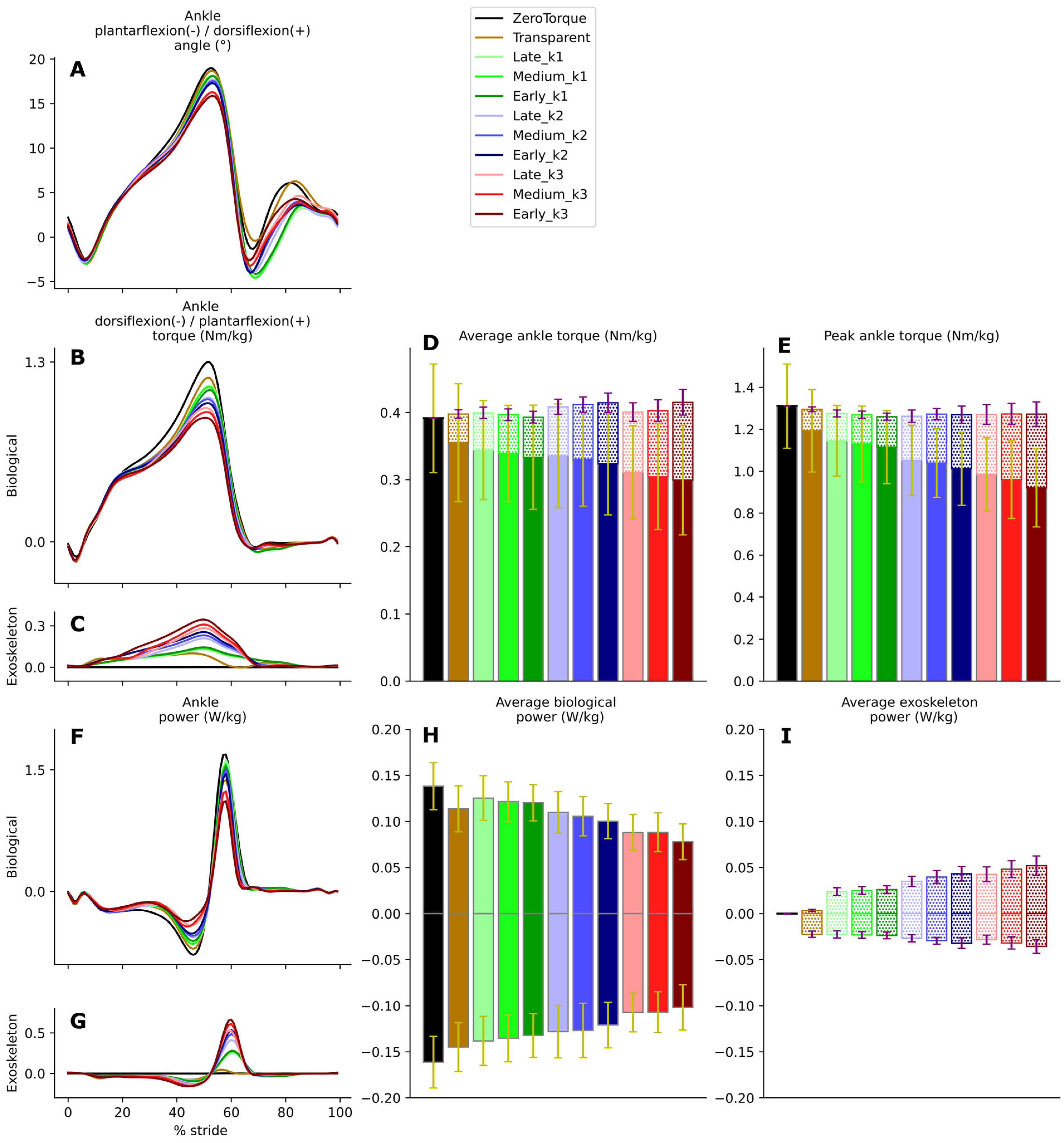
The key result validating that the AN-EXTRA-Push was performing its primary intended function is found in
Figure 3, showing that the exo was able to provide an adjustable level of ankle plantarflexion assistance based on the choices of brake engagement timing and elastic tendon stiffness values. Depending on the experimental condition, exo torque peaks ranged from 10.3% to 28.5% of total ankle plantarflexion torque peaks at the extremes, with the other condition spaced in between. In a clinical use case, the therapist could utilize this range to set the appropriate assistance level of training to follow the assist-as-needed paradigm in rehabilitation. The tested tendon stiffness values had a larger effect on the assistance level of the exo compared to the brake engagement timings, which can be consistently observed across most tests in our statistical analysis.
With the increased exo contribution, the biological contribution was reduced in equal measure, leading to a largely unchanged total ankle torque. The peak biological ankle plantarflexion torque reduction due to external assistance in our study was 28.5%. For comparison, the peak biological ankle plantarflexion torque reduction observed in a study featuring healthy participants using an exo with a human-in-the-loop optimized controller was 31% [
7]. This reduction was achieved after multiple days of individualized controller HILO and user adaptation [
23]. Further increases in assistive torque levels did not lead to additional metabolic cost savings, which was the primary optimization criterion. With a generic assistive torque trajectory, developed in a pilot study leading up to studies [
7,
23], the peak biological torque reduction was 28%. Similar peak values were reported in other literature primarily focused on human augmentation ([
24,
25,
26,
27], in one case even reaching 47% [
28], but that demanded significant changes in joint kinematics).
Among the devices focused on rehabilitation use cases, reported assistive torque values are lower: in a study by Orekhov et al. [
29], only 0.03 Nm/kg of assistive torque provided with an active ankle exo to patients with cerebral palsy was enough to promote higher gait speed and lower the soleus muscle activation. The device used was battery powered, weighing 2.07 kg, with the majority of the weight accounted for by the battery pack strapped to the user’s waist.
An example of an exo aimed at stroke rehabilitation can be found in [
30]. Awad et al. created a soft robotic exosuit capable of assisting with ankle plantarflexion and dorsiflexion torque. The battery-powered Bowden cable-based device weighed 3.1 kg. The majority of the weight was worn at the waist, with about 0.9 kg distributed down the leg. Using this exosuit, stroke survivors walked with improved symmetry, lower metabolic energy consumption, and a more healthy-like gait pattern. These results were achieved at only 0,15 Nm/kg (i.e., 12% total) assistive plantarflexion ankle torque.
In this study, AN-EXTRA-Push users modified their gait pattern to accommodate for the added exo plantarflexion torque. Changes in kinematics that we observed closely resemble those reported in the literature, where they were put under similar external torque conditions. During the stance phase, increased plantarflexion torque generally resulted in decreased ankle dorsiflexion angle [
31,
32,
33,
34]. After push-off, ankle plantarflexion angle was generally increased [
31,
32,
33,
35]. At other joints, kinematics changes were minor, if at all noticeable [
7,
24,
27,
31,
32,
33,
34,
35].
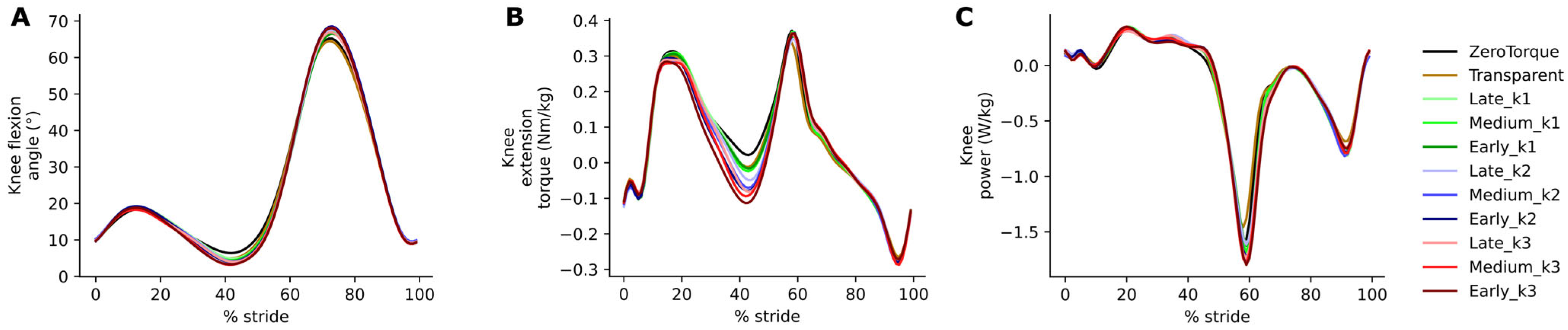
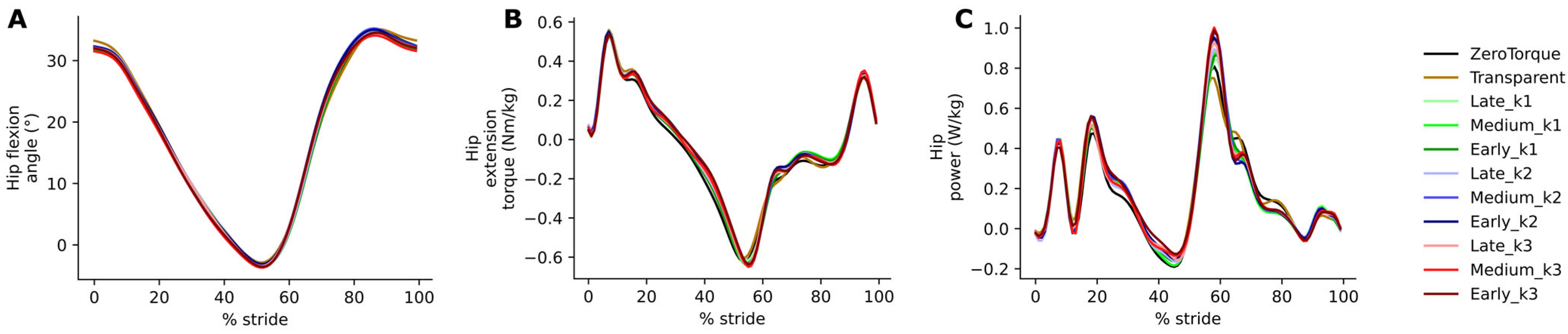
The effects of AN-EXTRA-Push assistance were also almost entirely isolated, as intended by the design, to the ankle joint of the leg equipped with the exo. No changes were observed at the hip. Study participants walked with slightly more extended knees on both legs during the stance phase. On the side equipped with the exo, additional knee flexion during early parts of the swing was also observed, likely to compensate for the slight shift in ankle angle towards plantarflexion and still ensure sufficient toe clearance.
The move towards increased knee extension is likely explained by the well-known ankle plantarflexion–knee extension coupling [
36,
37]. During stance, the external plantarflexion torque at the ankle results in tibia rotation since the foot is constrained by the treadmill surface and cannot move. The change is likely mirrored on the contralateral side by the users to maintain symmetry.
Ankle exos from the literature usually initiate assistance later on in the stance phase and therefore do not encounter this phenomenon. A previously mentioned rare example of an active exo by Awad et al., where earlier assistance starts were investigated, can be found in [
30]. For some stroke survivors that participated in that study, earlier assistance helped improve their gait pattern, while for others it had the opposite effect.
With the added external plantarflexion torque, AN-EXTRA-Push provided additional power to the ankle joint—negative during early and midstance and positive during push-off. The total power integrated over the gait cycle remained largely unchanged. Study participants utilized the additional external power of the exo to reduce their own biological power generation. These observations are consistent with the literature [
4,
7,
27,
31,
32,
33,
34].
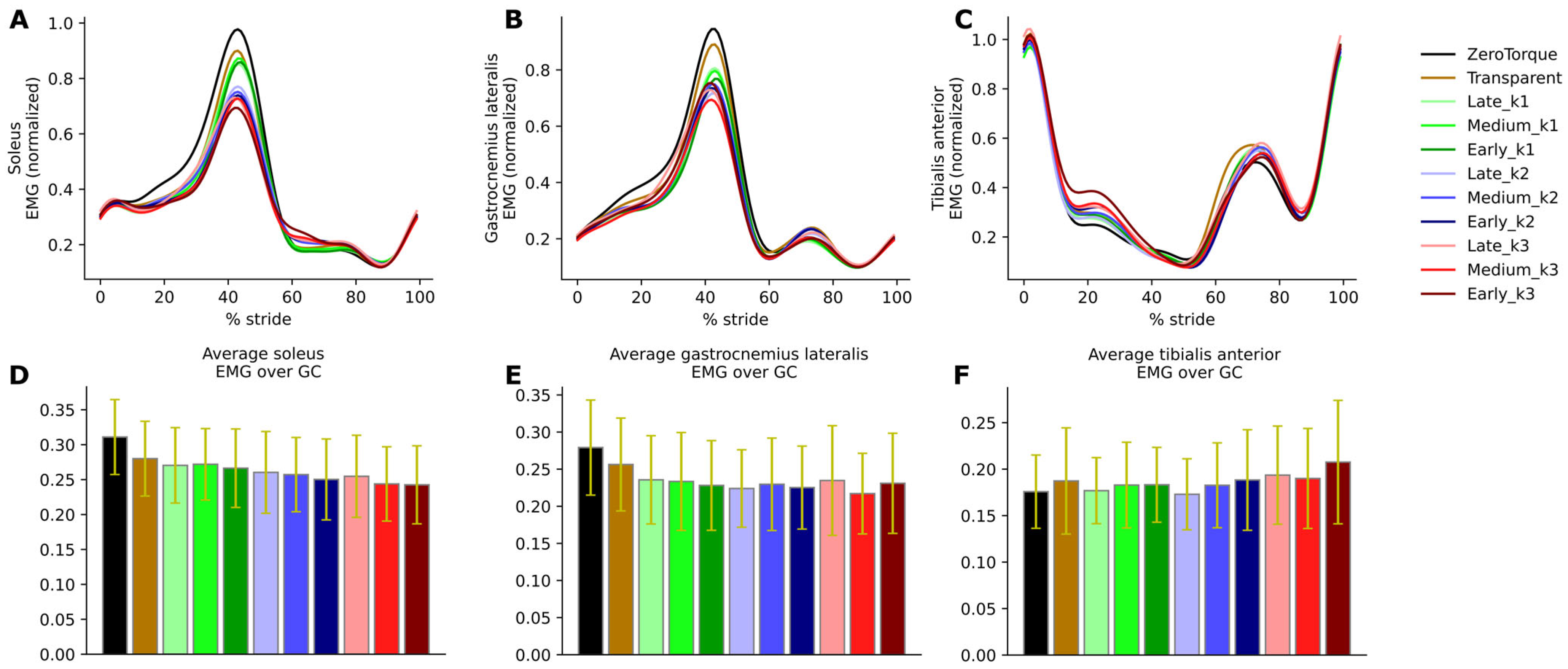
Observed changes in ankle biomechanics are also well explained by our EMG measurement results. Soleus muscle excitation was reduced in proportion to the AN-EXTRA-Push assistance level. Similar results are consistently reported across studies from literature, where external plantarflexion torque was applied to healthy humans during gait [
4,
7,
24,
27,
31,
33,
35]. From the point of view of designing a device for training in rehabilitation, the hope is that the externally provided additional plantarflexion torque will, in cases of users with neuromuscular deficits, supplement the lack in plantarflexion torque generation abilities while promoting optimal patient effort through a well-selected assistance level, following the principle of the assist-as-needed paradigm.
Just like the AN-EXTRA-Push mechanism, the soleus muscle and the Achilles tendon span the ankle joint, and the forces that are exerted by the muscle result in plantarflexion torque. The medial and lateral heads of the gastrocnemius muscle also connect to the Achilles tendon and, together with the soleus muscle, share the vast majority of the burden of biological plantarflexion torque generation. In contrast to soleus and AN-EXTRA-Push, gastrocnemius spans the knee as well as the ankle joint. It is therefore not surprising that the correlation between EMG measurements and AN-EXTRA-Push assistance levels holds firmer for the soleus compared to the gastrocnemius muscle. In similar fashion to our reasoning, in the literature, most studies with plantarflexion torque exo-assistance report reduced gastrocnemius activity [
7,
24,
31,
33,
35], but not all do [
4,
27].
In the same literature, increased tibialis anterior (the main dorsiflexor and antagonist to soleus) muscle activity is also often reported [
4,
7,
27,
35,
38]. This can in most cases be attributed to co-contraction of the plantarflexor-dorsiflexor agonist–antagonist pair. Co-contractions increase the ankle joint stiffness and are likely present due to the exo user seeking additional stability. In HILO studies focused on metabolic energy consumption minimization, increases in co-contraction were observed with higher assistance levels [
7] but would generally lessen with user training [
31]. Even at maximum assistance levels, no co-contractions have been observed in our study. The only relevant increase in tibialis anterior activity connected to AN-EXTRA-Push assistance coincides with the swing phase when the foot was returned from the overly plantarflexed position after push-off.
The importance of proper assistance timing is often discussed in the literature but is not formally or explicitly defined [
3,
12,
13,
39,
40]. In recent HILO studies, the timing of, e.g., the start, the peak, or both of the assistance torque trajectory was varied until a torque trajectory with minimal metabolic cost was found [
6,
7,
8]. Similar optimization techniques were also attempted to minimize chosen muscle activity metrics [
9,
10]. In both cases, once the optimization was completed for the individual, the chosen control law (torque trajectory deemed optimal) was applied to the exo for the rest of the experiment.
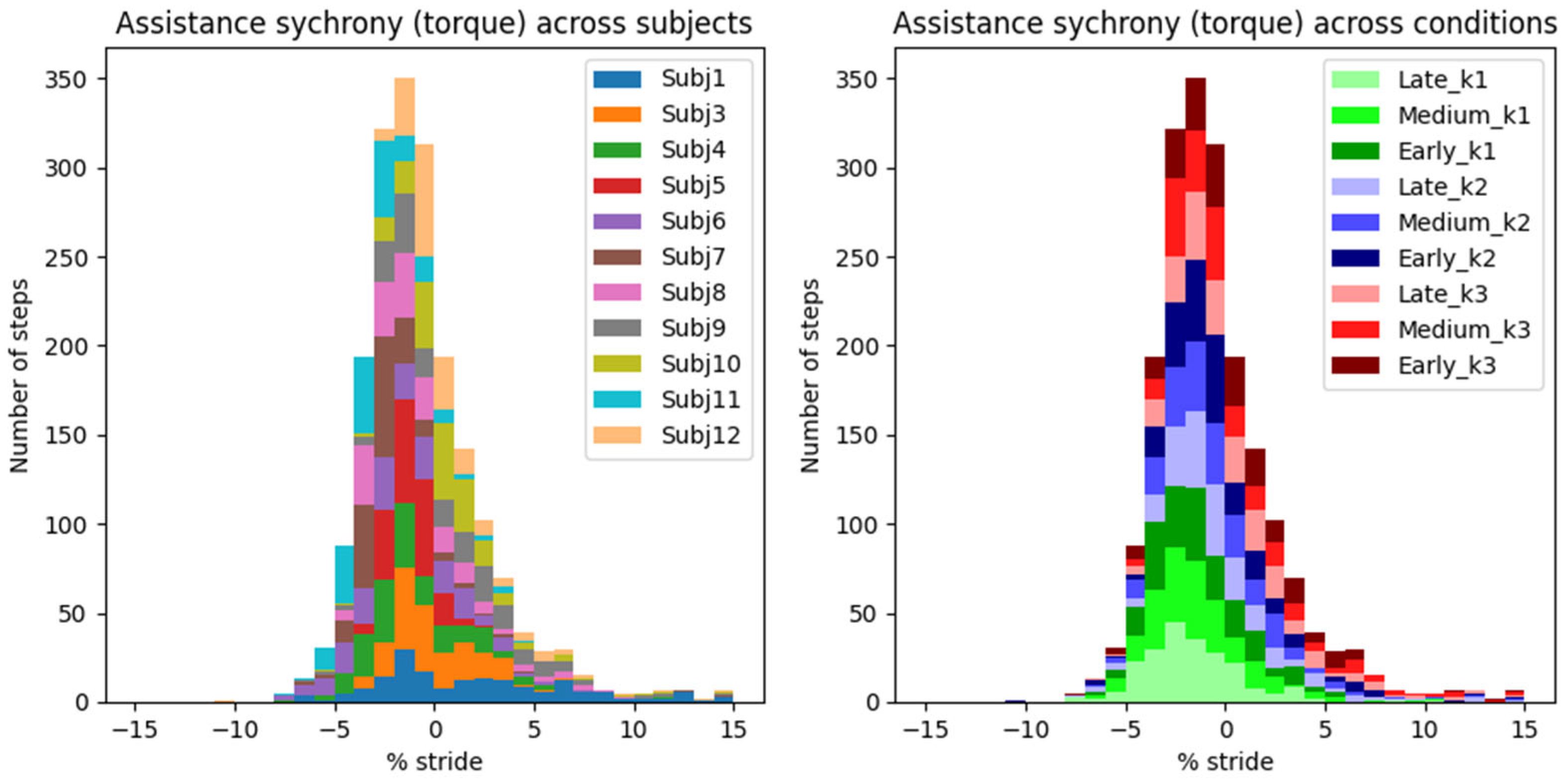
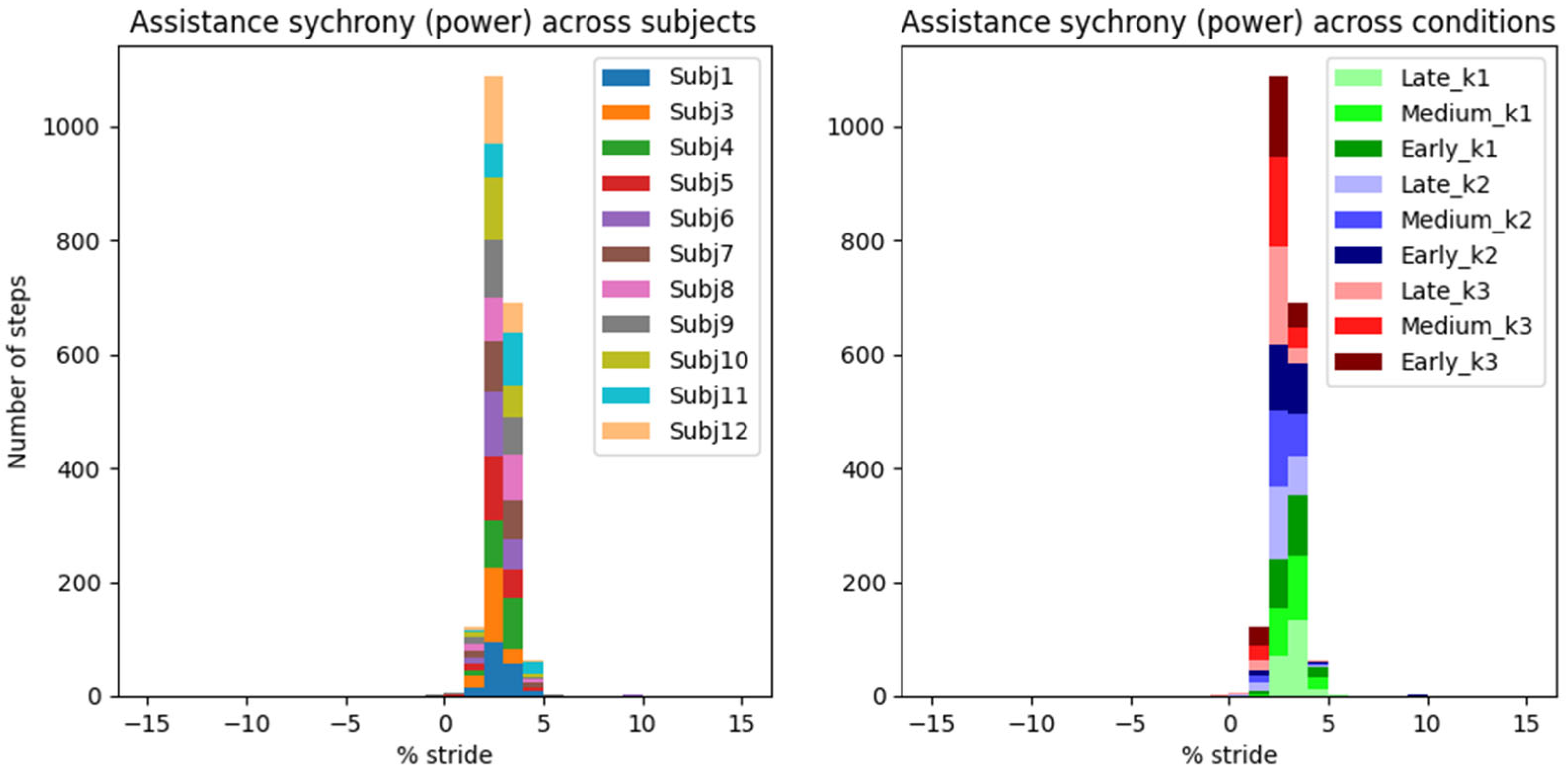
The “control law” of AN-EXTRA-Push is baked into its mechanical design, so the peak timing of the torque trajectory cannot be optimized for, only evaluated. Our newly proposed metric of assistance synchrony is helpful in that regard but does not allow for direct comparisons with studies from literature, since it hasn’t been reported. We attempted to calculate the assistance synchrony metric for a few relevant example studies in the field based on publicly accessible experimental data.
In [
7], which features HILO-minimizing metabolic energy cost and uses an active ankle exo, the peak exo torque reached 9.5% GC, and the peak exo power reached 1.4% GC after the peak of biological ankle plantarflexion torque and power, respectively. In [
33], an example of a proportional myoelectric controller without optimization is presented. Peak exo torque and power occurred 9.8% GC and 2.0% GC after peak biological torque and power, respectively. In [
4], a passive ankle exo is featured. This time, the peak exo torque and power both preceded their biological counterparts by around 1.2% GC. In all three examples, the exo torque trajectories resembled the scaled-down biological torque trajectories. Both soleus muscle activity and metabolic cost were reduced in all cases. Based on these results, we estimate that a well-synchronized biomimetically shaped torque trajectory should likely reach its peak between −1% GC and 10% GC after the biological torque peak. The timing constraints for the power appear much stricter at −1% GC to 2% GC after the biological power peak.
In our study, the peak exo torque occurred on average 0.96 ± 0.07% (mean ± standard error of mean) GC before peak biological torque. Peak exo power occurred 2.41 ± 0.04% (mean ± s.e.m.) GC after peak biological power. These timings are very similar to those in [
2] and highly repeatable across different assistance levels and users.
In a rehabilitation setting, we argue that this high degree of repeatability is especially important, since the device should behave in a safe and robust way at all times. The assistance synchrony achieved by AN-EXTRA-Push even in spite of intersubject repeatability bodes well for use with patient populations, where a lot of intrasubject variability in gait patterns is expected. This stands in contrast to HILO-optimized controllers, usually researched in the context of human augmentation, which rely on regularity of gait.
Study Limitations and Generalizability of Results
We acknowledge that our study has limitations. The main limiting factor in subject recruitment was the fact that the AN-EXTRA-Push prototype used in this study could only be used by people with EU shoe sizes 42 to 46. This resulted in a cohort of only males with similar anthropometric characteristics, not representative of the general population. For future studies and potential integration of the device in clinical protocols, a new version of the device will be produced, featuring a large range of shoe size options and shank attachment point locations. With it, we will be able to adjust for much more anthropometric variability, enabling us to recruit a more diverse and gender-balanced study cohort.
Participants were forced to diverge from their preferred gait pattern due to how the treadmill was constructed. The belts of the split-belt treadmill were spaced 24 mm apart, which imposed a wider than preferred gait pattern onto the participants. The non-instrumented belt was only 81 cm long, which was sufficient to enable walking with a typical preferred stride length [
41], but restricted whole-body anteroposterior movement. Additionally, all limitations concerning the ecological validity of treadmill walking for the purpose of improving overground gait [
42] are inherent to our device.
While wearing the AN-EXTRA-Push orthosis, a reflective marker could not be placed at the location on the shank that is prescribed by the Plug-In Gait model. The marker was instead placed on the orthosis, and the model was modified accordingly.
In spite of the homogeneity of this study cohort, we observed large intersubject variability in measured gait patterns, including during the ZeroTorque condition. AN-EXTRA-Push assistance during active conditions affected all participants in substantially similar ways, with minor deviations from the results reported for the average gait cycles shown in
Figure 3,
Figure 4,
Figure 5 and
Figure 6. Crucially, the exo assistance remained synchronous with the participants’ own efforts across their different gait patterns, as explicitly shown in
Figure 7 and
Figure 8.
This study only included able-bodied individuals, so any clinical translation is so far speculative. While the effects of AN-EXTRA-Push were consistent with our expectations and comparable to state-of-the-art ankle exos used in rehabilitation [
29,
30], clinical studies on patient groups with specific impairments are the necessary next step.